Abstract
Herbal toothpaste with natural ingredients is more acceptable in public opinion than chemical-based synthetic formulations in the current oral dental care scenario due to their safety and efficacy in reducing dental caries and preventing other dental disorders to which this generation is prone. In this composition, we use Clove oil, Tulsi oil, Mango leaf powder, all of which have never been used together before in any research. These extracts have anti-ulcer, anti-caries, anti- bacterial, as well as certain unique properties including anti-oxidant and anti-fungal. The physicochemical evaluations of selected plants were within the pharmacopeial limit and phytochemical screening in ethanol and water extracts showed the intensive presence of constituents such as Alkaloids, Glycosides, Tannins, Volatile oils. The formulated Herbal toothpastes were evaluated to determine important physical characteristics such as pH, extrudability, spreadability, foamability, and Abrasiveness, Shape retention in order to develop a more effective and stable product. All invitro evaluations of prepared herbal toothpaste showed better result. The developed formulation showed anti-bacterial activity against bacterial culture and produced a better zone of inhibition. The purpose of this project is to make and test herbal toothpaste. This research proves that our herbal-based toothpaste formulation with natural ingredients is as excellent as it gets in terms of performance.
Keywords
Herbal toothpaste, Anti-bacterial, Dental care.
Introduction
1.1 Cosmetics
As per Drug and Cosmetics Act 1940 and Rules 1945 “Cosmetics is defined as any article intended to be rubbed, poured, sprinkled or sprayed on or introduced into, or otherwise applied to the human body or any part therefore cleansing, beautifying, promoting attractiveness or altering the appearance. [1].
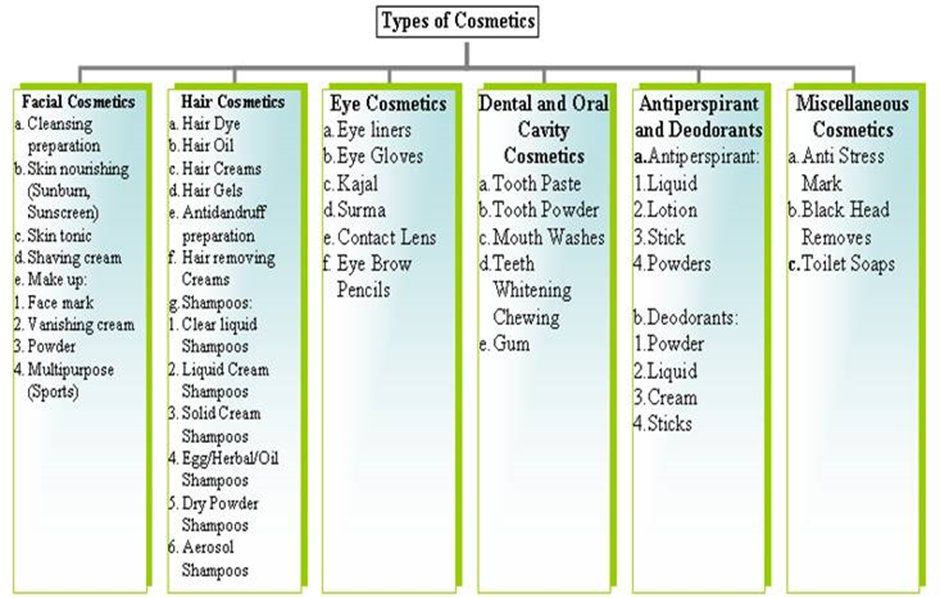
Figure 1.1 Classification of Cosmetics
1.2 Oral And Dental Cavity
Anatomy of the oral cavity includes the lips, hard palate (the bony front portion of the roof of the mouth), soft palate (the muscular back portion of the roof of the mouth), retromolar trigone (the area behind the wisdom teeth), front two-thirds of the tongue, gingiva (gums), buccal mucosa (the inner lining of the lips and cheeks), and floor of the mouth under the tongue.[2]
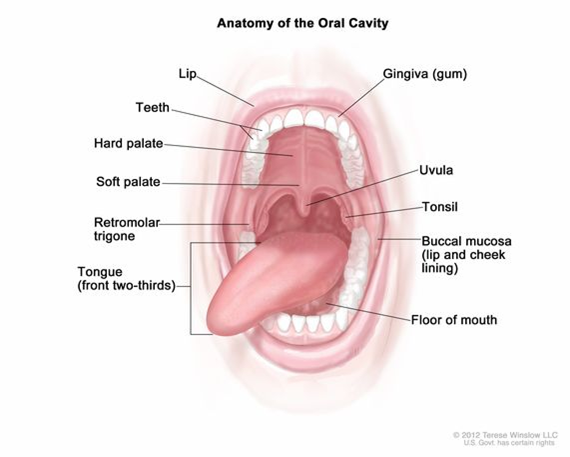
Figure 1.2 Anatomy of oral cavity
1.2.1 Parts of the oral cavity
- Lips
- Buccal mucosa
- Tongue
- Teeth
1.2.2 Teeth
The teeth are the strongest and most rigid substances in the human body. A normal adult has 32 teeth and is divided into incisor, canine, molars and premolars. There is a third molar, which is called the wisdom teeth, which appear in a person’s late teens or early twenties.Each tooth consists of a crown with one or more tips, a neck, and a root. The Pulp cavity is the centre part of the tooth, filled with blood vessels, nerves and connective tissues and is surrounded by an enamel, which protects the tooth against scratch, cut and the invasion of bacteria present in the mouth.
1.2.3 Types of teeth
We have different types of teeth, and each type serves an important purpose. There are four types of permanent teeth in humans.
- Incisors
- Canines
- Premolars
- Molars
A. Incisors
Your incisors are the most visible teeth in your mouth. Most people have four incisors on the upper jaw and four on the lower. These include your front two teeth and the teeth on either side of them. Each incisor has a single narrow edge, which helps cut into food when you bite.
B. Canines
Canine teeth get their name because they resemble a dog’s fangs. They’re pointier than other types of teeth. Most people have four canine teeth — one in each quadrant (upper right, upper left, lower right, lower left). Canine teeth help you tear into foods like meat and crunchy vegetables. Sometimes, people call canines “eye teeth” because of their position directly under your eyes.
C. Premolars
Also called bicuspids, premolars sit between your canines and your molars (the teeth in the back of your mouth). Premolar teeth have features of both canines and molars. They help you tear, crush and grind food into smaller pieces.
D. Molars
Your molar teeth are in the very back of your mouth. Most of your chewing, about 90% takes place here. Most adults have 12 molar teeth, three in each quadrant. Molar teeth include wisdom teeth (third molars). So, if you’ve had your wisdom teeth removed, or if you were born without them, then you probably have eight molars altogether. [3]
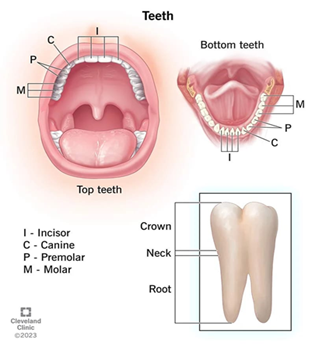
Figure 1.3 Teeth
1.3 Common Problems Associated with Oral Cavity
- Tooth decay
- Periodental disease
- Trench mouth
- Tooth erosion
- Stain teeth
- Dental cavities
A. Tooth decay
Tooth decay also known as dental caries or cavities. It is a breakdown of teeth due to acids made by bacteria. The most common bacteria associated with dental cavities are Streptococcus mutan and Streptococcus sobrinus and Lactobacillus.
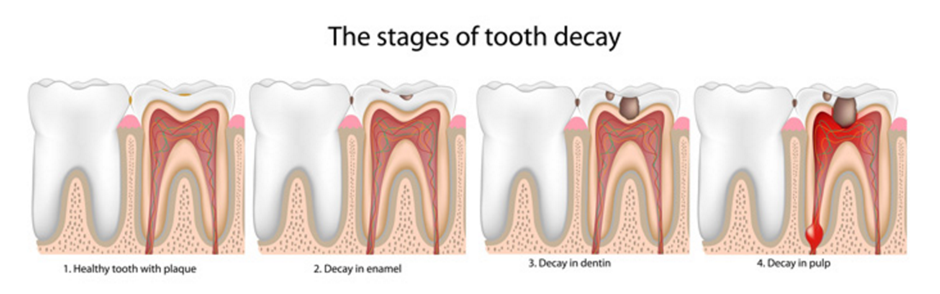
Figure 1.4 Stages of tooth decay
B. Periodental disease
Periodental disease is a serious gum infection that damage the soft tissue and destroy the bone that support the teeth. Periodental disease can cause teeth to loosen or lead to tooth loss. It is caused by Actnomyces.
They are of two types:
C. Trench mouth
It is a severe gum infection caused by build up of bacteria. It is caused by Prevatella intermedia. It generally occurs in HIV infected patients.
D. Tooth erosion
It is defined as the irreversible loss of tooth structure due to chemical dissolution by acids not of bacterial origin. The most common cause of erosion is by acidic foods and drinks of pH below 5-5.7. It is caused due to accumulation of food particles, accumulation of bacteria in gelatinous mass called as plaque, release of lactic acid and anaerobic fermentation of sugars in saliva.
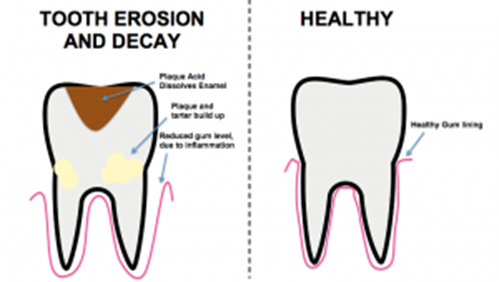
Figure 1.5 Tooth erosion
Stain teeth
Teeth can become discoloured by stains on the surface or by changes inside the tooth.
- Extrinsic: This occurs outside the layers of teeth
- Intrinsic
- Age related factors

Figure 1.6 Stain teeth
Dental cavities
Permanently damaged areas in teeth that develops into tiny holes. Causes include bacteria, snacking, sipping sugary drinks and poor teeth cleaning. [4]
1.4 Herbal Formulations
Herbal formulations means a dosage form consisting of one or more herbs or processed herbs in specified quantities to provide specific nutritional, cosmetic benefits meant for use to diagnose, treat, mitigate diseases of human beings or animals, alter the structure or physiology of human beings or animals. Herbal formulation contain an active substance or herbal substance in combination with one or more herbal preparations. Herbal formulation are obtained by subjecting herbal substances to treatments such as extraction, distillation expression, fractionation, purification, concentration or fermentation include comminuted or powdered. [5]
-
-
- ADVANTAGES
- These medicines are used to treat the cause of the issue you are having and not just mask the symptom, because herbal medicines contain vitamins, antibodies and other health promoting agents.
- It helps to strengthen the overall body not just combat illness.
- Less toxic or having less side effects in contrast to synthetic drugs.
- Effective, specific, stable, and potent.
1.4.2 DISADVANTAGES
- They may cause other problems if you’re taking other medicines.
- You may experience a bad reaction or side effects after taking a herbal medicine.
- Not all herbal medicines are regulated.
- Evidence for the effectiveness of herbal medicine is generally very limited.
1.5 Herbal Cosmectics
Herbal cosmetics are formulated using different cosmetic ingredients to form the base in which one or more herbal ingredients are used to cure various skin aliments. Plants are highly used for the development of new drug products for cosmaceuticals and pharmaceutical applications. The demand of herbal medicine is increasing rapidly due to their skin friendliness and lack of side effects. The best thing of the herbal cosmetics is that it is purely made by the herbs and shrubs and thus is side effect free. The natural content in the herbs does not have any side effects on the human body; instead provide the body nutrients[6].
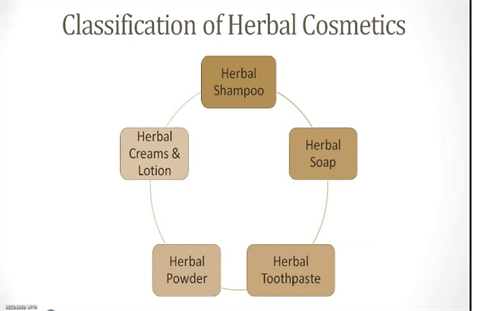
Figure 1.7 Classification of herbal cosmetics
1.6 Herbal Toothpaste
Tooth paste is a paste or gel dentifrice used with a toothbrush as an accessory to clean and maintain the aesthetics and health of our teeth promoting oral hygiene. Nowadays most of the toothpaste contains chemicals that produce harmful effect to the teeth and gums , hence herbal toothpaste is a wise and healthier choice for those who would like to minimize the amount of chemicals that could potentially endanger general health. Herbal ingredients have been present in oral care products, more commonly produced in south Asian countries.[7]
1.6.1 Benefits
- Natural toothpastes can eliminate the bacteria in your mouth naturally with out using harmful chemicals.
- Natural toothpastes are free of artificial flavoring and dyes.
- The herbal toothpaste formulated which can satisfy all the required condition to keep the mouth fresh and prevent tooth decay by bacteria.
- Toothpaste have anti-cavity properties
2. Review Of Literature
- Karandikar S M, Pandit V A et al (1997)[5] Have reported that polyherbal formulations are products from medicinal plants. Pharmacological effects of many plants have been studied in various laboratories in India. However. there are many limitations regarding safety and efficacy of these preparations. Knowledge about active principles of herbal preparations are not well defined, information on toxicity and adverse effects of these formulations are lacking. They also explained about selection of plant materials based on quality which will increase the acceptability of herbal preparation as therapeutic agents.
- Shah C.S et al (1985-86)[6] Had undertaken a study on the natural herbs and their products used for aromatic value in cosmetic preparations. The increased demand for the natural product has created new avenues in cosmeceuticals market.
- Mangilal T and Ravikumar M et al(2016)[7] Had undertaken a study of on herbal toothpaste containing natural ingredients are more acceptable in public belief than chemical based synthetic formulations due to their safety and efficacy in reducing dental caries .They also demonstrate that the extract of natural ingredients posses many activities like anti-cancer, anti-ulcer, anti-caries, anti-bacterial etc.
- Silva GO, Abeysundara AT et al (2017)[26] Showed that preliminary phytochemical analysis and screening of extracts of plants. Phytoconstituents individually or in the combination, determine the therapeutic value of a medicinal plant. Alkaloids, flavonoids, phenolics, tannins, saponins, steroids, glycosides, terpenes etc are some of the important phyto chemicals with diverse biological activities .It states that the pharmacological activity of a plant can be predicted by the identification of the phytochemicals.
- Marongiu, Bruno et al (2005)[29] Reported in detail the extraction of essential oil and identification of clove oil by clevenger apparatus. Eugenol is the main volatile compound extracted oil from clove bud ( Eugenia caryophyllus), and used in traditional medicine, as a bactericide, fungicide, anesthetic, and others. Its extraction was performed using hydro distillation (CLEVENGER) which is the most common extraction technique.
- Dange VN, Magdum C.S et al (2008)[33] Showed that herbal toothpaste containing natural ingredients are more acceptable in public belief than chemical based synthetic formulations due to their safety and efficacy in reducing dental caries, and preventing other dental issues.
- Nishad U, Ali M et al (2020)[35] Have reported that this formulated herbal toothpaste complies with all the required condition and can prevent tooth decay by bacteria. They also demonstrated the various evaluation methods of formulated herbal toothpaste includes determination of pH, spreadability, extrudability, foamability, viscosity, abrasiveness, shape retention etc.
Prashant GM, Chandu GN et al (2007)[49] He demonstrates The antibacterial activity against Staphylococcus aureus reveals that formulated herbal toothpaste exhibited notable activity with zone of inhibition. They undertaken a study about agar well diffusion method by using streptococcus mutans as culture micro oraganism.
3.AIM And Objective
3.1 Aim of the study
Oral diseases like caries and periodontitis affect people all over the world and cause a lot of stress. Toothpaste seems to be the most important chemical product used to keep teeth clean. Toothpaste with chemical agents are mostly used to keep the teeth clean, however the fluoride content in most of the chemical toothpaste have negative side effects, such as altered taste and discoloured teeth, and their deleterious effects on endocrine function including fertility are uncertain. In order to overcome these problems while using toothpaste containing chemical agents herbal toothpaste was used. Herbal preparations are more effective compared to conventional formulations. The present work is to develop toothpaste containing herbal ingredients to be applied in the treatment of tooth decay and bad breath. The main aim of study is to make herbal toothpaste by using herbal plants such as Mangifera indica, Syzygium aromaticum and Ocimum tenuiflorum. All of these plants have effective antibacterial activity.
3.2 Objectives of the study
1. Collection and authentication of the plants Mangifera indica, Syzygium aromaticum and Ocimum tenuiflorum.
2. Study of physiochemical parameters of selected plant materials.
a. Ash values(Total ash ,Acid insoluble ash, water soluble ash).
b. Extractive values (Water soluble extractive value ,alcohol soluble extractive value).
c. Moisture content.
3. Extraction of plant material .
4. Qualitative analysis of the extract (Phytochemical screening) for the presence of various phytoconstituents.
5.Formulation of herbal toothpaste
6. Evaluations of prepared herbal toothpaste
4.MATERIALS AND METHODS
4.1 List Of Materials
Table no:1 Plant material used for the development of formulation.
|
SL.NO
|
Botanical Name
|
Vernacular Name
|
Source/Supplier
|
|
1.
|
Mangifera indica
|
Mango
|
Indoor ventures
|
|
2.
|
Syzygium aromaticum
|
Clove
|
Indoor ventures
|
|
3.
|
Ocimum tenuiflorum
|
Holy basil
|
Indoor ventures
|
Table no:2 Chemical ingredients used for the development of formulation
|
SL.NO
|
Materials/Solvents
|
Manufacturer/Supplier
|
|
1.
|
Sodium lauryl sulphate
|
Burgoyne Burbidges &Co, Mumbai India
|
|
2.
|
Sodium benzoate
|
Medilise chemicals, Kannur, Kerala, India
|
|
3.
|
Sodium saccharine
|
Burgoyne Burbidges &Co, Mumbai India
|
|
4.
|
Glycerin
|
Medilise chemicals, Kannur, Kerala, India
|
|
5.
|
Calcium carbonate
|
Medilise chemicals, Kannur, Kerala, India
|
|
6.
|
Peppermint oil
|
Fortune chemicals Melmuri, Kerala, India
|
Table no 3: Equipments used for the formulation.
|
SL.NO
|
Equipments
|
Supplier/Manufacturer
|
|
1.
|
Digital weighing balance
|
SHIMADZU AY 220
|
|
2.
|
Clevenger apparatus
|
Borosil
|
|
3.
|
Muffle furnace
|
Set win
|
|
4.
|
Desiccator
|
Kawanan international, Kannur
|
|
5.
|
Hot air oven
|
CAT-NO-RHO-14/ROTEK
|
|
6.
|
Mortar and pestle
|
Universal agencies
|
|
7.
|
Digital pH meter
|
Jain-LT-10/Labtronics
|
|
8.
|
Incubator
|
Medwin Diagnostics
|
4.4 Plant collection
The fresh leaves of Mangifera indica, Syzygium aromaticum and Ocimum tenuiflorum were collected from Kasargod and Kannur district, Kerala (India) in the month of March 2023. The plant material were identified and authenticated by Dr. Harikrishnan. E, Assistant Professor Department of Botany, Payyanur Collage, Payyanur, Kannur, Kerala. Mangifera indica, Syzygium aromaticum and Ocimum tenuiflorum were dried under shade.
4.4.1 Plant profile
a. Mangifera Indica

Figure 4.1 Mangiferra indica
Scientific classification of Mangifera indica
Kingdom Plantae – Plants
Subkingdom Tracheobionta – Vascular plants
Superdivisionm Spermatophyta – Seed plants
Division Magnoliophyta – Flowering plants
Class Magnoliopsida – Dicotyledons
Subclass Rosidae
Order Sapindales
Family Anacardiaceae – Sumac family
Genus Mangifera – mango
Species Mangifera indica L. – mango
Vernacular names
Sanskrit Ambrah, Madhuulii, Madhuula, Madhuulaka;
English Mango
Hindi Aam
French mangot, mangue, manguier;
Portuguese manga, mangueira
Dutch manja
Tamil Ambiram, Mambazham, Mambalam, Mangai
Punjabi Amb, Wawashi
Gujarati Ambo, Keri, Marvo (unripe)
Kashmiri Amb
Malayalam Amram, Choothaphalam, Manga, Manpalam, Mavu
Marathi Amchur, Amba
Synonyms
Mangifera amba Forssk, Mangifera anisodora Blanco, Mangifera austroyunnanensis Hu, Mangifera balba Crevost & Lemarié, Mangifera cambodiana (Pierre) Anon.[8]
Morphological characteristics
MI is a large evergreen tree in the anacardiaceae family that grows to a height of 10-45 m, dome shaped with dense foliage, typically heavy branched from a stout trunk. The leaves are spirally arranged on branches, linear-oblong, lanceolate – elliptical, pointed at both ends, the leaf blades mostly about 25-cm long and 8-cm wide, sometimes much larger, reddish and thinly flaccid when first formed and release an aromatic odour when crushed. The inflorescence occurs in panicles consisting of about 3000 tiny whitish-red or yellowish – green flowers. The fruit is a well known large drupe, but shows a great variation in shape and size. It contains a thick yellow pulp, single seed and thick yellowish – red skin when ripe. The seed is solitary, ovoid or oblong, encased in a hard, compressed fibrous endocarp.
Phytochemical constituents
Mangiferin, followed by phenolic acids, benzophenones, and other antioxidants such as flavonoids, ascorbic acid, carotenoids, and tocopherols.
Medicinal uses
Antibacterial, Antioxidant, Immunomodulation, Hypertensive, Antiseptic, Astringent, Anti-asthmatic.[9]
b. Syzygium Aromaticum

Figure 4.2 Syzygium aromaticum
Scientific classification of syzygium aromaticum
Kingdom Plantae
Sub kingdom Tracheobionta
Super division Spermatophyta
Division Magnoliphyta
Class Magnoliopsida
Subclass Rosidae
Order Myrtales
Family Myrtaceae
Genus Syzygius
Species aromaticum
Vernacular names
Sanskrit Lavanga, Devapuspa, Varala, Bhadrasriya
Hindi Laung, Lavang
Malayalam Krayampu, Grampu
Marathi Luvang
Tamil Grambu, Kirampu, Kirambu, Lavanga,
Kannada Daevakusuma
Telgu Lavangalu, Devakusumamu
Bengali Lavanga
Gujarati Lavang
Punjabi Laung
Oriya Labanga
Urudu Laung, Loung
Morphological characteristics
The Clove tree is a slow growing, evergreen tree that ranges greatly in maximum height, from a relatively small, shrubby treelet at 8m to a medium sized tree of up to 20m. It has a dense conical crown when young, but becomes cylindrical or pyramidal when mature., Propagation, Propagated from softwood cuttings and seeds. Fresh seeds should be sown immediately as viability rapidly declines. Seed germination occurs in 4 to 5 weeks and seedlings can be tranplanted when they reach 25cm in height.
Phytochemical constituents
Quercetin, ursolic acid, ,eugenol followed by ?-caryophyllene , ?-humulune and eugenyl acetate as the main components. Gallotannic acid, and two crystalline principles; ?- and ?- caryophyllenes, methyl furfural, gum, resin, and fibre.[10]
Medicinal uses
Antibacterial, Antifungal, insecticidal, antioxidant, Antiseptic in oral infections, Toothache, Dental plaque, Antiviral.[11,12]
3. Ocimum Tenuiflorum

Figure 4.3 Ocimum tenuiflorum
Scientific classification of Ocimum tenuif
Kingdom Plant
Division Magnoliophyta
Class Magnoliopsida
Order Lamiales
Family Lamiaceae
Genus Oscimum
Species O. tenuiflorum
Synonym
Holy Basil, Sacred Basil, Tulasi.
Vernacular names
Sanskrit Tulasi, Tulsi,
Surasa, Krishnam, Vishnu-priya
Hindi Kala, Tulasi
English Holy basil
Bengali Krishna tulasai
Tamil Thulasi
Morphological characteristics
Tulsi belonging to family Labiatae, is an aromatic herb, is native across the Eastern World tropics and distributed as a cultivated herb and weed. The herb is an erect with many branched sub-shrub.
Height: 30-75 cm.
Stems: Hairy.
Leaves: Green with petiole, ovate or oblong, 5 cm long and slightly toothed, acute with entire or serrate mar…
Phytoconstituents
Isothymusin,ursolic acid, eugenol, and sinapic acid,methyl cinnamate, camphor and thymol,saponins, flavonoids, triterpenoids, and tannins.[13,14]
Medicinal uses
Antimicrobial, Antiviral, Antifungal, Antimalarial, Antiprotozoal, Teeth whitening, Anti asthmatic, Anti stress, Antiemetic.[15,16]
4.5 Pharmacognostic Study
A. Determination of moisture content
The grams of the leaves were weighed in a tared evaporating dish. It was dried out at 105°C with intermittent weighing. The drying and weighing at 1 hour interval was continued until the difference between two successive weighing was not more than 0.25%. Constant weight was reached when two consecutive weighing after drying for 30 minutes and cooling for 30 minutes in desiccators, showed not more than 0.01 gm difference. The percentage of moisture present in the sample was calculated.[17,18]
B. Determination of ash value
The ash value is an important parameter for the evaluation of crude drugs, due to the variation of value within fairly wide limits. The ash value of any organic material is composed of inorganic materials like metallic salts and silica.
The following three different methods were adopted.
• Total ash
• Acid insoluble ash
• Soluble ash
Ashing involves the oxidation of components of the products and a high ash value indicates the contamination, substitution, adulteration, or carelessness in the preparation of crude drugs for marketing[19].
i. Total ash
Three grams of ground air dried material was accurately weighed out in a crucible previously ignited for 30 minutes. The material was spread in an even layer and ignited at a temperature of 500-600°C until it was white indicating the absence of carbon; cooled in a desiccator and weighed. The content of total ash per gram of air dried material was calculated.
ii. Acid insoluble ash
To the crucible containing the total ash, 25 ml of 2N hydrochloric acid was added, covered with a watch-glass and boiled gently for 5 minutes. The watch-glass was rinsed with 5 ml of hot water and this liquid was also added into the crucible. The insoluble matter was collected on an ashless filter paper and washed with hot water until the filtrate was neutral. Filter-paper containing the insoluble matter was transferred to the original crucible, dried on a hot plate and ignited to constant weight. The residue was allowed to cool in a desiccator for 30 minutes and then weighed. The content of acid insoluble ash per gram of air-dried material was calculated[20,21].
iii. Water soluble ash
To the crucible containing the total ash, 25 ml of water was added and boiled for 5 minutes. The insoluble matter was collected in a sintered-glass crucible, washed with hot water and ignited in a crucible for 15 minutes at a temperature not exceeding 450°C. The weight of this residue in milligram was subtracted from the weight of total ash. The content of water soluble ash was calculated per gram of air-dried material. The results are given in Table no. –

Figure 4.4 Ash

Figure 4.5 Muffle furnace
C. Determination of extractive value
This method determines the amount of active constituents in a given amount of plant material when extracted with a solvent. As mentioned in different official books, the extractive value is used as a means of evaluating crude drugs which are not readily estimated by other means. For example, lowering from the prescribed values indicates the addition of exhausted or unwanted material with the original drug or incorrect processing of the drug.[22]
i. Alcohol soluble extractive value
Macerated 5 grams of coarsely powdered air dried leaf (Mangifera indica, Eugenia caryophyllata, Ocimum tenuiflorum) separately with 100 ml ethanol in a stoppered flask for 24 hours, with occasional shaking during the first 6 hours and then allowed to stand undisturbed for another 18 hours. Filtered rapidly, by taking precaution against loss of alcohol. Then 25 ml of the filtrate was evaporated to dryness in a tared flat bottomed shallow dish, dried at 105°C and weighed. Calculated the percentage w/w ethanol soluble extractive with reference to air dried material. The results are given in Table no.[23]
ii. Water soluble extractive value
Macerated 5 grams of coarsely powdered air dried leaf(Mangifera indica, Eugenia caryophyllata, Ocimum tenuiflorum) separately with 100 ml chloroform water in a stoppered flask for 24 hours, with occasional shaking during the first 6 hours and then allowed to stand for another 18 hours. Filtered rapidly, then 25 ml of the filtrate was evaporated to dryness in a tared flat bottomed shallow dish and dried at 105°C and weighed. Calculated the percentage w/w of water soluble extractive with reference to air dried material[24].

Figure 4.6 Extraction
4.6 Preparation of plant extracts
The collected leaves are carefully washed under water and was dried at shade for 10-15 days. These shade dried plant material were homogenized to a coarse powder using an electronic blender and then stored at air tight container until further use .The organic solvents such as ethanol and water was used for extraction .10 gm of homogenized coarse leaf powder of Mangifera indica, Syzygium aromaticum, and Ocimum tenuiflorum was soaked in different conical flasks containing 10 ml of ethanol and water .Then it is allowed to stand 30 minutes with occasional shaking, finally each sample extract was filtered through whatmanNO:1 filter paper .This filtrate used to detect various biologically active constituents present in various solvent extract.[25]
4.7 Phytochemical Screening
4.7.1 Preliminary phytochemical screening of the various extracts[26,27,28]
A. Chemical test for alkaloids
- Mayer's test: To the leaf extract add 2 ml of Mayer's reagent shaken, a dull white or creamy precipitate indicates the presence of alkaloids
- Dragendorff's test: Take 1 ml of extract in a test tube and few drops Dragendroff's reagent appearance of orange or red colour reveals the presence of alkaloids.
- Hager's test: Add 2 ml of Hager's reagent to the leaf extract, formation of yellow colour precipitate confirms the presence of alkaloids.
B. Chemical test for glycosides
- Legal's test: The extract was dissolved in pyridine, to this add freshly prepared sodium nitroprusside solution then add sodium hydroxide solution to make this alkaline, Formation of pink red colour indicates the presence of cardiac glycoside.
- Keller killiani: To the extract add sulphuric acid and glacial acetic acid and ferric chloride solution. Formation of blue colour indicates the presence of glycoside.
- Borntrager's test: The extract boiled with dilute sulphuric acid filtered and to this add benzene and shake well, the inorganic layer was separated, to this add ammonia solution slowly. Formation of pink, red or violet colour indicate the presence of free hydroxyl antraquinone.
C. Chemical test for flavonoids
- Lead acetate test: To the extract add lead acetate solution. Formation of yellow colour precipitate reveals the presence of flavonoids.
- Alkaline reagent test: To the extract add sodium hydroxide solution, a yellow colour is formed which becomes colourless on the addition of dilute acetic acid indicate the presence of flavonoids.
D. Chemical test for carbohydrates
- Molish's test: To the filtrate add Molish's reagent and add slowly concentrated sulphuric acid along the side of the test tube.Formation of violet ring at the junction indicate the presence of carbohydrates.
- Benedict's test: To the filtrate add Benedict's reagent and heat. Orange red precipitate indicates the presence of reducing sugar.
- Fehling's test: To the filtrate add dilute HCL, then it is heated with fehling's A and B. Formation of red precipitate indicates the presence of reducing sugar.
E. Chemical test for saponins
- Foam test: A small quantity of extract diluted with 20 ml of water in a cylinder. The suspension was shaken for 15 min. Formation of foam indicates the presence of saponins.
F. Chemical test for phytosterols
- Liberman Burchard's test: Extract is treated with chloroform and the solution is filtered. Then boiled the solution with a few drops of acetic anhydride solution and cooled. Then concentrated sulphuric acid was added through the side of the test tube. Formation of a brown ring at the junction indicates the presence of phytosterol
- Salkowski's test: Extract is mixed with chloroform and filtered. The filtrate was treated with few drops of concentrated sulphuric acid, shaken well and allowed to stand. Formation of yellow our indicates the presence of terpenes.
G. Chemical test for Tannins and phenolic compounds
- Ferric chloride test: A small quantity of extract is diluted with water, then it is treated with ferric chloride solution and formation of bluish black indicates the positive result.
- Gelatin test: The extract was dissolved in water and filtered. To this add sodium gelatin containing sodium chloride. Noted for the presence of milky white precipitate.
- Lead acetate test: The extract dissolved in water and added lead acetate solution. Positive result shown when bulky white precipitate formed.
- Decolourisation test: The extract dissolved in water treated with dilute potassium permanganate solution, decolourisation of potassium permanganate shows positive results.
H. Chemical test for proteins and amino acids
- Million's test: To the extract add a few ml of Million's reagent and observe for the presence of white precipitate which turns to red.
- Biuret test: To the extract add sodium hydroxide solution and heat. Then add a few drops of copper sulphate solution, forming a purplish violet colour.
Ninhydrin test: To the extract add ninhydrin reagent and heat. Formation of blue colour indicates the presence of amino acid.

Figure 4.7 Preliminary phyto chemical screening
4.8 Extraction Of Plant Material
4.8.1 Extraction of clove flower buds[29,30]
Extraction of clove using Clevenger Apparatus consist of one round bottom flask of 500 ml which is connected with another two way round flask which holds raw material. The top flask is connected with condenser through the connecter. The separating funnel is used for the separation of essential oil and water. Buds (50 gm)of Syzygium aromaticum then washed the buds with water . After collection and 50 g boiled with 500 ml of distilled water in a Clevenger apparatus until oil distillation ceased after 5-6 hrs. The oil obtained after extraction was collected.
4.8.2 Extraction of holy basil[31,32]
The leaves were dried in order to reduce the initial moisture content (90 % of humidity), in an oven with air renewal and circulation. The drying temperature was 50 °C and was kept constant for 5 hours until constant moisture. After drying, the leaf’s were grinded in order to increase the contact surface reducing the resistance to oil extraction. The grinded leaves were placed in sealed plastic bags, protected from light and moisture .The extraction was done in a Clevenger apparatus, coupled to a bottom flask of 500 ml. It was added 30 g of crushed leaves of basil and 300 mL of water into the flask. The extraction time was fixed at 4 hours. The extract oil was diluted in hexane and filtered after separation oil was collected after extraction.
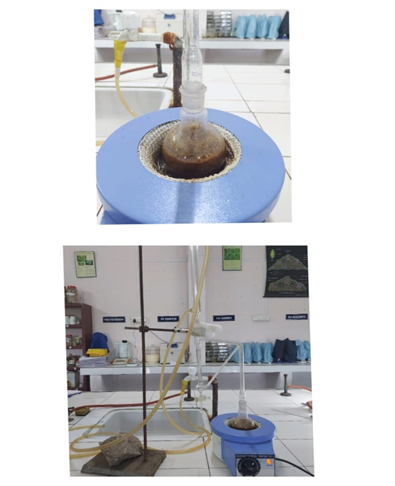
Figure 4.8 Extraction using Clevenger apparatus
4.9 Formulation Of Herbal Toothpaste[33,34]
Table no 4: Formula of the composition of herbal toothpaste
|
SL.NO
|
Ingredients (gm)
|
Quantity (w/w) %
|
Functions
|
|
1
|
Tulsi oil
|
2ml
|
Teeth whitening
|
|
2
|
Clove oil
|
2ml
|
Treat bad breath
|
|
3
|
Mango leaf powder
|
2g
|
Antibacterial agent
|
|
4
|
Sodium lauryl sulphate
|
1.5g
|
Detergent
|
|
5
|
Sodium benzoate
|
0.1g
|
Preservative
|
|
6
|
Sodium saccharine
|
0.2g
|
Sweetener
|
|
7
|
Glycerin
|
40ml
|
Humectant
|
|
8
|
Calcium carbonate
|
44g
|
Abrasive
|
Procedure
- The solid ingredients calcium carbonate, sodium lauryl sulphate, glycerin, sodium benzoate, sodium saccharin were weighed accurately as mentioned in the formula.
- These ingredients were mixed in a mortar and pestle, then triturated with precisely weighed glycerin until a semisolid substance was created.
- Accurately weighed herbal ingredients like Tulsi oil, clove oil and mango leaf powder were added to the base and triturated to form a paste.
4.9.2 Evaluation of herbal toothpaste[35,36,37]
A. Physical Evaluation
Physical parameters such as colour and appearance were evaluated.
B. Determination of pH[38]
Take 1gm of the toothpaste in a 150ml beaker and add 10ml of freshly boiled and cooled water. Stir well to make a thorough suspension and determine the pH of the suspension using pH meter.

Figure 4.10 Determination of pH using digital pH meter
C. Foamability [39]
2g of toothpaste was measured and mixed with 5ml of water in a measuring cylinder. Shake it for 10 minutes and final volume was taken.
Foamability = Final volume – Initial volume

Figure 4.11 Determination of foamability using measuring cylinder
D. Determination of spreadability[40]
Slip and drag characteristics of paste are used to determine the spreadability technique. About 1-2g of herbal toothpaste was weighed and placed between two glass slides (10 x10) that were stacked one on top of the other, and then slides were moved in opposing directions. After 3 minutes measure the amount of toothpaste that has spread. Repeat three times.
S = M x L/T
M – Weight placed
L – Length moved by glass slide
T – Time taken to separate upper slide from lower

Figure 4.12 Determination of spreadability
E. Extrudability [41,42]
In this method, the formulated paste was filled in standard capped collapsible aluminum tube and sealed by crimping to the end. The weights of tubes were recorded. The tubes were placed between two glass slides and were clamped. 500g was placed over the slides and then cap was removed. The amount of the extruded paste was collected and weighed. The percent of the extruded paste was calculated.
F.Abrasiveness[43,44]
Extrude the content 15-20 cm long on through butter paper. Repeat the same process for at least ten collapsible tubes. Press the contents of the entire length with fingertip for the presence of sharp and hard edged abrasive particles.
H.Fragrance test[45,46]
It was based on individual observation for its acceptability. 5 people were asked for acceptability of fragrance and their opinion was taken and fragrance was evaluated based on the below described criteria.
- The fragrance was good, as good as the fragrance of reference toothpaste.
- The fragrance of the toothpaste was not so good but comparable to the reference toothpaste.
- The fragrance of the toothpaste was poor than reference toothpaste.
I. Shape retention[47,48]
Toothpaste was squeezed out from the tube and put entirely on a toothbrush and the state of the toothpaste after it was allowed to stand for 10 seconds was evaluated based on the,
- Shape just after the toothpaste is squeezed out on the toothbrush is maintained.
- Shape just after the toothpaste is squeezed at on the toothbrush is almost maintained.
- The toothpaste squeezed from the toothbrush and cannot maintain its shape.

Figure 4.13 Determination of shape retention using toothbrush
4.10 Screening For Antimicrobial Activity[49,50]
4.10.1 Invitro- antimicrobial activity
Antibacterial activity was determined by agar well diffusion method using amoxicillin as standard. Activity of herbal toothpaste was tested against E.coli and lactobacillus.
- Media: Nutrient agar medium
The media was prepared by the addition of 2% agar (Bacteriological grade) selected strains were preserved by sub-culturing them periodically on nutrient agar slants and storing them under frozen condition.
- Ingredient
Beef extracts -0.3%
Peptone – 0.5%
Agar-1.5%
- Preparation
The ingredients were dissolved in distilled water with aid of heat and pH was adjusted to 7.2-7.6 using alkali or dilute acid.
- Sterilization
15-20 ml of nutrient agar was transferred to petri plates and covered. It was then autoclaved at a temperature of 121 degree celcius and pressure of 15 psi for not less than 20 minutes.
- Sample used : Formulated herbal toothpaste
- Standard drug: Amoxicillin
- Culture used:
Lactobacillus
The bacteria lactobacillus is prepared from curd. The curd is filtered and the broth is incubated at 25 degree celcius for 24 hours.
E. coli
E. coli was cultured by adding 1-2 grams of sugar to 100 ml of clear beef broth and boil for several minutes. E. coli could grow well in any medium that has a little protein and some carbohydrate.
The micro organisms are cultured and stored in our pharmaceutical biotechnology laboratory.
- Working condition
The entire work has done using horizontal laminar flow hood so as to provide aseptic conditions. Before Commencement of the work air sampling was carried out using a sterile nutrient agar plate and exposing it to the environment inside the hood .After incubation it was checked for the growth of micro organism and absence of growth confirmed aseptic working condition.
- Method
Agar well diffusion method was used. Agar plates were prepared aseptically to get a thickness of 5-6 mm. The plates were allowed to solidify and inverted to prevent condensate falling on the agar surface. The test micro organisms was spread on the agar plates by means of sterile cotton swab. The wells were then punched into agar medium and filled with herbal toothpaste and standard drug. The plates were incubated for 24 hours at 37 degree celcius. Observation were made for zones of inhibition around the formulation and compared with that of standard.
5. RESULT AND DISCUSSION
5.1 Plant collection and drying
Collected plant materials were identified and authenticated by Dr. Harikrishnan. E, Assistant professor, Department of Botany, Payyanur collage, Payyanur, Kannur, Kerala. Mangiferra indica, Syzygum aromaticum and Oscimum tenuiflorum.
5.2 Preparation of plant extracts
The collected plant materials are subjected to extraction using various solvents such as ethanol and water. The extracts obtained after maceration process was the used for phytochemical studies to choose the most suitable solvent for further extraction.
5.3 Physico-chemical parameters
After the collection of plant materials, they were shade dried and powder costly in an electronic blender and stored in air tight containers until further use. Physico-chemical parameters of plant were tabulated in table no. Parameter such as ash value, extractive values and moisture content were estimated.
Table no 5: Physico-chemical parameters of Mangiferra indica, Syzygum aromaticum and Oscimum tenuiflorum
|
SL NO
|
Test
|
Mangiferra indica
|
Syzygum aromaticum
|
Oscimum tenuiflorum.
|
|
1
|
Total Ash (%w/w)
|
14%
|
16%
|
2%
|
|
2
|
Acid insoluble Ash(%w/w)
|
1.5%
|
0.5%
|
1.5%
|
|
3
|
Water insoluble Ash (%w/w)
|
2%
|
1%
|
2%
|
|
4
|
Water soluble extractive value (%w/w)
|
5%
|
7.4%
|
4.4%
|
|
5
|
Alcohol soluble extractive value (%w/w)
|
5.6%
|
3.2%
|
3.4%
|
|
6
|
Moisture content
|
15.8%
|
5.6%
|
5.6%
|
Table no 6: Phytochemicals present in ethanol and aqueous extracts of Mangiferra indica, Syzygum aromaticum and Oscimum tenuiflorum
|
Sl no
|
Chemical constituents
|
Mangiferra indica
|
Syzygum aromaticum
|
Oscimum tenuiflorum.
|
|
|
|
Water extract
|
Ethanol extract
|
Water extract
|
Ethanol extract
|
Water extract
|
Ethanol extract
|
|
1
|
Alkaloids
|
+
|
+
|
_
|
+
|
+
|
+
|
|
2
|
Carbohydrate
|
_
|
_
|
_
|
+
|
_
|
_
|
|
3
|
Flavanoids
|
+
|
+
|
_
|
+
|
_
|
_
|
|
4
|
Saponins
|
+
|
_
|
_
|
_
|
+
|
+
|
|
5
|
Glycosides
|
+
|
+
|
_
|
_
|
_
|
_
|
|
6
|
Tannins
|
+
|
+
|
+
|
+
|
_
|
+
|
|
7
|
Phenols
|
+
|
+
|
_
|
_
|
+
|
_
|
5.4 Formulation of herbal toothpaste
In the present study herbal toothpaste were prepared.

Figure 5.1 Formulation of herbal toothpaste

Figure 5.2 Packed herbal toothpaste
5.5 Evaluation of herbal toothpaste
Table no 7: Physical evaluation
|
S. NO
|
Parameters
|
Observation
|
|
1.
|
Colour
|
Beige brown
|
|
2.
|
Odour
|
Characteristics
|
|
3.
|
Taste
|
Sweet
|
|
4.
|
Smoothness
|
Smooth
|
The formulated herbal toothpaste were visually inspected for their colour, odour, state, and its constituency was determined .Result are shown in the table 7.
Table no 8: Evaluation result
|
SL NO
|
Parameters
|
Observation
|
|
1
|
pH determination
|
8.58
|
|
2
|
Foaming determination
|
10 ml (good)
|
|
3
|
Spreadability
|
3.3cm/sec
|
|
4
|
Abrasiveness
|
Good abrasives
|
Table no 9: Extrudability
|
SL NO
|
Extrudability
|
Mean of Three tube
|
|
1
|
Net wt. of formulation in tube (gm)
|
13.01gm
|
|
2
|
Wt. of toothpaste extruded (gm)
|
10.9gm
|
|
3
|
Extrudability amount percentage
|
83.78%
|
Table no 10: Fragrance test
|
SL NO
|
Observer
|
Opinion
|
|
1
|
Tester 1
|
A
|
|
2
|
Tester 2
|
B
|
|
3
|
Tester 3
|
A
|
|
4
|
Tester 4
|
C
|
|
5
|
Tester 5
|
A
|
5.5.1 Shape retention
Toothpaste was squeezed out from the tube and put entirely on a toothbrush and the state of the toothpaste after it was allowed to stand for 10 seconds was evaluated as, Shape just after the toothpaste is squeezed out on the toothbrush is maintained.
5.6 In-Vitro Anti -Microbial Activity Evaluation
The anti microbial activity of the developed formulation was done by using agar well diffusion method. The anti-bacterial activity was measured in terms of zone of inhibition. The zone of inhibition obtained for herbal toothpaste was compared with the standard Amoxicillin The developed formulations showed anti-bacterial activity against Lactobacillus and E.coli produced a better zone of inhibition of about 6mm,7mm respectively which was near to the zone of inhibition produced by standard Amoxicillin (10mm,8mm).

Figure 5.3 Preparation of culture media
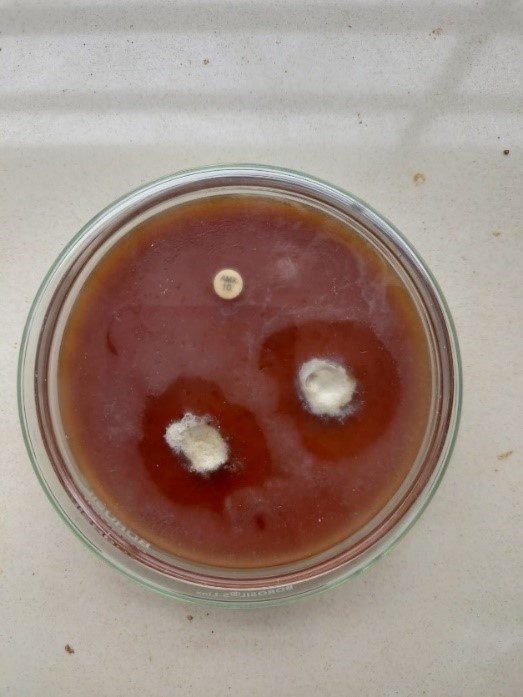
Figure 5.4 Zone of inhibition of formulated herbal toothpaste against lactobacillus

Figure 5.5 Zone of inhibition of formulated herbal toothpaste against E.coli
Table no.11 Result showing Anti-bacterial activity of Herbal Toothpaste formulation
|
SL.NO
|
Bacteria
|
Diameter of the zone of inhibition(mm)
|
|
Amoxicillin
|
Herbal toothpaste
|
|
1.
|
Lacto bacillus
|
10
|
6
|
|
2.
|
E.coli
|
8
|
7
|
Result showing Anti- bacterial activity of Herbal toothpaste formulation
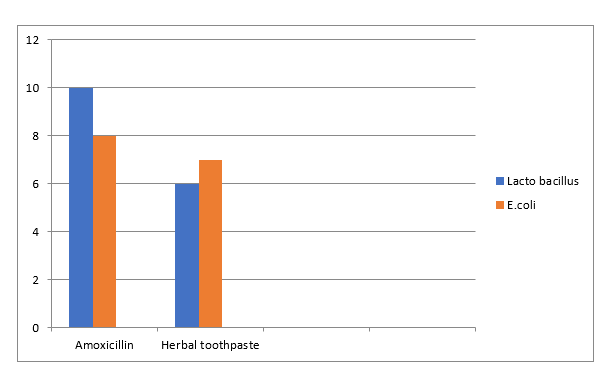
Figure 5.6 Result showing anti-microbial activity of Herbal toothpaste by using Lacto bacillus and E.coli
DISCUSSION
The present work is to develop toothpaste containing herbal ingredients to be applied in the treatment of tooth decay and bad breath. Continuous usage of synthetic agents containing toothpaste causes various side effects such as altered taste, discoloured teeth etc. in order to overcome these problems associated with synthetic agents herbal toothpaste were considered as the better choice. There is a greater possibility in the development of herbal formulation as it is safe, effective, convenient and economic.
The main aim of the study is to make herbal toothpaste using herbal plants such as Mangifera indica, Syzygium aromaticum and Ocimum tenuiflorum. The plants were selected on the basis of their therapeutic potential, antibacterial activity, selective therapeutic action on oral cavity and ease of availability. The leaves of Mangifera indica, Ocimum tenuiflorum and flower buds of Syzygium aromaticum were collected from Kasaragod and Kannur district, Kerala (India) in the month of March 2023. The plant material was identified and authenticated by Dr. Harikrishnan. E, Assistant Professor, Department of Botany, Payyanur College, Payyanur, Kannur, Kerala. The collected plant materials were carefully washed under running tap water followed by sterilized distilled water and were air dried at room temperature for 10 days. The dried plant materials were homogenized to a fine coarse powder and were subjected to maceration technique by using solvents water and ethanol. The extracts obtained after maceration was subjected for phytochemical analysis. By analysing the result obtained for preliminary phytochemical analysis, it was confirmed that the ethanolic extract of Mangifera indica was found to be rich in alkaloids, flavanoids, saponins, glycosides, tannins and phenols, and the ethanolic extract of Syzygium aromaticum was found to be rich in alkaloids, carbohydrates, flavanoids and tannins. The water extract of Ocimum tenuiflorum was found to be rich in alkaloids, saponins and phenols. The literature survey and the phytochemical analysis conducted during the study reveals that the selected plants are rich source of important phytoconstituents and are able to exert excellent therapeutic activity The physicochemical parameters like ash value, extractive value, moisture content was determined. Total ash vale of Mangifera indica, Syzygium aromaticum and Ocimum tenuiflorum was found to be 14 %,16% and 2% and Acid insoluble ash was found to be 1.5%, 0.5% and 1.5%, Water soluble ash was found to be 2%, 1%, and 2% respectively. Water soluble extractive value of Mangifera indica, Syzygium aromaticum and Ocimum tenuiflorum was found to be 5%,7.4% and 4.4 % and the Alcohol soluble extractive value was found to be 5.6%, 3.2% and 3.4% respectively. The moisture content of Mangifera indica, Syzygium aromaticum and Ocimum tenuiflorum was found to be 15.8%, 5.6% and 5.6% respectively. The higher water-soluble extractive value indicates presence of more number of water-soluble contents in the plants. The herbal toothpaste was formulated by using natural ingredients such as of Clove oil (Treat bad breath) and Tulsi oil (Teeth whitening) extracted from Ocimum tenuiflorum and Syzygium aromaticum by using Clevenger apparatus, and the mango leaf powder were used. Pharmaceutical dosage form is said to be incomplete without excipients. Natural ingredients were mixed along with suitable Detergent, Preservative, Sweetener, Humectants and abrasive. The developed formulation was subjected further evaluation studies. The prepared herbal toothpaste was examined by its Appearance, pH, Foamability, Spreadability, Extrudability, Abrasiveness, Fragrance test, Shape retention. It was found that formulation was Beige brown in colour, have characteristic odour, sweet taste and smooth. The smoothness was tested by rubbing the paste formulation between the fingers. The pH of the herbal toothpaste was found to be 8.58 which lies in alkaline pH range do not produce any irritation upon buccal cavity, and it complied with BIS limit. The spreadability of herbal toothpaste was good. The internal part of all collapsible tubes has no signs of corrosion or damage during the normal storage conditions at a temperature of 45 degree celcius for 10 days. The foamability of herbal toothpaste was good (10ml). The herbal toothpaste shows good abrasiveness and 83.78% of extrudability. The antibacterial activity of the developed herbal toothpaste was done by agar well diffusion method using Ampicillin as the standard drug. The developed formulations showed anti-bacterial activity against Lactobacillus and E.coli produced a better zone of inhibition of about 6mm,7mm respectively which was near to the zone of inhibition produced by standard Ampicillin (10mm,8mm).It was concluded that the developed herbal toothpaste posses all the desirable properties of an ideal herbal toothpaste.
SUMMARY AND CONCLUSION
The aim of the study is to formulate the herbal toothpaste using the leaves of Mangifera indica, Ocimum tenuiflorum and flower buds of Syzygium aromaticum. Physicochemical and phytochemical investigation of the leaves of Mangifera indica, Ocimum tenuiflorum and flower buds of Syzygium aromaticum have been reported here in this thesis work The preliminary phytochemical screening of these plant shows the presence of major constituents such as alkaloids, flavanoids, saponins, glycosides, tannins and phenols have been known to be biologically active and thus partially responsible for the antimicrobial activity. The herbal toothpaste formulated by using extracts of Ocimum tenuiflorum and Syzygium aromaticum and mango leaf powder. From the result revealed that the prepared herbal toothpaste was good in appearance, easily spreadable, good abrasiveness, extrudability, foamability. The invitro antibacterial evaluation was done by using agar well diffusion method and antibacterial activity was measured in terms of zone of inhibition. The formulation shows a better zone of inhibition against Lactobacillus and E.coli bacteria which was near to the zone of inhibition produced by standard ampicillin. Following conclusion can be drawn from the results obtained in the present work of investigation. This herbal toothpaste is having prominent function in the maintaining of the oral hygiene and preventing dental caries and are safer with minimum side effect than chemical based synthetic toothpaste. The formulated herbal toothpaste has good scope in future in nature remedies research, reduced cost and improve dental health of public.
REFERENCES
- Pallingston, J (1998). Lipstick: A Celebration of the World's Favorite Cosmetic. St. Martin's Press. ISBN 978-0-312-19914-2.
- Lovegrove JM. Dental plaque revisited: Bacteria associated with periodontal disease. J N Z Soc Periodontol 2004;87:7–21.
- Ellis GL et al. Tumors of the Salivary Glands. AFIP Atlas of Tumor Pathology. 4th series. Washington, DC: American Registry of Pathology in collaboration with the Armed Forces Institute of Pathology; 2008
- Moudgil A, Srivastava RN, Vasudev A, Bagga A, Gupta A.Fluorosis with crippling skeletal deformities. Indian Pediatr 1986;23:767–73.
- Karandikar S M, Pandit V A & Kulkarni S D, Pharmacoepidemiological study of use of herbal medicines in the elderly in Pune. Bhara/i Vidyapee/h Bull. 1(1997) 9.
- Shah C.S., Quadry J.S. “Text book of Pharmacognosy” B. S. Shah Prakashan, Ahemdabad 5th edition 1985-86, 2007-289
- Mangilal T and Ravikumar M. Preparation and Evaluation of Herbal Toothpaste and Compared with Commercial Herbal Toothpastes: An In-vitro Study. International Journal of Ayurvedic and Herbal Medicine. 2016; 6: 2266 –2251.
- Barreto, J.C.; Trevisan, M.T.S.; Hull, W.E.; Erben, G.; De Brito, E.S.; Pfundstein, B.; Würtele, G.; Spiegelhalder, B.; Owen, R.W.Characterization and Quantitation of Polyphenolic Compounds in Bark, Kernel, Leaves, and Peel of Mango (Mangifera indica L.).J. Agric. Food Chem. 2008,56, 5599–5610.
- Doughari J. H, Manzara S.; In vitro antibacterial activity of crude leaf extracts of Mangifera indica Linn, African Journal of Microbiology Research; 2008; 2; 67-72.
- Boulos, L. in Medicinal plants of North Africa, Reference publications, Algonac, MI. 1983.Cai, L. and Wu, C. D. Compounds from Syzygium aromaticum possessing growth inhibitory activity against oral pathogens. J. Natural Products 1996, 59, 987-990.
- Abushouk, A.I.; Negida, A.; Ahmed, H.; Abdel-Daim, M.M. Neuroprotective mechanisms of plant extracts against MPTP induced neurotoxicity: Future applications in Parkinson’s disease. Biomed. Pharmacother. 2017, 85, 635–645.
- Abushouk, A.I.; Ismail, A.; Salem, A.M.A.; Afifi, A.M.; Abdel-Daim, M.M. Cardioprotective mechanisms of phytochemicals against doxorubicin-induced cardiotoxicity.Biomed pharmacother. 2017,90,935-946
- Patwardhan B, Warude D, Pushpangadan P, Bhatt N. Ayurveda and traditional chinese medicine: A Comparative overview. Evidence-Based complementary and alternative medicine. 2005;2(4):465-473.
- Ghosh, G.R. Tulasi (N.O. Labiatae, Genus- Ocimum) . New Approaches to Medicine and Health (NAMAH). 1995;3: 23–29.
- Balaji R, Prakash G, Suganya devi P, Aravinthan K M, Antioxidant activity of methanol extract of Ocimum tenuiflorum (dried leaf and stem), IJPRD 2011; 3(1):20-27.
- Sudha P, Smita S Zinjar de, Shobha Y Bhargava, Ameeta R Kumar, Potent a -amylase inhibitory activity of Indian Ayurvedic medicinal plants. BMC Complementary and Alternative Medicine 2011; 11(5):1-10. 7.
- AOAC Official Methods: 13th ed., p. 507-532 (1980) Japan Food Industrial Technology Society: Analytical methods of food, Kohrin, p. 4-69 (1982).
- Air Pollution Engineering Manual (Second Edition). Danielson, J.A. (ed.). U.S. Environmental Protection Agency, Office of Air Quality Planning and Standards. Research Triangle Park, NC. Publication No. AP-40. 1973.
- Rani P. and Khullar N: Antimicrobial evaluation of some medicinal plants for their anti-enteric potential against multidrug resistant Salmonella typhi. Phytotherapy Research. 2003; 18(8): 670–673.
- Kadam , V.B. ( 2009 ) “Determination of ash values of three endangered medicinal plants of South G ujarat Forest ”. Plant Archives, 9 (1), 27 – 29.
- Mitscher, L.A., Park, Y.H. and Clark, D.1980: Antimicrobial agents from higher Research Article, 2, (2). 8. R.K.Momin and KadamV.B.(2011) “Determination of ash values of Some Medicinal Plants of Genus Sesbania of Marathwada Region in Maharashtra ”Journal of Phytology , 3(2),52-54.
- Payal R.Dande, Vikram S.Talekar, G.S.Chakraborthy International Journal Research Pharmaceutical Sciences Vol.1, Issue-3,296-299, 2010.
- Gupta A. et al. (2013) phytochemical comparision between pet ether and ethanolic extracts of Bacopa monnieri, Evolvulus alsinoides and Tinospora cordifolia. Pakistan J Bio Sci. 7. Han X., Shen T, Lou H., (2007), International Journal of Molecular Science., 8,950-988
- A. K. Azad, M. Awang, M. M. Rahman. (2012) Phytochemical and Microbiological Evaluation of a Local Medicinal Plant Bacopa monnieri (l.) Penn. International Journal of Current Pharmaceutical Review and Research, 3(3), 66-78.
- Abdullah S, Mudalip SK, Shaarani SM, Pi NA. 2010. Ultrasonication extraction of oil from Monopterus albus: effect of different ultrasonic power solvent volume and sonication time. J. Appl. Sci. 10: 2713-2716.
- Silva GO, Abeysundara AT, Aponso MM. Extraction methods, qualitative and quantitative techniques for screening of phytochemicals from plants. American Journal of Essential Oils and Natural Products. 2017;5(2):29-32
- Gami Bharat, Parabia MH. Pharmacognostic Evaluation of Bark and Seeds of Mimusops elengi. International Journal of Pharmacy and Pharmaceutical Sciences 2010; 2(4):110-113.
- Naqvi SA, Khan MS, Vohora SB. Antibacterial, antifungal, and antihelminthic investigations on Indian medicinal plants. Fitoterapia 1991; 62:221-228.
- Marongiu, Bruno et al, Piras, Alessandra, Porcedda, Silvia, Scorciapino, Andrea: Chemical Composition of the Essential Oil and Supercritical CO2 Extract of clove Engl. and of Acorus calamus L. Journal of Agricultural and Food Chemistry 2005; 53(20): 7939-43
- Ali, I., Naqshbandi, M. F., Husain, M. 2019. Cell migration and apoptosis in human lung cancer cells by Clove (Syzygium aromaticum) dried flower buds extract. Journal of Taibah University for Science. 13(1): 1163-1174.
- Tongnuanchan, P. Benjakul .Essential Oils: Extraction, Bioactivities,and Their Uses for Food Preservation. J. Food Sci.( 2014) ; 1231–1249.
- M.G.V. Silva, F.J.A. Matos, P.R.O. Lopes, F.O. Silva, M.T. Holanda, Composition of essential oils from three Ocimum species obtained by clevenger appartatus steam and microwave distillation and supercritical CO2 extraction, ARKIVOC, (2004) 66-71.
- Dange VN, Magdum C.S, Mohite SK and Nitlikar M. Review on Oral Care Product: formulation of toothpaste from various and extracts of tender twigs of neem, J of Pharm Res. 2008; 1(2): 148-154.
- Mridul Haque Journal of chemical and Pharmaceutical Research, 2014, 6(3): 1279-1285.
- Nishad U, Ali M, Maurya A. Formulation and Evaluation of a polyherbal Toothpaste using Medicinal Plants. J .Pharm. Sci. & Res, 2020; 12(1): 105-11..
- Singh K., Singh P. and Oberai G. Comparative studies between herbal toothpaste (dantkanti) and nonherbal toothpaste (2016); 4(2):53-56.
- Grace X.F. et.al., Preparation and evaluation of herbal dentifrice. Int. Res. J. Pharm. (2015), 6(8):509-511.
- Shende V, Telrandhe R. Formulation and evaluation of Tooth Gel from Aloe vera leaves extract Int J Pharm Drug Analysis. 2017;5(10): 394-398.
- Review on formulation and evaluation study on herbal toothpaste by Nikita M. Rathi, Shital V. Sirsat, Sanket S. Toshniwal, Nikita T. Zagare, Shaikh Fazil Shaikh Mahamad 2022 | Volume 7, Issue 4 April 2022 | ISSN: 2456-4184.
- Joel Ogboji IY, Chindo , Aliyu Jauro DEA, Boryo, Lawal NM. Formulation, physicochemical evaluation and antimicrobial activity of green toothpaste on streptococcus mutans, International Journal of Advanced Chemistry, International Journal of Advanced Chemistry 2018;6(1):108-11.
- Prabhu K. Halakatti, Anita Desai, Mahantesh Moogi, Mahantesh Patted, Ashok Gumtaj, Pallavi Jakati arm Adv Res, 2022; 5(8): 1639-1645,on evaluation of multi herbal toothpaste.
- Mangilal T, Ravikumar M. Preparation and Evaluation of Herbal Toothpaste and Compared with Commercial Herbal Toothpastes: An In-vitro Study. Int J Ayurvedic Herbal Med. 2016;6(3):2266–73.
- Pavan Deshmukh, Roshan Telrandhe*, Mahendra Gunde, Formulation and Evaluation of Herbal Toothpaste: Compared With Marketed Preparation, INTERNATIONAL JOURNAL OF PHARMACEUTICS & DRUG ANALYSIS, VOL.5 ISSUE 10, 2017; 406 – 410.
- Mamatha D, Naveen Kumar G. Preparation, Evaluation and Comparision of Herbal Toothpaste with Markedly Available Tooth Pastes, IOSR Journal of Pharmacy and Biological Sciences. 2017;12(6):1-06.
- Kavita Varma Shukla, Deepika Kumari*Formulation Development and Evaluation of Herbal Toothpaste for Treatment of Oral Disease Journal of Drug Delivery & Therapeutics. 2019; 9(4-s):98-104.
- Urmila Nishad 1 , Meraj Ali 2 , Anupama Maurya 3, Formulation and Evaluation of a Polyherbal Toothpaste using Medicinal Plants; Urmila Nishad et al /J. Pharm. Sci. & Res. Vol. 12(1), 2020 ; 105-111.
- K.L. Senthilkumar , S. Venkateswaran , A. Vasanthan , P. Chiranjeevi , N Mohamed ,S. Dinesh , K.L.S. Neshkumar. Formulation development and evaluation of novel herbal toothpaste from natural source International Journal of Pharmaceutical Chemistry and Analysis 2022;9(1):17–21.
- Mahendran Sekar, Muhammad Zulhilmi Abdullah Formulation, Evaluation and Anti.microbial Properties of Polyherbal Toothpaste Int J Curr Pharm Res, Vol 8, Issue 3, 105-107.
- Prashant GM, Chandu GN, Murulikrishna KS, Shafiulla MD. The effect of mango and neem extract on four organisms causing dental caries Streptococcus Mutans ,Streptococcus Salivavius, Streptococcus Mitis and streptococcus Sanguis: An in Vitro study. Indian J Dent Res, 2007; 18: 148–51.
- Gibraiel F., Rajput M., Rajput M.S., Singh M., Saxena N., Vishal A., Kumar A. In vitro study to investigate the antimicrobial efficacy of different toothpastes and mouthrinses. Res J Pharm BiolChem Sci. 2014;5:245–257


 Shanthi M. V.*
Shanthi M. V.*
 Anaswara B.
Anaswara B.

























 10.5281/zenodo.14382608
10.5281/zenodo.14382608