The aim of study was to extract, screen, formulate and evaluate Tinospora crispa ointment via phytochemical techniques and phytochemical methods. The objectives of this work were to understand the pharmacogenetics of Tinospora crispa, to determine suitable procedures and solvents for the extract, to understand the phytochemical screening of the extract, to formulate an ointment of Tinospora Crispa extract and to evaluate ointment on the basis of various evaluation parameters. The plant Tinospora crispa was selected on the basis of a literature review. Then it was collected from an online store and extracted by Soxhlet extraction. The extract was subjected to phytochemical screening and after that ointment was formulated using drug extract and various excipients, after which various evaluation parameters were tested. On the basis of various literature surveys the plant Tinospora crispa was selected and authenticated by the Nims Institute of Pharmacy. The plant was extracted by Soxhlet extraction using methanol. Various phytochemical tests revealed the presence of saponins, tannins, steroids, carbohydrates and fixed oils. On the basis of various evaluation tests the ointment was found to be safe and effective for treating various skin problems. Tinospora crispa is a remarkable herb that can be used to treat many types of illnesses. Therefore, additional research is needed in addition to clinical studies to demonstrate the health advantages of this herb. The results of this study support the use of T. crispa ointment in the treatment of a variety of skin conditions, including acne, eczema, dark undere circles, and skin allergies.
Phytochemical screening, Ointment, Formulation, Evaluation
Not only in the present but also in the distant past, trees and plants have been essential to human life. Early man relied on them for both his spiritual requirements, such as magic or ritualistic practices, as well as his physical needs, such as sources for food, housing, clothes, medicine, adornment, and tools. Locally grown medicinal herbs are typically accessible local and traditional treatments are nontoxic, safe, affordable, and socially and culturally acceptable for every reason1. Numerous researchers have extensively studied the genus Tinospora and claim that it contains a number of phytochemicals with notable medicinal efficacy. Tropical lowland areas are home approximately 70 genera and 450 species of plants that make up the Menispermaceae plant family. These shrubs are rarely climbing or twining plants. Leaves are alternate or lobed, flowers are small chimes, and seeds are usually hooked or uniform. This family is a rich source of alkaloids and terpenes2. Both the traditional medical system and Ayurveda highlight the plant's healing properties. The plant can be found in the tropical region of India from Kumaon to Assam and further north via West Bengal, Bihar, Deccan, Konkan, Karnataka, and Kerala, up to 1,200 m above sea level. It is a common shrub that grows over hedges and small trees in deciduous and dry woodlands. It enjoys a variety of soil types, from acidic to alkaline, and requires a moderate amount of soil moisture3.
Plant profile:
Kingdom: Plantae Division: Magnoliophyta Class: Magnoliopsida, Order: Ranunculaceae Family: Menispermeaceae Genus: Tinospora
Common names4:
Latin:
Tinospora cordifolia (willd.) Hook.F. & Thomson English:
Gulancha, Indian Tinospora
Sanskrit:
Guduchi, Madhuparni, Amrita, Chinnaruha, Vatsadaani, Tantrika, Hindi: Giloya, Guduchi
Bengal:
Gulancha
Telugu:
Tippatiga Tamil: Shindilakodi Marathi: Shindilakodi Gujarathi: Galo Kannada: Amrita balli

A

B

C
Fig. no. 1: A: Leaves of T. Crispa B: Flowers of T. Crispa C: Stem of T. Crispa
Botanical description:
Large, glabrous, deciduous, climbing shrub T. crispa. The stem structure is fibrous, and the transverse slice reveals a yellowish wood with wedge-shaped wood bundles containing large vessels that are radially organized and spaced apart by narrow medullary rays. The stem has rosette-like lenticles, and the bark ranges in color from creamy white to gray and is deeply spiralled. The leaves are cordate and membranous in texture. Unisexual, small, yellow, and in the axillary position, the flowers have a raceme length of 2 to 9 cm and are borne on leaflet branches. Female flowers are often solitary, whereas male blooms are grouped. Curved seeds are present. Fruits have a solitary seed and are meaty. Fruits ripen in the winter, and flowers ripen in the summer5.
Morphological Description:
A large, widely spreading climbing deciduous shrub with many coiling branches is called Tinospora crispa. The following types of morphology can be observed in various Tinosporan regions.
Stem:
This plant has a long, filiform, fleshy, climbing stem that is fairly succulent in appearance. The branches give rise to aerial roots. The bark is deeply left spirally and ranges in tint from creamy white to gray6.
Arial Root:
There are aerial roots, and the fundamental structure of these aerial roots ranges from a tetra to a penta-arch. However, the cortex of the root is split into an inner parenchymatous zone and an exterior thick walled zone7.
Leaves:
Simple, alternate, exstipulate, round, pulvinate, heart-shaped, partially twisted, and halfway circular leaves of this plant have a length of approximately 15 petioles. Oval, 10–20 cm long, 7 nerved, profoundly cordate at the base, and membranous are the characteristics of the lamina8.
Flowers:
Unisexual, receptive, and greenish yellow in hue, flowers only bloom when a plant has no leaves. Female flowers are observed in single inflorescences, while male flowers are grouped. There are two series of three sepals each, totaling six. The inner sepals are smaller than the outer sepals. Additionally, six petals are membranous, free, and smaller than the sepals. The flowering season lasts from March to June9.
Fruit:
These fruits are orange?red in color, fleshy, have an aggregate of one to three smooth, ovoid drupelets on a thick stem, and have a subterminal style scar. Fruits grow in the winter10.
Seed:
There have been reports of curved seeds of this species. As a result, this family is also known as the moonseed family. The embryo instantly assumed a curved shape, much like the shape of seeds. Additionally, the endocarp has different ornamentations and has crucial taxonomic characteristics.
METHODS
MATERIAL
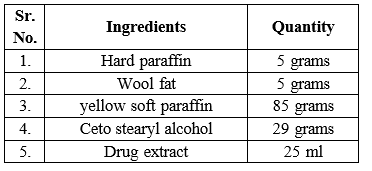
For Formulation
- Tinospora crispa plant extract
- Wool fat
- Hard paraffin
- Soft paraffin
- Cetostearyl alcohol
Chemicals-
All the chemicals used for the study were of analytical grade and were purchased from R.S. Enterprises, Jaipur (Rajasthan).
Other Materials
- Beaker
- Conical flask
- Cotton
- Butter paper
- Spatula
- Forceps
- China dish
- Filter paper
Research Methodology
Selection of plants:
The plant Tinospora crispa was selected on the basis of various literature surveys and its future prospects in the herbal industry.
Collection of plants:
The whole plant powder of Tinospora crispa was collected from an online herbal store called Indianjadibooti.com in March 2023.
Authentication:
The plant species were identified and authenticated by the Department of Pharmacognosy of NIMS Institute of Pharmacy and reference no. is given as NU/Nip/2023/524.
Storage: -
The dried powder of Tinospora crispa plants was preserved in tightly closed airtight containers and stored in a suitable cool and dry place.
Extraction: -
- Thirty grams of dried and powdered Tinospora crispa plants were weighed properly.
- The powder was then extracted with 100 ml of alcoholic solvent (methanol) using Soxhlet extraction for 18 hrs.
- The extract obtained was then concentrated in a water bath and after cooling the extract was stored in a refrigerator.

Fig. no. 2: Soxhlet apparatus
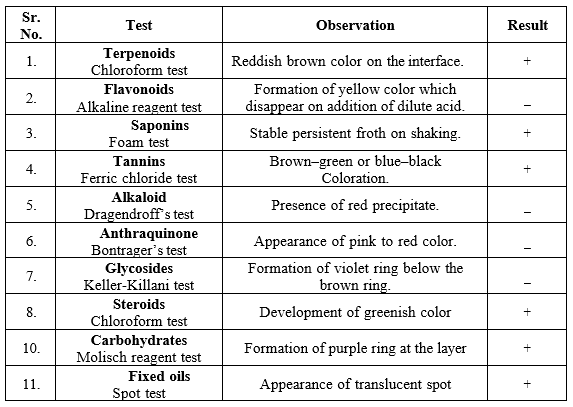
Phytochemical Screening
Phytochemical examinations of the extracts were carried out according to standard methods.
Alkaloid
extracts were dissolved individually in dilute hydrochloric acid and filtered. The filtrate was used to test for the presence of alkaloids.
- Mayer’s test-
Filtrates were treated with Mayer’s reagent (potassium mercuric iodide). The formation of a yellow precipitate indicates the presence of alkaloids.
- Wagener’s test-
filtrates were treated with Wagener’s reagent (iodine in potassium iodide). The formation of a brown/reddish precipitate indicates the presence of alkaloids.
- Dragendroff’s test-
filtrates were treated with dragendroff’s reagent (potassium bismuth iodide). The formation of a red precipitate indicates the presence of alkaloids.
- Hager’s test-
filtrates were treated with Hager’s reagent (saturated picric acid solution). The presence of alkaloids was confirmed by the formation of a yellow precipitate.
Flavonoids
- Alkaline reagent test-
Extracts were treated with a few drops of sodium hydroxide solution. The formation of an intense yellow color, which becomes colorless upon the addition of dilute acid, indicates the presence of flavonoids.
- Lead acetate test-
Extracts were treated with a few drops of lead acetate solution. The formation of a yellow precipitate indicates the presence of flavonoids.
Tannins
- Ferric chloride test-
Approximately 0.5 ml of extract was boiled in 10 ml of water in a test tube. A few drops of 0.1?rric chloride were added and the samples were observed for brownish green or blue?black coloration. This indicates the presence of Tannins.
Phenols
- Ferric Chloride Test-
Extracts were treated with 3-4 drops of ferric chloride solution. The formation of a bluish black color indicates the presence of phenols.
Saponins
- Froth test
extracts were diluted with distilled water to 20 ml and shaken in a graduated cylinder for 15 minutes. The formation of a 1 cm layer of foam indicates the presence of saponins.
- Foam test-
First, 0.5 g of extract was shaken with 2 ml of water. The persistence of foam produced for ten minutes indicates the presence of saponins.
Glycoside
- Keller- Killiani test-
First, 0.5 ml of extract was boiled with 5 ml of distilled water and 2 ml of glacial acetic acid containing 1 drop of 0.5?rric chloride solution was added. This solution was mixed with 1 ml of concentrated sulfuric acid. The formation of a violet ring below the brown ring and a greenish ring in the acetic layer just above the brown ring and gradual spreading throughout this layer indicated the presence of cardiac glycoside.
- Molisch’s reagent test-
Two to three drops of Molisch reagent were added to the extracts and mixed well. A few drops of conc. sulfuric acid were added carefully. The formation of a reddish-purple ring at the junction of the two layers indicates the presence of glycosides.
Terpenoids-
- Chloroform test-
The extract was mixed with 2 ml of chloroform, and concentrated H2SO4 (3 ml) was carefully added to form a layer. A reddish brown coloration of the interface is formed to indicate the presence of terpenoids.
Carbohydrate-
a. For the Fehling’s reagent test, 2 ml of the given extract was added to a clean test tube. Then 2 ml of Fehling’s solution A and Fehling’s solution B were added. The solution was kept in a boiling water bath for approximately 10 minutes. If a red precipitate formed, then the presence of carbohydrates was confirmed.
b. Benedict’s reagent test-
One milliliter of the extract was mixed with 2 ml of Benedict’s reagent and heated in a boiling water bath for 3 to 5 minutes. The development of a brick-red precipitate of cuprous oxide confirmed the presence of carbohydrates.
- Molisch’s reagent test-
Two to three drops of Molisch’s reagent must be added to a small amount of the extract in a test tube and mixed well. Now, a few drops of concentrated sulfuric acid must be added drop wise along the walls of the test tube to facilitate the formation of a layer and avoid mixing. The development of a purple ring at the layer formed by the concentrated acid is a positive indicator of carbohydrates.
Steroids-
- Chloroform test-
The development of a greenish color when 2 ml of the extract was dissolved in 2 ml of chloroform and treated with concentrated sulfuric acid and acetic acid indicates the presence of steroids.
Fixed oils-
- Spot test-
In this test, the given sample to be tested was rubbed between the folds of filter paper. The appearance of translucent spots confirms the presence of fats in the given sample.
Anthraquinones
- Bontrager’s test-
One gram of extract was mixed with 5-10 ml of dilute HCl and boiled in a water bath for 10 minutes. The solution was filtered, and the extract of the filtrate was treated with CCl4 or benzene and an equal amount of ammonia solution. After shaking, the appearance of a pink to red color indicated the presence of an anthraquinone moiety.
Formulation:
- First, all the ingredients were weighed properly as per the manufacturer’s requirements.
- Twenty-nine grams of cetosteryl alcohol and 5 g of hard paraffin were weighed and stored in a china dish.
- Both the ingredients were melted in a water bath.
- Five grams of wool fat and 85 g of soft white paraffin were added to the melted mixture.
- After the ointment base was prepared, 5% plant extract was added to 95% ointment base and mixed well.
- The ointment was then stored in airtight containers and kept in a cool and dry place.

Fig. no. 3: Tinospora Crispa Ointment
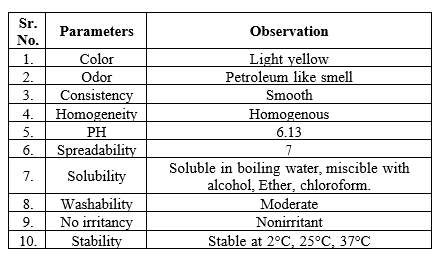
Evaluation
The following parameters were evaluated for the formulated ointment.
- Color and odor-
Physical parameters such as color and odor were examined by visual examination.
- Consistency-
Examined by applying it to the surface of the skin.
- Homogeneity-
The homogeneity of the formulated gels was examined by visual inspection for the presence of any aggregates.
- pH Value-
The pH values of the prepared formulations were measured by using a digital pH meter. The solution of ointment, cream, and gel was prepared by using 100 ml of distilled water and was set aside for 2 hrs. The pH of the solution was determined in triplicate, and the average value was calculated.
- Spreadability-
The spreadability was determined by placing an excess of sample between two slides, which were compressed to a uniform thickness by placing a definite weight for a certain time. The time required to separate the two slides was measured as spreadability. A shorter separation time for two slides results in better spreadability.
The spreadability was calculated by the following formula:
S=M×L/T,
where
S= Spreadability
M= Weight tide to the upper slide
L= Length of glass slide
T= Time taken to separate the slides
- Solubility-
Soluble in boiling water, miscible with alcohol, ether or chloroform.
- Washability-
The formulations were applied to the skin, and then the extent of washing with water was checked.
- Non-irritancy Test-
The prepared formulations were applied to the skin of humans, and their effects were observed.
- Stability study-
Physical stability tests of the formulations were carried out for four weeks at various temperatures, such as 2 °C, 25 °C and 37 °C.
- Viscosity-
The viscosity of the formulations was checked using a Brookfield Viscometer.
Results
Selection of Plants
The plant was selected on the basis of various literature surveys. A total of 42 articles were considered of which 12 were review articles and 30 were research articles on the basis of which the plant Tinospora crispa was selected.


 Savita Yadav*
Savita Yadav*
 Dr Gaurav Dubey
Dr Gaurav Dubey
 Shailendra Singh Chandel
Shailendra Singh Chandel
 Aditi Omprakash Jyotishi
Aditi Omprakash Jyotishi









 10.5281/zenodo.11228397
10.5281/zenodo.11228397