Abstract
Herbal formulation is a dosage form consisting of one or more than fresh herbs of processed herbs in particular quantities to provide specific nutritional, cosmetics benefits and are meant to diagnose, treat, alleviate disease of human being. Poly herbal capsule is a form of ayurvedic medicine that is defined as totally dried raw materials which is powdered very minutely to a small size and then filtered to obtain fine powder and made as granules and filled in capsules. It is used to treat variety of diseases and conditions. The Aim of the present work is to reveal the pharmacognostic, phytochemical, antidiabetic activity for the formulation and evaluation of polyherbal capsules. The required materials were collected from Kasaragod and subjected to successive solvent extraction. Later pharmacognostic studies like microscopical evaluation, ash value and extractive value were carried out. The preliminary phytochemical studies were carried out in different solvents like Water, Petroleum ether, Chloroform, Ethanol, Methanol and Ethyl acetate. Water and ethanolic extract was used for the study of antidiabetic activity.
Keywords
Andrographis paniculata, Tamarindus indica, Syzygium aqueum, Passiflora edulis.
Introduction
Medicinal plants are one of the sources of natural products for the treatment and management of debilitating diseases. Plants which have one or more of its parts having substances that can be used for treatment of diseases, are called Medicinal plants. Medicines derived from plants are widely famous due to their safety easy availability and low cost.
HERBAL MEDICINE
Herbal medicine is defined as a branch of science in which plant based formulations are used to alleviate diseases. It is also known as botanical medicine or phytomedicine. Later phytotherapy has been introduced as a more accurate synonym of herbal or botanical medicine. With the advent of the allopathic system of medicine, herbal medicine gradually lost its popularity among people. The WHO has recently defined Traditional medicine (including herbal drugs) has comprising therapeutic practices that have been in existence, often for 100 of years, before the development and spread of modern medicine and as still un used today. Traditional medicine is a synthesis of therapeutic experience of generations of practicing physicians of indigenous system of medicines. Traditional preparations comprise medicinal plants, minerals and organic matter etc. Herbal drugs constitute only those traditional medicines which primarily use medicinal plant preparation for therapy. Herbal medicines are complex compound with multiple synergistic mechanisms of action that modulate pathophysiological function. It has become a popular form of health care. Even though several differences exist between herbal and conventional pharmacological treatment, herbal medicine can be tested for efficacy using conventional trial methodology. Several specific herbal extracts have been demonstrated to efficacious for specific conditions.
2) POLYHERBAL FORMULATIONS
Polyherbal formulation refers to a medicinal preparation that contains a combination of two or more herbs or herbal extracts. These formulations are created by blending multiple herbs with complementary or synergistic properties to enhance therapeutic efficacy or address multiple health concerns simultaneously. Polyherbal formulations plan has been utilized generally around the earth because of its restorative and remedial application. Polyherbalism confers some benfits not available in single herbal formulations it is evident that better therapeutic effect can be reached with a single multi constituent formulation. For this, a lower dose of herbal preparation would be needed to achieve desirable pharmacological action, thus reducing the risk of deleterious side effects. Besides, PHF’S bring improved convenience for patients by eliminating the need of taking more than one different single herbal formulation at a time, which indirectly leads to better compliance and therapeutic effect. All these benefits have resulted in the popularity of PHF in the market when compared to single herbal formulation
MATERIALS AND METHODS
Collection of plant materials
The fresh leaves of Andrographis paniculata, Syzygium aqueum, Passiflora edulis and Tamarindus indica were collected from Kasargod and Kannur district, Kerala (India) in the month of April 2024.
Authentification of plant material
The plant material was identified and authenticated by Smitha K o Assistant professor (Horticulture), college of Agriculture, Kerala Agricultural university, Padannakad, Kasargod, Kerala.
Extraction of plant material
Extraction of Andrographis paniculata, Tamarindus indica, Passiflora edulis and Syzygium aqueum was carried out separately by maceration. The coarsely powdered leaves of Andrographis paniculata, Tamarindus indica, Passiflora edulis, Syzygium aqueum were macerated with solvents like petroleum ether, ethanol, water separately. The extract obtained after maceration process was then used for phytochemical studies to choose the most suitable solvent for extraction.
Preliminary phytochemical screening
The extracts of selected plants were subjected to preliminary phytochemical screening to detect the various phytoconstituents such as alkaloids, glycosides, flavonoids, phenols, tannins, proteins and amino acids.
Formulation of polyherbal antidiabetic capsule
Antidiabetic capsules containing leaf extract were prepared by manual capsule filling method. Other ingredients such as lactose used as diluents, Magnesium stearate as lubricant and Microcrystalline cellulose act as diluents and disintegrants. Methyl Paraben act as preservatives. All the excipients along with API weighed as shown in table and passed through sieve no.44. Then, all ingredients were mixed following geometric mixing excluding glidant and lubricant thoroughly for 15 minutes. Glidant and lubricant were later added and pushed to a capsule having size “0” and have an average net content weight of 400mg.
|
SL NO.
|
INGREDIENTS
|
F1
|
F2
|
F3
|
|
1
|
Drug
|
400
|
400
|
400
|
|
2
|
Microcrystalline cellulose
|
14
|
12
|
11
|
|
3
|
Magnesium stearate
|
25
|
27
|
23
|
|
4
|
Methyl paraben
|
0.2
|
0.3
|
0.5
|
Physical characterstics:
Bulk density, Angle of repose, Hausner's ratio, Carr's index, and particle size distribution was determined for evaluating the physical characteristics of the powder.
a. Bulk density
10g of mixture was taken in a graduated measuring cylinder and tapped on a wooden surface. Bulk density is calculated by using the formula Bulk density= weight taken/ bulk volume Tapped density= weight of churna taken volume/ (tapped)
b. Angle of repose
Angle of repose was determined by using funnel method. The powder was allowed to flow through a funnel fixed on a stand to form a heap. The height and the radius gives the angle of repose. Angle of repose Tan ? =Tan (h/r) Where, h =height of heap, r =radius of heap
c. Compressibility/Carr's Index
This is calculated using the formula: Car's Index- Bulk density (Tapped)- Bulk density (Untappedy/Bulk density (Tapped) * 100
d. Hausner's Ratio
The formula used to determine Hausners ratio is
Hausner's ratio = Bulk density (tapped) /Bulk density (untapped)
INVITRO ANTIDIABETIC ACTIVITY
Inhibition of alpha-amylase enzyme
A starch solution (0.1% w/v) was obtained by stirring 0.1g of potato starch in 100 ml of 16mM of sodium acetate buffer. The enzyme solution was prepared by mixing 27.5 mg if alpha-amylase in 100 mol of distilled water. The colorimetric reagent is prepared by mixing sodium potassium tartrate solution and 3, 5- dinitro salicylic acid solution 96mM. Both control and plant extracts were added with starch solution and left to react with alpha-amylase solution under alkaline conditions at 25°C. The reaction was measured over 3 times. The generation of maltose was quantified by the reduction of 3, 5- dinitro salicylic acid. This reaction is detectable at 540 nm.
Calculation of 50% Inhibitory concentration (IC50)
The concentration of the plant extracts required to scavenge 50% of the radicals (IC50) was calculated by using the percentage scavenging activities at five different concentrations of the extract. Percentage inhibition (1%) was calculated by,
I%= (Ac-As)/Ac × 100, (Shai et al., 2010).
Where Ac is the absorbance of the control and As is the absorbance of sample.
EVALUATION OF POLYHERBAL CAPSULE
a) Weight variation test
weight variation test is used to determine the variation in the amount of powder contained in each herbal capsule. Twenty capsules were taken and weighed. The average weight is calculated and compared with individual capsule weight. Percentage variation was calculated as per USP (2010) specification. The herbal capsule should not be less than 90% and not more than 110% of theoretically calculated weight of each unit.
b) Disintegration test
It is the measurement of time required under a given set of conditons in which the selected capsules was disintegrated into the particles that pass through 10 mesh screens within a specific time.
c) Determination of moisture content
Loss on drying is a test that determines the presence of moisture or volatile matter in sample. An excess of water will encourage microbial growth. Presence of moisture content provides the information concerning shelf life and quality of drug.
Moisture content % = (Final weight of sample / initial weight of sample) x 100
d) Determination of pH
1g of capsule powder was taken and dissolved in 100ml of demineralised water. The pH value of solution is determined by using digital pH meter. The electrodes were immersed in the test solution and the pH was measured.
RESULT AND DISCUSSION
Extraction of plant material
The extraction of dried leaves of Andrographis paniculata, Syzygium aqeuem, Tamarindus indica, Passiflora edulis were carried out by maceration process by using suitable solvent. The extracts obtained were collected and concentrated which then weighed and kept in a desicator until it was used for further studies.
Preliminary phytochemical screening
Phytochemicals present in water and ethanolic extracts of the crude drug.
Physical Evaluation: Results of the moisture content of the crude drug.
|
SL NO
|
Plant
|
Plant part
|
Loss on drying (g)
|
Percentage loss on drying (%w/w)
|
|
1
|
Andrographis paniculata
|
Leaf
|
10.64±0.36
|
8.70±0.54
|
|
2
|
Tamarindus indica
|
Leaf
|
30.54±2.47
|
24.92±1.28
|
|
3
|
Syzygium aqueum
|
Leaf
|
31.40±2.87
|
20.60±1.54
|
|
4
|
Passiflora edulis
|
Leaf
|
29.30±1.41
|
25.44±0.45
|
|
5
|
Polyherbal mixture
|
Leaf
|
13.50±0.11
|
10.15±0.25
|
Results of Total ash value, Acid insoluble ash value and Water-soluble ash value of crude drug.
|
SL NO
|
Plant
|
Plant part
|
Total ash value
(%w/w)
|
Acid insoluble ash value (%w/w)
|
Water soluble ash value (%w/w)
|
|
1
|
Andrographis paniculata
|
Leaf
|
8.62±0.76
|
2.10±0.55
|
1.50±0.67
|
|
2
|
Tamarindus indica
|
Leaf
|
7.95±0.63
|
0.516±0.18
|
5.25±0.92
|
|
3
|
Syzygium aqueum
|
Leaf
|
15.8±0.12
|
13.2±0.88
|
3.8±0.47
|
|
4
|
Passiflora edulis
|
Leaf
|
6.1±0.52
|
2.9±0.54
|
3.29±0.14
|
The results of extractive values
|
SL NO
|
Plant
|
Plant part
|
Water soluble extractive value
(%w/w)
|
Alcohol soluble extractive value
(%w/w)
|
|
1
|
Andrographis paniculata
|
Leaf
|
88.27±0.78
|
55.47±0.89
|
|
2
|
Tamarindus indica
|
Leaf
|
30.22±5.40
|
25.82±4.77
|
|
3
|
Syzygium aqueum
|
Leaf
|
83.6±0.30
|
67.58±0.72
|
|
4
|
Passiflora edulis
|
Leaf
|
21.94±0.85
|
18.46±0.98
|
Fluorescence analysis
The fluorescence analysis was determined as described earlier and the results are
|
Name of plant
|
Before treatment
|
After treatment with 50% HCL
|
After treatment with 50% NAOH
|
|
Ordinary light
|
Short UV
|
Long UV
|
Ordinary light
|
Short UV
|
Long UV
|
Ordinary light
|
Short UV
|
Long UV
|
|
Andrographis paniculata
|
Light green
|
Colourless
|
Light yellow
|
Light green
|
Colourless
|
Light green
|
Light green
|
Colourless
|
Light
Brown
|
|
Tamarindus indica
|
Yellowish brown
|
Light brown
|
Dark brown
|
Brownish yellow
|
Light yellow
|
Colourless
|
Dark red
|
Light yellow
|
Yellowish brown
|
|
Syzygium
aqueum
|
Yellowish brown
|
Light green
|
Dark brown
|
Dark brown
|
Light
Brown
|
Black
|
Light orange
|
Light yellow
|
Colourless
|
|
Passiflora edulis
|
Light green
|
Light yellow
|
Yellowish green
|
Dark brown
|
Dark green
|
Light green
|
Yellowish green
|
Light yellow
|
Dark green
|
|
Polyherbal mixture
|
Olive green
|
Light yellow
|
Colourless
|
Dark brown
|
Colourless
|
Colourless
|
Light orange
|
Light yellow
|
Fluorescent Green
|
Physical characterstics
Evaluation of physical properties of polyherbal formulation
|
SL.NO
|
Parameters
|
Observation
|
|
1
|
Bulk density
|
0.3615±0.013
|
|
2
|
Tapped density
|
0.4032±0.002
|
|
3
|
Angle of repose
|
32.56±0.142
|
|
4
|
Carr’s index
|
10±0.341
|
|
5
|
Hausner’s ratio
|
1.11±0.013
|
Evaluation of polyherbal capsule
|
SL NO
|
FORMULATION
|
WEIGHT VARIATION
|
pH
|
MOISTURE CONTENT
|
DISINTEGRATION TIME (MIN)
|
|
1
|
PHF1
|
441mg±0.22
|
6.10
|
13.21±0.01
|
13.15±0.01
|
|
2
|
PHF2
|
443mg±0.01
|
6.3
|
13.50±0.02
|
13.26±0.02
|
|
3
|
PHF3
|
445mg±0.02
|
6.25
|
15.50±0.04
|
13.56±0.01
|
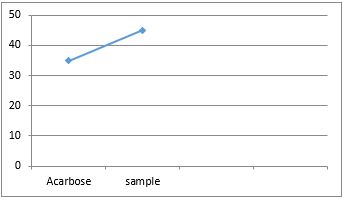
Anti-diabetic Activity
In vitro antidiabetic activity from alpha amylase method.
|
SI.NO
|
Sample
|
IC50 values (µg/ml)
|
|
1
|
Acarbose (standard)
|
34.89 (µg/ml)
|
|
2
|
Formulation
|
45.04 (µg/ml)
|
DISCUSSION
Herbs play a major role in the treatment than allopathic medicines because of fewer side effects, low cost and easy availability. The research work was done on that basis and the selected plants for the formulation was literally proved for the therapeutic use of antidiabetic purpose. The present study was undertaken with an intention to develop an antidiabetic polyherbal capsule. Diabetes mellitus is a chronic disorder found in all parts of the world and is becoming a serious threat to mankind. The leaves of Andrographis paniculata, Tamarindus indica, Syzygium aqueum and passiflora edulis were collected from Kasargod district, Kerala, India in the month of March 2023. The capsule having fine powder of herbs in appropriate ratio was subjected standardisation by means of various physical and chemical methods. The pH was determined inorder to avoid any irritation and moisture content was determined to find any increase in weight caused by moisture absorption. Finally the value obtained was within the standard. The antidiabetic activity can be measured by inhibition of alpha amylase enzyme. From this investigation by considering the results, it was found to be effective for the treatment of diabetes.
ACKNOWLEDGEMENT
This thesis has been kept on track and seen through to completion with the support and encouragement of numerous people. It is a delightful moment for us to express our heartfelt gratitude and sincere thanks to our esteemed guide Mrs. Priya Abraham, Professor, Department of Pharmacognosy, Rajiv Gandhi Institute of Pharmaceutical science and research for her constaAAnt guidance, valuable suggestions and encouragement. We are deeply indebted to Prof. Dr. M. Paridhavi, M Pharm, PhD, FABAP, Principal, Rajiv Gandhi Institute of Pharmaceutical science and research, for his valuable advice and support to make the study successful. We also extend our sincere thanks to all teachers, who helped us for the completion of this dissertation.
REFERENCES
- Niranjan A, Tewari S, Lehri A. Biological activities of Kalmegh (Andrograpis paniculata Nees) Indian J Nat Proc Resour. 2010;1:125–
- Zhang XF, Tan BK. Anti-diabetic property of ethanolic extract of Andrographis paniculata in streptozotocin-diabetic rats. Acta Pharmacol Sin. 2000;21:1157–64.
- Manaharan, T., Appleton, D., Cheng, H. M. &Palanisamy, U. D. (2012a). Flavonoids isolated from Syzygium aqueum leaf extract as potential antihyperglycaemic agents. Food Chemistry
- Salles B.C.C., da Silva M.A., Taniguthi L., Ferreira J.N., da Rocha C.Q., Vilegas W., Dias P.H., Pennacchi P.C., Duarte S., Rodrigues M.R., et al. Passiflora edulis Leaf Extract: Evidence of Antidiabetic and Antiplatelet Effects in Rats. Biol. Pharm. Bull. 2020;43:169– 174. doi: 10.1248/bpb.b18-00952 3.
- Bhadoriya SS, Ganeshpurkar A, Narwaria J, Rai G, Jain AP. 2011. Tamarindus indica: extent of explored potential. Pharmacogn Rev. 5:73–81. doi:10.4103/0973-7847.79102.
- Mohammed B et al., medicinal plants with potential antidiabetic activity- A review of ten years of herbal medicine research (1990-2000). Int J Diabetes Metabol. 2006; 14: 1-25.
- Subbulakshmi G and Naik M. Indigenous foods in the treatment of diabetes mellitus. Bombay hospital J.2001; 43(4): 548-61.
- Subramani Parasuraman; Gan Siaw Thing; Sokkalingam Arumugam Dhanaraj (2014).Polyherbal formulation: Concept of ayurveda" Pharmacogn. Rev. 8 (16): 73– 80.
- M.A. Bhutkar et al., Invitro assay of alpha amylase inhibitory activity of some indigenous plant. International Journal of Chemistry and Science.2012;10(1):457- 462.
- Alarcon AFJ et al., Study of the anti-hyperglycemic effect of plants used as antidiabetics. J Ethno Pharm. 1998; 61:101-10. 1


 Adhithyakrishna K.* 1
Adhithyakrishna K.* 1

 10.5281/zenodo.14011459
10.5281/zenodo.14011459