Formulation And Evaluation of Different Polymer Coated Spherules from Granules
Fluidized bed drying (FBD), advanced drug delivery systems (ADDS), granulation, spheronization, and bed coating during sliding (BCDS).
The most widely used method for creating spherules (particles with a spherical form) is spherization. This, in contrast to granules and pellets, yields spherules with a higher waft home and an excessive capacity for drug loading. This is so because the shape of granules and pellets isn't always spherical. [1] Additionally, the spherules offer the chance to alter their surface characteristics by polymer film coating. During the spherule production process, surface coatings enhance the functional characteristics of the particles, including their appearance, drug release, and integrity. Spherules require less coating solution since they have a lower surface area to volume ratio than granules. The most popular method for spheronization is fluidised bed drying (FBD), in which the droplets are dried in air under circulation to form spherules with irregular shapes and surface roughness due to rapid drying. In order to supply homogenous spherules, opportunity procedures that may be used in local and large-scale tactics are thus needed. Granulation can be carried out via the sieve method that is visible through sizing. Wet granulation and "bed coating during sliding (BCDS)" are two low-cost methods of producing spherules because they can be scaled up and integrated into routine pharmaceutical unit operations. As the polishing of coated starch particles to granules occurs during sliding, which can result in the conversion of granules to spherules, the spheronization by BCDS can produce uniform-sized particles. The spherules' surface can be modified by applying a polymer film coating. For that reason, polymers with unique physical properties could be employed. A polymer produced from cellulose, ethylene cellulose (EC) is often used in formulations with continuous release. Hydroxypropyl methylcellulose (HPMC) expands after absorbing stomach fluid. This study examines the impact of polymer coating, BCDS, and wet granulation on aspirin stability. The granules, spherules, and coated spherules are easily manufactured; nevertheless, the stability of aspirin is impaired. Spherules of sustained-release aspirin coated in ethanol for the treatment of COVID-19; suitable for use in an emergency. Cellulosic derivatives utilized either alone or in conjunction with other macromolecules, such as carboxy methyl cellulose,
MATERIALS AND METHODS
Materials
The supplies that were obtained were hydroxy propyl methyl cellulose (HPMC) K15, lactose, acetone, starch, ethanol, and aspirin from Sudarshan Scientific Laboratories in Nandgaon, Maharashtra. All of the chemicals and reagents used were of laboratory quality. Each reagent and buffer was prepared following standard protocol. Drug release tests and UV/Visible spectrum analysis (SHIMADZU V-730 UV Visible spectrophotometer) were carried out by use of this equipment.
Methodology
This is an important step in the aspirin production process: the wet granulating of aspirin granules. Using a mortar and pestle, aspirin (14 grams), lactose (8 milligrams), and starch powder (76 milligrams) were combined and pounded into a fine powder. To create a cohesive mass, starch paste (5% w/v) was then added and thoroughly combined. To obtain wet granules, the cohesive material was passed through a number of sieves.11, 12,
Preparation of Polymer Coated Spherules
Ten grams of the moist granules, which had been meticulously weighed, were put into a 250 ml beaker. After that, the beaker was turned 45 degrees in the direction of the clock. To keep the granule bed moist, a 50:50 v/v ethanol and water mixture was sprayed over it while it rotated. To enhance the flow properties, a little quantity of starch powder was added while the granule bed was rotating. Seven to eight drops of starch solution, made by mixing four drops of starch powder, were added to further improve the binding of the small starch powder particles to form spherules On the granule bed, a solution of 5% starch paste and 7 milliliters of distilled water was sprayed or applied. To create spherules of a consistent size, the generated spherules were sieved via sieve numbers 25 and 45. A coating solution was prepared by dissolving 500 mg of polymer, 100 mg of dye, and 400 mg of talc in 25 milliliters of acetone. The spherule bed was continuously rotating as the coating solution was sprayed on top of it. To make dried polymer-coated spherules, the coated spherules were spread out on a petri dish and baked for 20 minutes at 60°C in a hot air oven.
Flow property determination
Measurements were made of characteristics like the angle of repose,Carr's index, and Hausner's ratio of spherules (retained in sieve numbers 25 and 45)
Microscopical evaluation of spherules and granules
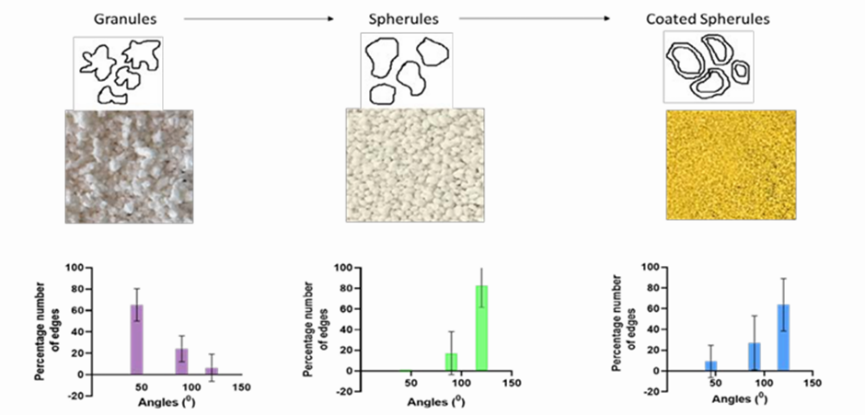
Using a projection microscope, the morphology and surface characteristics of granules and spherules were investigated. The granules and spherules that were generated were arranged individually on a glass slide and their edges and shapes were scrutinized with a projection microscope (10x). We looked into the shapes of fifteen randomly chosen particles. To gather and examine the particles, a projection microscope was employed. The number of edges in each of the three particles—450, 900, and 1200—was counted during the study.
Angle of Repose
The maximum angle that develops between the horizontal plane and the cone of the solid material pile, signifying the unrestricted flow of materials, is known as the angle of repose. To create a pile, spherules were let to freely flow down a funnel and onto graph paper. The following formula can be used to determine the angle of repose.13
Repose Angle (tan ?) = h/r
Where”r” is the pile’s radius, while “h” is its height.
Particle packing parameters
The primary application of bulk density is in spherule homogeneity determination. This promotes consistency in the size, closures, and capsules of the containers as well as in the choice of production tools and machinery. A ten milliliter measuring cylinder containing twenty grams of spherules was used to determine the bulk and tapped densities. After two hand taps on the flat surface, the volume filled by the spherules The bulk density was determined by measuring it. The volume occupied by spherules on a level table top following 100 tappings was recorded to estimate the tapped volume. The measuring cylinder containing spherules was linked to a tapped density device. From bulk and tapped volume, bulk and tapped density were calculated using the
Spherule weight / bulk volume = bulk density.
Spheruleweight/tapped volume=tapped density
Carr's Index
The Compressibility Index, also known as Carr's Index, takes into consideration interparticulate interactions to gauge spherules' propensity to cluster together. To calculate it, use the formula below.13, 14, 15,
[Tapped density – Bulk density/Tapped density] 100 is the Carr’s index.
Drug content evaluation
Precisely weighed aspirin spherules weighing fifty milligrams were dissolved in fifty milliliters of suitable phosphate buffer (6.4 or 7.4), chosen according to the coated polymer, agitated for thirty minutes, and filtered. In order to quantify absorbance at 265 nm, phosphate buffer was employed as a blank in the UV spectrophotometer.15
In vitro dissolution study
The amount of drug released from spherules in relation to time was measured using the USP type 2 basket device. 1500 mg of spherules were placed in a dissolving basket along with an appropriate pH 6.8 or 7.4 phosphate buffer. The device was kept at 37 ± 0.5°C, and the basket rotated at 75 rotations per minute, in order to maintain sink conditions. 1, 2, 3, 4, 5, 6, 7, 8, 9, and 10 times hours, five milliliter samples were extracted, and each interval was followed by a replacement of the same volume of buffer. At 265 nm, absorbance was measured with a UV spectrophotometer.15
Kinetic Modelling
Using the Zero order, First order, Higuchi model to suit the in vitro drug release data and Korsmeyer-Peppas models, the kinetics and mechanism of drug release from spherules were ascertained. model. By using R2 and n value, the model that fit best agreed.16
Zero order release:
The medication is administered steadily and in zero sequence. The cumulative percentage of pharmaceuticals released over a specific time period is displayed in the drug release data.16, 17
Qt = Q0 + K0t
Where,
Qt = Drug released in time ‘t’
Q0 = Initial drug content
K0 = Rate constant for release of zero order
First order release:
First order release states that the concentration determines the release. The release data was plotted against time as a log cumulative percentage of medication remaining.16, 17
Log Q0 – K1t/ 2.303 = log Qt
Where,
Qt =The release of drugs at time ‘t’
Q0 = Initial drug content
K1 = First-order release rate constant
Higuchi model:
The drug release data is presented as the cumulative fraction of drug release versus the square root of time. 16, 17
Qt = KH t½
Where,
Qt = quantity of medication released at time “t”
KH = Higuchi release rate constant.


 Rutik Kotwal*
Rutik Kotwal*
 Rahul Mohan
Rahul Mohan
 Dr.Rajendra Kawade
Dr.Rajendra Kawade

 10.5281/zenodo.14272721
10.5281/zenodo.14272721