Abstract
Over the past three decades, formulation technology has significantly advanced, particularly in drug delivery systems. Innovations include novel dosage forms and new uses for existing drugs, offering benefits like improved patient compliance, sustained drug concentration, reduced dosing frequency, targeted delivery, and minimized side effects. Transdermal drug delivery systems (TDDS) are key developments, allowing controlled, continuous medication administration through the skin, bypassing gastrointestinal degradation and hepatic first-pass metabolism, and enhancing bioavailability and patient compliance. The FDA approves roughly one transdermal product every 2.2 years, with the first patch approved four decades ago. This research examines the skin's role as a barrier, clinical trials, patents, commercialization, and the benefits and limitations of TDDS. Various TDDS methods are reviewed, highlighting their advantages, disadvantages, and potential applications. Recent advancements demonstrate TDDS's effectiveness and potential across diverse sectors, emphasizing their transformative impact on drug delivery and therapeutic practices.
Keywords
Sustained, Tablet, Captoprilusing, Cross-Linked Alginate
Introduction
Research in the field of sustained release delivery system is extremely fertile and produced many discoveries. Different drug has basic goal is to achieve steady state blood level that is therapeutically effective and non toxic for an extended period of time. The design of proper dosage form is an important element to accomplish this goal. Over recent years, as the expense and complication involved in marketing new entities have increased with accompanying recognition of the therapeutic use of controlled drug delivery, greater attention has been focused on the development of sustained or controlled drug delivery system.Sustained release, sustained action, prolonged action, controlled release, extended action and depot dosage form are term used to identify drug delivery system that are designed to achieve prolonged therapeutic effect by continuously releasing medication over an extended period of time after administration of a single dose. In the case of the oral sustained released dosage form, an effect is for several hours depending upon residence time of formulation in the GIT. Captopril belongs to class Angiotensin Converting Enzyme inhibitor (ACE inhibitor).The impact is on the renin-Angiotensin system, where it hinders the transformation of Angiotensin I, which is relatively inactive, into the active Angiotensin II. Interaction of Captopril with iron and other transition metals has been documented in literature. It has been postulated by the researchers that this interaction might be peculiar to Captopril within the class of ACE inhibitors due to the presence of a sulfhydryl functional group. Consequently, in cases where a patient necessitates an ACE inhibitor and is also taking iron salts, alternative agents to Captopril should be contemplated. Captopril is highly soluble in water and possesses a half-life of 1.9 hours. The primary objective of this research endeavour was the formulation of sustained release tablets of Captopril by employing different proportions of Xantham gum and Ethyl cellulose.
MATERIALS & METHODS:
MATERIALS:
Captopril was purchased from Balaji Drugs B20, Varachha Road, Surat – 395010 (GUJ)., HPMC K 100,Xantham gum, PVP K30 purchased from Balaji Drugs. Magnesium stearate, Aerosil 200 are from Research lab fine chem industries (Mumbai).
Formulation of Captopril sustained release tablet:
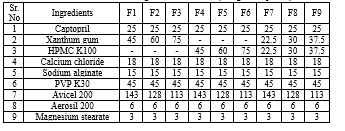
Sustained release tablets of Captopril were prepared by direct compression technique using varying proportions of polymers in combination. The composition of sustained release Captopril tablets is given in table -1. All the ingredients were individually passed through a 60 mesh sieve, except glidant and lubricant. For each formulation required quantities of Captopril, polymer (xanthan gum ), were accurately weighed according to the composition and mixed in a polybag for about 30 to 45 minutes. s. The obtained blend was lubricated with aerosil and magnesium stearate for another 5 minutes. The appropriate amount of the mixture was weighed and then compressed using 8 station rotary tablet press equipped with 11 mm flat faced punches at a constant compression force required to produce hardness of tablets about 2-4 kg/ cm2.
Total weight of the tablet= 300mg
Each tablet contains= 25mg of the Captopril
Table 1: Formulation Design for Each Batch (Drug +Excipients)
Pre-compression Parameters:
Angle of repose
The angle of repose of granules was determined by funnel method. It is a maximum angle possible between the surface of the pile and horizontal plane. The diameter of the powder cone was measured and angle of repose was calculated using the following equation:
Tan ?=h/r
Where h and r is the height and radius of the powder cone.
Carr’s compressibility index
The carr’s are determined by compressibility index. ?rr’s index can be calculated by using the following formula
Carr’s Index(%) = TBD – LBD/ TBD × 100
Post-Compression Parameters
Sustained release tablets of Captopril were prepared by direct compression technique. Total nine formulations were prepared. The tablet weight variation, hardness, friability and content uniformity for each formulation are shown in table 1. The prepared sustained release tablet were evaluated for their physical properties like hardness and friability, swelling index and drug content.
Hardness and friability:
For each formulation, the hardness and friability of 6 tablets were determined using the Monsanto hardness tester and roche friabilator.
Friability= Initial weight– Final weight / Initial weight x100
% Friability of tablets less than 1% is considered acceptable.
Swelling index
The swelling index of Captopril tablet is determined in 0.1 N HCL (pH 1.2) at R.T. After each interval the tablet was removed from beaker and then remove the excess buffer by using filter paper and weighed again upto7 hrs. The swelling index was calculated by following equation:
Swelling index (SI) = (Wt – W0)/ W0 x 100
Where Wt = Weight of tablet at time t
W0 = Initial weight of the tablet
Drug Content
The drug content of Captopril is following procedure take a 20 tablet and crush it then take a powder containing 0.1gm of Captopril with 150ml of phosphate buffer pH 6.8 to produce 200ml and filter this mixture by using whatman filter paper. Dilute 10ml of filterate to 100ml with water and measure the absorbance of filter a teat 205nm. The drug content of tablet was found to vary between 96.50% to 98.10%.
In vitro dissolution study
the dissolution profiles of formulation shows the comparative release profile with different concentration of different polymer from batches formulation (F1) to formulation (F9). In Vitro drug release studies of sustained release tablets of Captopril were done in USP type II dissolution test apparatus (Electro lab TDT-08, India) at 37°C ± 0.5°Cand 50 RPM speed in 900 ml of dissolution medium(0.1 N HCL). Dissolution medium consisted of 0.1N HcL for first 2 hrs and phosphate buffer pH 6.8 from 3 to 8 hrs. Take a Five mili litre sample were taken by filtration at predetermined time intervals then after each sampling the volume of dissolution medium was replaced by 5ml of phosphate buffer solution(pH 6.8). The amount of drug present in Captopril released is determined by UV spectroscopy. In formulation F1, F2 and F3 HPMC K100 is used as sustained released polymer. Then determination of Preformulation study and physiochemical properties of SR tablet of Captopril was conducted and results are shown in following table.4. In- vitro dissolution study results F1 showed 58.55% drug release within 8 hrs. F2 showed 68.59% and F3 showed 74.62% cumulative drug release in 8 hrs. Thus sustained release profile was not achieved. so the next batch F4 the HPMC K100 are used as a polymer. Then F4 showed 65.58% and F5 showed 50.34% and F6 showed 72.90% drug release within 8 hrs. Then in F7, F8, F9 both xanthum gum and HPMC K100 are used ratio as 1:1. Hence f7 showed 77.01% and F8 showed 76.66 and F9 showed 57.39% drug release within 8 hrs. These result indicated that xanthum gum and HPMC K100 could control the release of Captopril up to 8 hr.
Zero Order Kinetics
A Zero order release of a drug is determined by the following equation:
Qt-Q0=K0t
Where Qt =Amount of drug release dissolved in time ‘t’
Co=Initial amount of drug concentration in solution.
Kot=Zero order rate constant.
First Order Kinetics:
A first order release would be predicted by the following equation
Log Qt=Log Q0-Klt/2.303
Qt=Amount of drug release in time ‘t’
Co= Initial concentration of drug in solution.
Klt=first order rate constant
Higuchi’s Model:
This model states that the drug release from the matrix device by diffusion has been described by the following diffusion equation,
Ft=Q=VD5/T(2C-5Cs)Cst
Where, Q=Total amount of drug release dissolved in time ‘t’.
Co= Diffusion Coefficient of drug in the release matrix.
Cs= Solubility of drug in the matrix.
5= Porosity of matrix
t=Tortuosity
T=Time (hr)
Stability study:
The stability study of this formulation was evaluated only through the stability studies in three different phases. The purpose of this stability testing was to obtain a stable product which assures drug safty and efficacy up to the end of shelf life at defined storage condition like Room temperature, cool and in freezer upto three months. The prepared sustained release Captopril tablet formulation ( F7) of Captopril were placed on plastic tubes containing desiccant and stored at ambient conditions like R.T and cool temperature. Total 99.87% Drug content are present in API.
METHODS:
Drug-excipient compatibility studies:
Drug and excipients compatibility studies by using following instrument: Infra-red (IR) spectroscopy was conducted on FT IR 8201 PC Spectrophotometer ( Tokiyo, Japan) and the spectrum was recorded in the wavelength region of 4000 to 400 cm?1. The procedure consist of dispersing a sample (mixture of drug and excipients) in KBr and compressing into discs by applying a pressure of 5 tons for 5 min in a hydraulic press. The pellet was placed in the light path and the spectrum was obtained.
RESULTS AND DISCUSSION:
Pre compression parameters
Table 2: Pre-compression parameters for sustained release tablet of Captopril [F1 to F9]
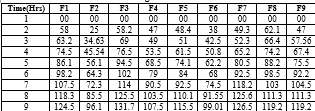
Table 3: Post-compression parameters for sustained-release Captopril tablets (Formulations F1 to F9).
Swelling Index of tablets of batch [F1 to F9]
The expansion characteristics of a pharmaceutical unit were assessed through the analysis of its weight gain. The degree of swelling exhibited by Captopril tablets was examined in a 0.1 N hydrochloric acid solution. The tablets were placed in a petri dish containing pH 6.8 phosphate buffer and observed at specific time points of 1, 2, 4, 6, and 8 hours. Subsequently, each tablet was carefully removed, dried with a tissue paper to eliminate excess moisture at each designated time interval.
Table 4: Swelling index of formulations


Fig.1: Swelling index of Captopril SR tablet
In-vitro release study:
Table 5: In vitro dissolution profile of Captopril.

Fig. 2: In-vitro Release study

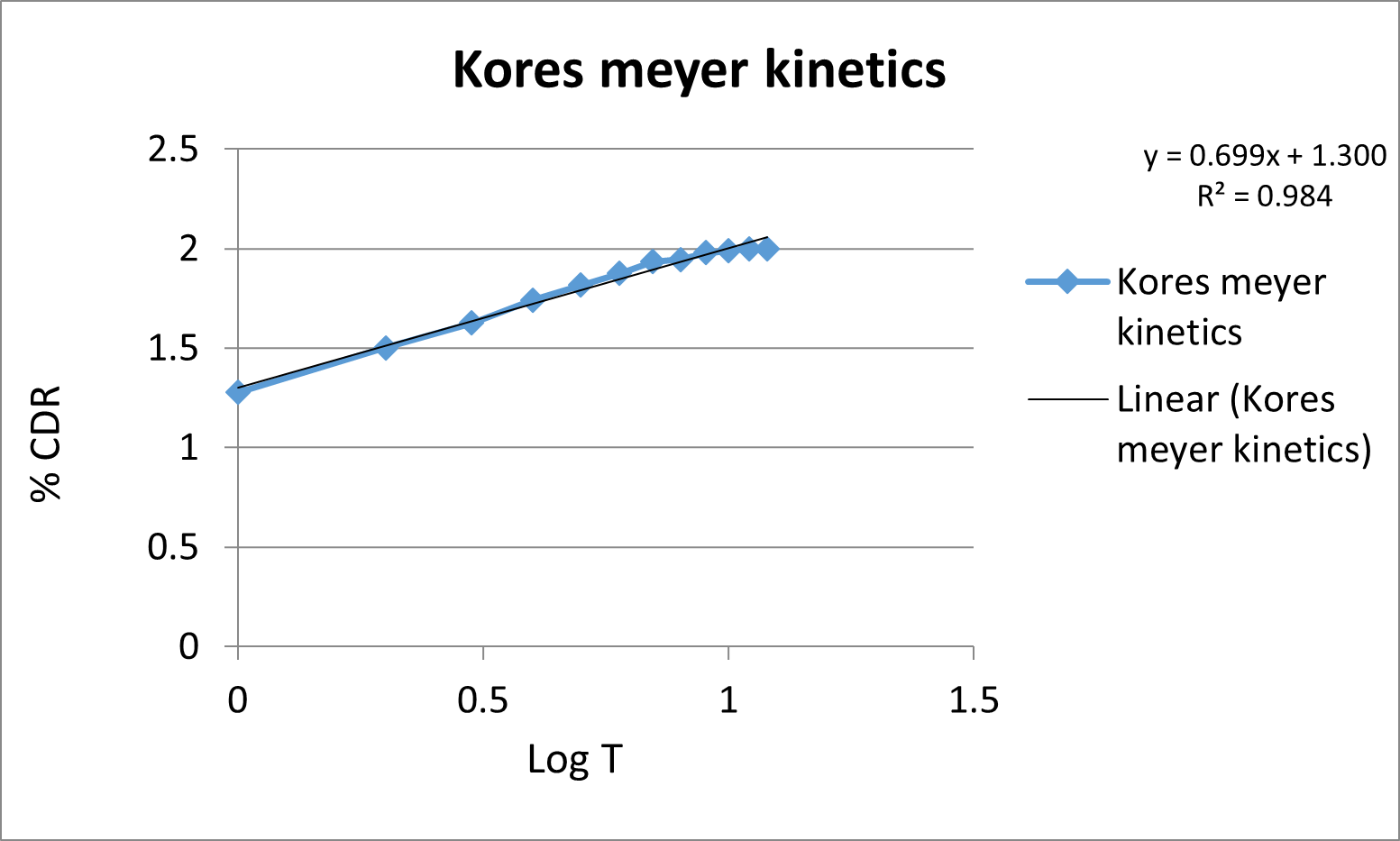
Kinetic study:
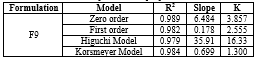
The release kinetics data for the various formulations of sustained release captopril tablets are presented in the following table, with correlation coefficients (R?2;) for Zero order, First order, Higuchi model, and Korsmeyer-Peppas model:
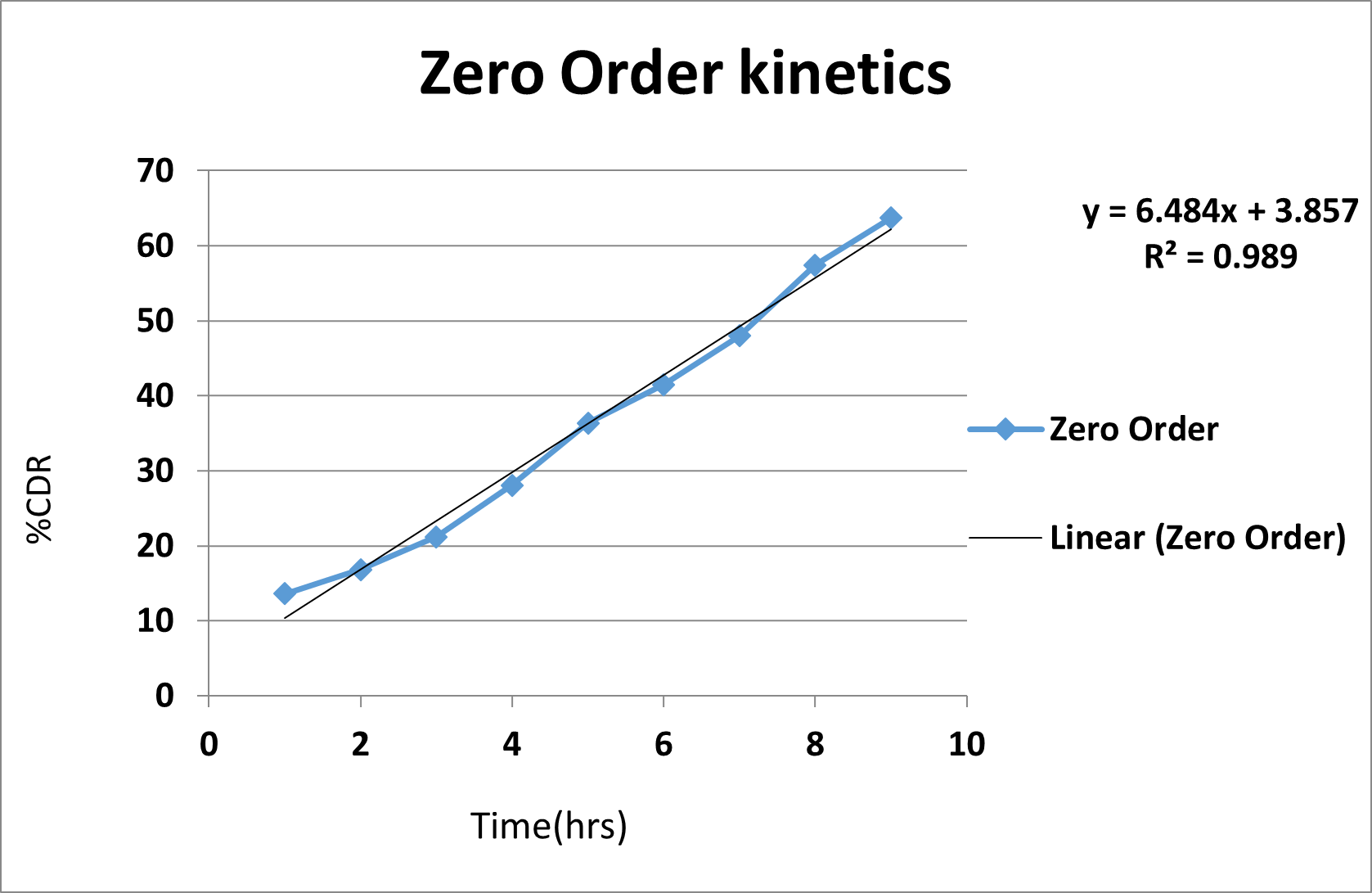
(A)

(B)

(C)
(D)
Fig. 3:Drug release kinetic study (A) Zero order drug release kinetics, (B) First order release kinetics, (C) Higuchi model drug release kinetics, (D) Korsemyer peppas model drug release kinetics.
Table 5. Release kinetics data optimized Captopril sustained release tablet (f9)

The in vitro release data of F9 was fitted with various kinetics equations. From the table.5. it was shown that the regression coefficient (r2=0.989) value was more in zero order and korsmeyer model (r2=0.984). The ‘n’ was found to be 1.300 which indicates that the drug release follows Fickian diffusion.
COMPATIBILITY STUDY OF DRUG:
Infrared spectrophotometer is useful analytical technique utilized to check the chemical interaction between drug and excipients used in formulation. The sample 1mg was powdered and mixed with 10mg of dry powdered potassium bromide. The scanning in the wavelength region of 4000-400 cm?1. IR spectroscopy studies revealed that the pure Captopril showed typical band at1743.33 cm-1. Due to C=0 stretching of carboxylic group and a band at 1594.84 cm-1 due to C=O stretching of amide group, C-H stretching of 2877.27 cm-1. There is no significant shifts of reduction in intensity of the FTIR bands of Captopril were observed as shown in figure.4.
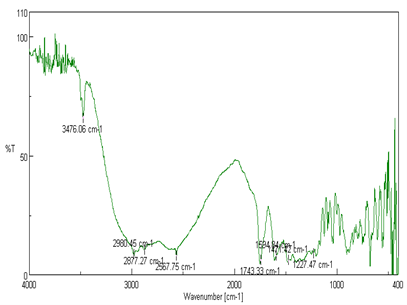
Figure 4: FTIR Spectra of Captopril
Differential Scanning Calorimetry(DSC):

Fig.5. DSC Thermogram of Captopril Differential scanning Calorimetry

Fig.6. DSC of Captopril+ Excipients
Thermal analysis of the drug was conducted using Differential Scanning Calorimetry (DSC). The DSC curve of Captopril exhibited a sharp exothermic peak at 105.07°C corresponding to its melting point, indicating its crystalline nature and the purity of the sample. The DSC thermogram is presented in the figure.
CONCLUSION
From the above results it is concluded that sustained release tablet of Captopril containing Different ratio of Xanthum gum and HPMC K100 By direct compression method. The results state that the xanthum gum and HPMC K100 control the Captopril release effectively for 12hrs. It is concluded that sustained release of Captopril over a period of 12 hours was obtained with formulation (F9) containing high amount of xanthum gum and HPMC K100. From the present research it is concluded that a successful sustain release tablet of Captopril can be formulated.
REFERENCES
- Copper J, Gunn C. Powder flow and compaction. In: Carter SJ, eds. Tutorial Pharmacy. New Delhi, India: CBS Publishers and Distributors; 1986:211-233.
- Darandale A, Aher A,Sustained release dosage form a concise review, International journal of pharmaceutics and drug analysis,2017;153-160.
- Agarwal G, Agarwal S, Oral sustained release tablets an overview with a special emphasis on matrix tablet, American journal of advanced drug delivery ,2017; 60-64
- Karvekar M, Khan A, A brief review on sustained release matrix type drug delivery system, Journal of pharmaceutical research 2017;282-283.
- Tapaswi RD, Verma P. Matrix tablets: An approach towards oral extended release drug delivery. Int J Pharma Res Rev. 2013; 2(2):1224.
- Debjit Bhowmik, Chiranjib B., Recent Trends in Sustained Release Matrix Drug Delivery System: A review, Page No. 1- 21.
- Ratnaparkhi M. P., Gupta Jyoti P., Sustained Release Oral Drug Delivery System, An Overview, International Journal of Pharmacy Research and Review, March 2013, Volume-2, Issued-3, Page No. 11-21.
- Ojha Kumar Abhishek, A Review- Sustained Release Drug Delivery Technology, World Journal of Pharmacy and Pharmaceutical Sciences
- Chaudhari UR., Review of Captopril Drug Formulation, Mechanism of action, Dosage, Use and Adverse drug reaction. Research Journal of Pharmaceutical Dosage Forms and Technology. 2021; 13(2):157
- Dinesh Kumar, Formulation And Evaluation Of Valsartan Sustained Release Matrix Tablets, Bulletin Of Pharmaceutical Research 2014;4(8), 81-85
- Ganesh G.K. Preparation And Evaluation Of Sustained Release Matrix Tablet Of Valsartan Using Natural Polymers, Indo-American Journal Of Pharmaceutical Research 2013;3(1),1310-1315
- Liberman, H.A. “Pharmaceutical Dosage Form Tablets’’ , 2nd edition, vol I, 136.
- Handbook Of Pharmaceutical Excipients, Fourth Edition, Page No.812-814.


 Nilesh Mhaske*
Nilesh Mhaske*
 Sudarshan Rashinkar 2
Sudarshan Rashinkar 2












 10.5281/zenodo.13117591
10.5281/zenodo.13117591