Abstract
This study focuses on creating and assessing herbal bath bombs to utilize their medicinal and cosmetic benefits for skin cleansing, detoxification, and moisturizing. Bath bombs, which contain citric acid and sodium bicarbonate, react with water to produce a fizzy effervescence, releasing carbon dioxide and aromatic scents. The research includes ingredients like neem oil, turmeric, rose oil, and Epsom salts to enhance therapeutic effects. The methodology involves preparing bath bombs and evaluating their physical appearance, effervescence time, skin compatibility, pH levels, stability, and antimicrobial properties. Results show that formulation "B" excels in stability, pH balance, effervescence duration, and lack of skin irritation. The study concludes that herbal bath bombs, with their skin-nourishing and aromatherapy benefits, offer significant advantages for mental and physical well-being. It also suggests potential future improvements in their formulation to address various skin types and needs.
Keywords
Bath bombs, muscle relaxant, skin nourishing, physical- mental wellbeing, skin repairing, improve sleep
Introduction
What is a Bath Bomb?
Bath bombs, also known as effervescent bath pills, are products designed to add colour, fragrance, and fizz to a bath. Recently, they have surged in popularity, with numerous variations available in retail and online stores. The chemistry of bath bombs is relatively straightforward, involving an acid-base reaction between citric acid and sodium bicarbonate. (1).This combination, when dry, remains inactive, but upon contact with water, it reacts to produce carbon dioxide, salt, and water. For example, sodium bicarbonate and citric acid react in the presence of water to create this effervescent effect (2).In today’s fast-paced world, achieving a relaxed state of mind and body can be challenging. Bath bombs offer an affordable means to support both mental and physical health. Through the use of moulds and dyes, herbal therapeutic bath bombs can be customized in various shapes, sizes, and festive colors, adding a playful and enjoyable element to bath time(3).
Objective of the Study
The objective of this study is to design bath bombs that capitalize on their medicinal and cosmetic properties, such as body cleansing, detoxification, and moisturizing. This involves assessing the safety and stability of these formulations, including pH levels and antimicrobial activity, to ensure they meet quality standards suitable for consumer use (3).
Reaction
Citric Acid + Sodium Bicarbonate + Water =Salt+ Water+ CO2
This reaction produces sodium citrate, water, and carbon dioxide. The carbon dioxide generates bubbles in the water, and the formulation's scent is released. A surfactant foaming agent creates additional foam, enhancing the bathing experience. Because the ingredients are diluted in water, sodium bicarbonate and citric acid do not cause skin irritation (2).
Cosmetic Chemistry
Educators continuously seek innovative ways to connect chemistry to real-world applications, often through food, medicine, and increasingly, cosmetics. The cosmetic chemistry industry, valued at over $100 billion, produces a range of products from shampoos to perfumes. Despite its everyday relevance, there are limited teaching experiments related to cosmetic chemistry in general courses. Notable educational activities include making cold cream, lotion, and soap, and exploring the acid-base properties of bath bombs. This study provides an experiment where students can create and analyse bath bombs, integrating fundamental principles of kinetics and cosmetic chemistry (1).
Introduction to Skin
The acidity of the skin's surface was first discovered by Heuss in 1892. Subsequent potentiometric measurements have shown that the skin's pH ranges from 4.2 to 5.6. The use of glass electrodes for skin pH readings has become common since the early 1950s. Various factors influence skin pH, including endogenous factors like age and sebum, and exogenous factors such as detergents and cosmetic products (4).
Table No. 01. Factors influencing skin pH
MATERIALS AND METHODS
Sodium Bicarbonate (Baking Soda)
Sodium bicarbonate offers several skin benefits, such as exfoliation and pore tightening. It helps remove dead skin cells, leaving the skin smooth and glowing. Additionally, it neutralizes body odors, making it an ideal ingredient for fragrant bath bombs (5).

Fig No.1- Baking Soda
Corn Starch
Corn starch synergistically enhances the effects of other bath bomb ingredients like baking soda, prolonging the effervescence. This results in a longer-lasting fizz, adding to the overall bathing experience (5).

Fig No.2- Corn Starch.
Magnesium Sulphate (Epsom salts)
Epsom salts, composed of magnesium and sulphate ions, are easily absorbed through the skin. They play critical roles in, inflammation reduction, muscle relaxation, and preventing arterial hardening. Sulphate in Epsom salts also help treat headaches (6).

Fig No.3. Magnesium sulphate
Neem (Azadirachta indica)
Neem oil, from the Meliciaceae family, is used to treat various skin issues, including wrinkles and erythema. Its antibacterial and moisturizing properties make it a common ingredient in external cosmetic applications (7).

Fig No.4- Neem
Rose Oil
Rose oil (Rosa damascena Mill, Rosaceae) is valued in medicine for its therapeutic properties, particularly in aromatherapy. It alleviates emotional distress, anxiety, tension, and stress-related insomnia. Rose oil also exhibits antimicrobial properties (8).

Fig No.5- Rose oil
Turmeric
Turmeric, derived from the rhizome of a ginger-like plant, contains curcumin and volatile oils with strong anti-inflammatory properties. Both in vivo and in vitro studies have demonstrated turmeric's ability to inhibit the activity of various carcinogens and mutagens. It also inhibits the growth of numerous bacteria, parasites, and fungi (9).

Fig No.6- Turmeric
Citric Acid
Citric acid, found in citrus fruits, is a tricarboxylic acid with a molecular weight of 210.14 g/mol. It is safe, affordable, biodegradable, and used for its sequestering, buffering, wetting, cleaning, and dispersing properties. Citric acid is essential in producing effervescent salts, confections, soft drinks, and medicinal citrates (10).

Fig No. 7 Citric Acid
EXPERIMENT AND RESULTS
Formulation Table
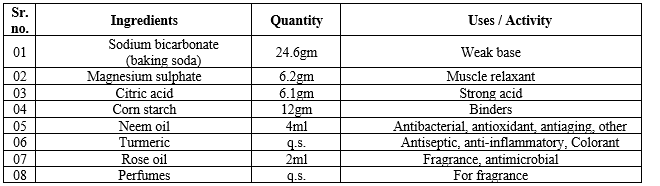
Table no. 02. Formulation table of bath bombs
Reaction
Citric Acid + Sodium Bicarbonate + Water = Salt + Water + CO2
METHODOLOGY
Preparation of Bath Bomb
A. Step One
- Weigh all dry ingredients.
- Add them to a dish and stir.
B. Step Two
- In a beaker, mix turmeric with rose oil.
- Add rose water to the turmeric solution.
- Slowly mix the liquid into the dry ingredients until slightly moist. Avoid adding too much water to prevent premature neutralization. Press the mixture into moulds and freeze for 30 to 45 minutes (11).
- Evaluation Tests
Physical Appearance
Physical appearance was assessed for three formulations (A, B, C) (7).
Table No.3: Physical Appearance Test
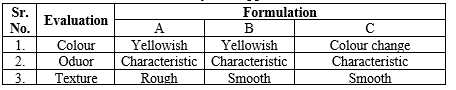
Fig no.8. Physical appearance of Bath Bomb

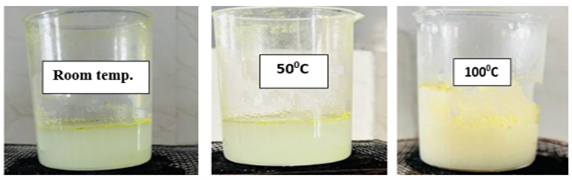
Effervescent Time
The effervescence time was measured by placing a bath bomb in 500 ml of room temperature distilled water until bubbling ceased (7).

Table No.4: Effervescent Time
Fig no. 9. Effervescent time
Skin Patch Test
The bath bomb was dissolved in a tub of water, and the test was considered successful if no irritation occurred after five minutes (7).
Table No.5: Skin Patch Test

pH Test
The pH of the solution containing the bath bomb was measured (7).
Table No.6: pH Test


Fig. no. 10. pH test
Stability Testing
Samples were stored at room temperature for two weeks, and any changes were noted (7).
Table No.7: Stability Testing

Antimicrobial Activity
Nutrient agar was prepared aseptically, and Staphylococcus aureus was used to test microbial growth. Three testing methods were employed
Negative control testing:
Nutrient agar was weighed, dissolved in distilled water, and placed into a petri dish.
Positive control testing:
Positive control testing involved weighing and dissolving nutrient agar in distilled water, then adding bacteria to the medium and pouring it into a petri dish.
Testing with bath bombs:
Nutrient agar was weighed and dissolved in distilled water before adding bath bombs formulation to the medium and pouring it into a petri plate. These methods were used to assess microbial growth with Staphylococcus aureus as the bacteria and nutrient agar as the culture medium (12, 13).
Table No. 8: Anti-microbial activity
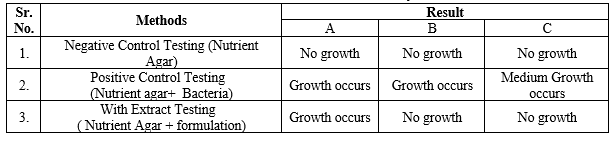
Fig. no.11. Anti-microbial activity
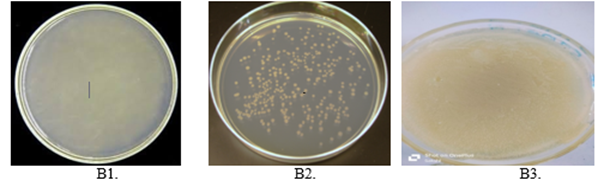
A. Nutrition agar, B. Nutrient agar+ bacteria, C. Nutrient agar + formulation
APPLICATION
- Bath bombs act as skin emollients and softeners, hydrating and pampering the skin.
- Natural and vegan-friendly, bath bombs avoid harsh chemicals and irritants.
- The citric acid and sodium bicarbonate in bath bombs provide a cleansing, deodorizing, and skin-repairing effect.
- The fragrances in bath bombs offer aromatherapy benefits, enhancing mood and relieving stress.
- Bath bombs create a luxurious and enjoyable bathing experience with their effervescence and pleasant aromas (7).
Storage Conditions
- Store in a cool, dry place.
- Avoid direct sunlight and contact with water.
MERITS
- Enhances relaxation.
- Moisturizes and hydrates the skin.
- Cleanses and repairs skin.
- Helps with muscle pain.
- Improves sleep quality.
DEMERITS
- May cause irritation or dryness in some cases.
- Artificial colours can cause irritation.
- Excessive use can alter skin pH.
FUTURE ASPECTS
- Innovation in formulations to enhance skincare benefits for aging, acne, and sensitive skin.
- Increased global adoption as more people become aware of the benefits.
- Customization for different skin types and mood enhancement.
CONCLUSION
From this we can conclude that the components and benefits of herbal bath bombs for skin health by their formulation and evaluation. Due to its muscle relaxant properties, skin benefits, and aroma therapy. Bath bombs contains oil such as coconut oil, neem oil, rose oil which are used to hydrate our skin, moisturize our skin. So we can use bath bombs at regular interval of time to fill better or more exited. We have three formulations: A, B, and C. upon comparing all three, formulation “B” is better than the other two. “B” has good stability and pH, a better effervescent time than the others, and it does not cause any skin irritation.
REFERENCES
- Cabassa, Meaghan, and Beth L. Haas. Sizzle and fizzle of bath bombs: an inexpensive and accessible kinetics experiment. Journal of Chemical Education 97.6, 2020, 1629-1632.
- Jugale P, Kadam A, Kadam A, Jetithor N, Kore P, Mohite S, Singh S. Preparation and Evaluation of Antifungal Bath Bomb of Ethanolic Extract of Betel Leaves. SGVU Journal of Pharmaceutical Research & Education. 5(1). 2020. 65-70.
- Himanshu Jaiswal, et al, formulation and evaluation of an herbal medicated bath bomb: a boon to cosmeceuticals 2023.
- Schmid-Wendtner, M-H., and Hans Christian Korting, The pH of the skin surface and its impact on the barrier function. Skin pharmacology and physiology, 19.6, 2006, 296-302.
- Draelos, Z. D. A Novel Approach to Enhancing the Quality and Appearance of Photoaged Skin. J Drugs Dermatol, 18(1), 2019. 28-31.
- Elbossaty WF Pharmaceutical Influences of Epsom salts. Am J Pharmacol Pharmacother. Vol.5 No.1:2, 2018.
- Thasni K.S, Silpa V.S., et al. A review on formulation and evaluation of herbal derived bath bomb. International Journal of Creative Research Thoughts, 10, 2022, 273-280.
- Hongratanaworakit, Tapanee. "Relaxing effect of rose oil on humans." Natural product communications 4.2, 2009, 291-296.
- Lal, Jaggi. Turmeric, curcumin and our life: A review. Bull. Environ. Pharmacol. Life Sic 1, 7, 2012, 11-17.
- Angumeenal, A. R., and D. Venkappayya. An overview of citric acid production. LWT-Food Science and Technology 50.2, 2013, 367-370.
- Darne, Chetan S., et al. Formulation and Evaluation of Antifungal and Muscle Relaxant Herbal Bath Bomb Containing Cinnamon Oil. International Journal of Pharmaceutical Sciences 1.08, 2023, 1-1.
- Gayatri H. Tiwaskar, Pawan Mahure, Prajakta Mowade, Apurva Tiwari, Krutika Sawarkar, Ajay G. Pise, Exploring The Spermicidal Properties of Extracted Neem Seed Oil: An Ex-Vivo Study Investigation Based on Tribal Knowledge , 2013, 12(10), 8369- 8370.


 Tejas Rajendra Burile*
Tejas Rajendra Burile*
 Ruchika Ravindra Umak
Ruchika Ravindra Umak
 Prachi Ganesh Yelne
Prachi Ganesh Yelne
 Sachin S. Padole
Sachin S. Padole
 Anil G. Dhawde
Anil G. Dhawde


















 10.5281/zenodo.12737110
10.5281/zenodo.12737110