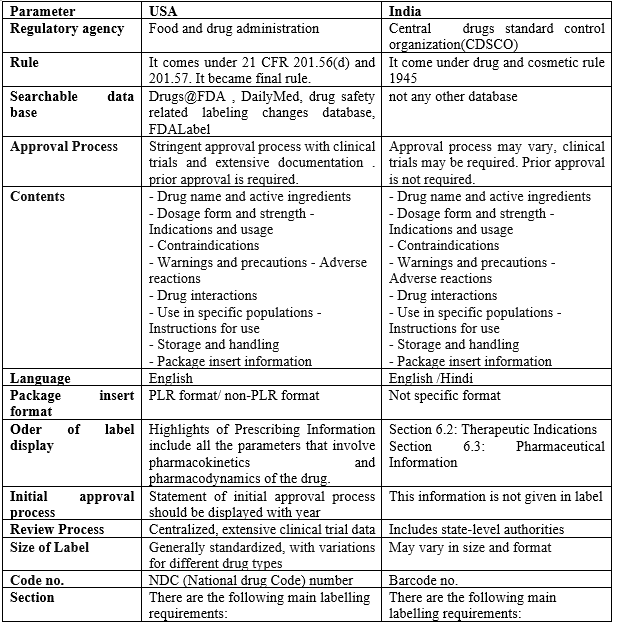
The pharmaceutical industry is heavily regulated worldwide, with drug labeling being a critical aspect of ensuring patient safety and effective usage. Drug labeling is a crucial aspect of pharmaceutical regulation, ensuring that essential information about a medication's safety, efficacy, and usage is communicated effectively to healthcare professionals and patients The Food and Drug Administration (FDA) in the USA and the Central Drugs Standard Control Organization (CDSCO) in India are the primary regulatory bodies responsible for ensuring drug safety and efficacy through labeling. This study aims to highlight similarities, differences, and potential areas for harmonization to enhance global pharmaceutical governance. A detailed comparative table is presented, summarizing key aspects of drug labeling regulations in the USA and India
Drug, Regulation, USA, Master, Files, Compliance, Standards, India
Drug labeling plays a critical role in ensuring the safe and efficacious use of medications. Drug labeling acts as a bridge between the manufacturer, healthcare professionals, and patients It provides healthcare professionals and patients with essential information about a drug's indications, dosage, contraindications, warnings, and potential side effects. Clear and comprehensive labeling empowers informed decision-making, promotes medication adherence, and minimizes the risk of adverse events. Regulatory authorities in different countries impose stringent labeling requirements to protect public health and ensure the safe and effective use of pharmaceuticals. This article delves into a comparative analysis of drug labeling requirements in the USA and India, two prominent pharmaceutical markets with distinct regulatory frameworks. The United States Food and Drug Administration (FDA) and the Central Drugs Standard Control Organization (CDSCO) are the respective regulatory bodies responsible for overseeing drug labeling in these countries.
Labeling and its objective :
Displaying instructions and information about a product on its innermost container or packaging or the product itself is important for drugs. Consumers absorb information from the label in three ways namely visually, auditory and kinaesthetically. Effective information and instructions on the label must be accurate in order to guide and educate the patients or consumers properly.
REGULATORY FRAMEWORK:
USA :
The Food and Drug Administration (FDA) is the primary regulatory body responsible for drug labeling in the USA. The Federal Food, Drug, and Cosmetic Act (FD&C Act) empowers the FDA to establish labeling standards for prescription and over-the-counter (OTC) drugs. The FDA enforces these regulations through its Center for Drug Evaluation and Research (CDER). The CDER publishes a series of guidance documents that provide detailed instructions on labeling content and format. The key components of the framework include:
- Food, Drug, and Cosmetic Act (FD&C Act):
Defines labeling requirements and empowers the FDA to enforce them.
- Title 21 of the Code of Federal Regulations (CFR) Parts 201 and 601:
Provide detailed specifications for labeling content and format for prescription drugs and over-the-counter (OTC) medications, respectively.
Offer additional recommendations and clarifications on specific aspects of labeling.
- New Drug Application (NDA) and Abbreviated New Drug Application (ANDA):
NDA is required for the approval of new drugs, including detailed labeling information .ANDA is used for generic drugs, requiring labeling that is identical to the reference listed drug.
India:
The Central Drugs Standard Control Organization (CDSCO) regulates drug labeling in India. The CDSCO, under the Ministry of Health and Family Welfare, is responsible for regulating drugs and cosmetics in India. The Drugs and Cosmetics Act, 1940, along with its subsequent amendments, sets out the legal framework for drug labeling in India. The CDSCO publishes guidelines on labeling formats and content. The CDSCO publishes guidelines outlining labeling requirements for different drug categories, including the Drugs and Cosmetics (Labels) Rules, 1970.
- Drugs and Cosmetics Act, 1940:
Establishes labelling requirements for drugs and cosmetics.
- Drugs and Cosmetics Rules, 1945:
Provide detailed specifications for labeling content and format.
A specific section within the Drugs and Cosmetics Rules dedicated to labeling requirements.
- Schedule D and Schedule Y:
Schedule D specifies the labeling requirements for imported drugs. Schedule Y outlines requirements for clinical trials, including labeling information for investigational drugs
LABEL CONTENT :
Similarities: Both the USA and India mandate labels to include essential information like:
- Product name and brand name (if applicable)
- Active pharmaceutical ingredient(s) and their strength
- Clinical pharmacology
- Therapeutic category
- Dosage form and route of administration
- Indications for use
- Contraindications and warnings
- Dosage and administration instructions
- Potential side effects
- Drug abuse and dependence
- Storage conditions
- Expiry date
- Manufacturer information
Differences: The level of detail and specific requirements may differ.
USA:
- Detailed risk information: Comprehensive sections on warnings, precautions, and adverse reactions are mandated.
- Pregnancy and lactation information: Specific categories detail risks associated with use during pregnancy and breastfeeding.
- Labeling for use by healthcare professionals: Detailed prescribing information.
- Highlights of Prescribing Information: This section provides a concise summary of the most critical information about the drug, including indications, usage, dosage, and safety information.
- Full Prescribing Information: This detailed section includes comprehensive information about the drug's pharmacology, clinical studies, contraindications, warnings, precautions, adverse reactions, and drug interactions.
- Medication Guide/Patient Information: This part of the label is intended for patients and includes information on the safe and effective use of the drug, potential side effects, and what to do in case of an adverse reaction.
India :
- Less emphasis on warnings: Warnings may be less prominent compared to the USA.
- Pregnancy and lactation information: While present, the details might be less comprehensive than US labels.
FORMAT:
USA:
The FDA has stringent requirements regarding the format and presentation of drug labels to ensure clarity and ease of use:
- Structured Product Labeling (SPL):
The FDA requires drug labeling to be submitted electronically in the SPL format, which standardizes the information and facilitates easy updating and dissemination.
Prescription drug labels must include a highlights section at the beginning, summarizing critical information such as indications, dosage, and warnings.
Specific guidelines on font size, style, and layout are provided to ensure readability. Important information such as warnings must be in bold or highlighted to draw attention.
Encourages clear and concise language to promote patient understanding.
In India, the format and presentation of drug labels are governed by the Drugs and Cosmetics Rules, 1945:
The label must be printed in a legible manner, ensuring that all information is clear and readable.
Labels must be printed in both English and Hindi, ensuring accessibility to a broader population.
The rules specify minimum font sizes for different sections of the label to ensure readability.
- Highlighting Critical Information :
Important information such as warnings and contraindications must be prominently displayed and highlighted.
Certain categories of drugs (e.g., prescription-only medications) may require specific color coding for easy identification.
REQUIREMENTS FOR SPECIFIC DRUG CATEGORIES:
Prescription Drugs
Both the USA and India require comprehensive labeling for prescription drugs, addressing the information needs of healthcare professionals.
Over-the-counter (OTC) Drugs
USA:
The USA utilizes a risk-based approach for OTC drugs. Labeling for established and less risky OTC products may follow a simplified format similar to the Drug Facts Label. However, those with higher risks may require more detailed information.
India:
Indian regulations generally require less detailed information on OTC drug labeling compared to the USA. However, the specific requirements may vary depending on the drug category and potential risks.
POST MARKETING UPDATE:
USA
- Mandatory reporting of adverse events: Manufacturers must report serious adverse events associated with their drugs.
- Label revisions: The FDA may require label changes based on new safety information or post-marketing studies.
- India
- Pharmacovigilance program: A system for monitoring adverse drug reactions exists, but reporting might not be as comprehensive as the USA.
- Label updates: The CDSCO can mandate revisions based on safety concerns, but the process might be less proactive compared to the FDA.
IMPACT ON PATIENT SAFETY:
Comprehensive and informative labeling plays a critical role in promoting patient safety. The USA's emphasis on standardized format, clear warnings, and post-marketing updates likely contributes to a higher level of patient safety awareness. However, India's focus on essential information can still offer adequate guidance for healthcare professionals.
COMPLIANCE STANDARD:
USA
The FDA enforces labeling compliance through inspections, pre-market approvals, and post-marketing surveillance. Manufacturers are responsible for ensuring their labels are accurate, complete, and up-to-date. The FDA can take enforcement actions against non-compliant labels, including warning letters, product seizures, or injunctions.
- Pre-Market Approval :
All new drugs must undergo a stringent pre-market approval process, including a thorough review of the proposed labeling.
- Post-Market Surveillance :
The FDA continuously monitors marketed drugs through post-market surveillance programs, ensuring ongoing compliance with labeling requirements.
- Inspections and Audits:
The FDA conducts regular inspections and audits of manufacturing facilities to verify compliance with labeling standards.
- Penalties and Recalls :
Non-compliance can result in penalties, product recalls, and other enforcement actions to protect public health.
India
The CDSCO enforces labeling compliance through inspections and post-marketing surveillance. Random sampling of marketed drugs is conducted to verify labeling accuracy. Non-compliant labels can result in penalties for manufacturers, including product recall or suspension of manufacturing licenses. However, enforcement actions for labeling violations may be less stringent compared to the USA.
- Pre-Market Approval :
All new drugs must receive approval from the CDSCO, including a review of the proposed labeling.
- Post-Market Surveillance:
The CDSCO conducts post-market surveillance to monitor the safety and efficacy of marketed drugs, including compliance with labeling requirements.
- Inspections and Audits :
Regular inspections and audits are conducted to ensure that manufacturing facilities comply with labeling regulations.
- Penalties and Recalls :
Non-compliance can lead to penalties, product recalls, and other enforcement actions to ensure public safety.


 Surbhi H. Makvana*
Surbhi H. Makvana*
 Shrikalp Deshpande
Shrikalp Deshpande
 10.5281/zenodo.11620505
10.5281/zenodo.11620505