Abstract
Regulatory quality systems play a critical role in ensuring public safety, product efficacy, and market efficiency across various industries. This comparative study aims to examine and contrast the regulatory frameworks in two significant economies: India and the United States of America. By analyzing the regulatory processes, enforcement mechanisms, and overall quality assurance practices in these countries, this research sheds light on the strengths and weaknesses of each system. The study begins by providing a comprehensive overview of the regulatory landscape in both countries, highlighting the key agencies responsible for overseeing diverse sectors, such as pharmaceuticals, medical devices, food and agriculture, and environmental protection. Subsequently, it delves into the legislative frameworks, guidelines, and standards that govern the approval, marketing, and post-market surveillance of products and services. The research analyzes the efficiency and transparency of the regulatory procedures in India and the USA, assessing factors such as time-to-market for new products, adherence to international standards, and opportunities for stakeholder engagement. It also evaluates the degree of coordination and cooperation between regulatory agencies, public-private partnerships, and efforts to combat corruption and undue influence on the decision-making process.
Keywords
Regulatory quality systems, medical devices, post-market surveillance, enforcement mechanisms, quality assurance practices.
Introduction
The term "regulatory system" includes a complete set of guidelines, organized processes, and clinically proven mechanisms that are designed to uphold and standardized the upcoming product [1]. The main motto of creating a regulatory system is to control the quality variation as well as maintain the specification of a complex system. Various domains like biological, financial, and technical are more-over government-specified areas, like business and commercialization so the quality system is all about safety, efficacy, and standardization. In this globe, two giant nations India and USA have their regulatory comities and they have remark able stake holding bodies responsible for manufacturing and supply chain management of pharmaceutical products. Albeit the regulatory systems of the two nations are well-established, there are some remarkable differences between them. [1,2] In the US, the Food and Drug Administration (FDA) is a prestigious directing body. FDA rules must be trailed by pharmaceutical products to guarantee their quality, safety, and efficacy. The FDA implements severe guidelines through different cycles, including pre-market approval, post- market surveillance, inspections, and enforcement actions [3]. The severe standards and broad assessment cycles of the American regulatory system can be credited for their importance in the pharmaceutical business [4] .The FDA needs broad clinical trials and data before endorsing new drugs. By doing this, the strictest safety and efficacy standards are fulfilled before pharmaceutical products are made accessible on the market. Moreover, to guarantee that GMPs and quality control standards are being adhered to, the FDA regularly inspects manufacturing destinations [5, 6] .The Central Drugs Standard Control Association (CDSCO), under the course of the Ministry of Health and Family Welfare, is responsible for managing pharmaceutical control. The CDSCO is liable for managing all parts of pharmaceutical item import, manufacture, distribution, and deals in India. The regulatory framework for the pharmaceutical industry in India is critical because it endeavours to combine the requirements for available pharmaceuticals at sensible rates with maintaining elevated expectations of quality. The flourishing generic pharmaceutical industry in India has given the country the moniker "pharmacy of the creating scene."[7,8] The CDSCO is a crucial participant; t in guaranteeing that generic drugs meet quality requirements by directing inspections, requiring licenses, and performing surveillance activities. In any case, it's important to remember that the validity and effectiveness of the Indian regulatory system have proactively been addressed. Because of allegations of subpar and fake pharmaceuticals, concerns have been raised about the overall quality assurance in the Indian pharmaceutical industry [9, 10].
OBJECTIVE:
The prime agenda of these comparative studies is to identify as well as analyze the area of similarity & dissimilarity and the approaches towards implementation of the new regulation. The purpose of these studies includes:
- Identifying best practices: The policymakers uphold in introducing new policies by comparing studies between two major countries. These will be more beneficial as the applicability is justified and produce improved and efficient guidelines.
- Learning from failures: The analytical data about shortcomings or the failure mode analysis outcome is beneficial for avoiding implementation mistakes of policies. Thus, it acts as a safeguard against potential risk. Analyzing the failures or shortcomings of regulatory systems in both countries can help avoid repeating similar mistakes and implement safeguards to prevent potential issues.
- Encouraging regulatory convergence: Analysis and differences and similarities can uphold the cooperation between two major countries in a regulatory sector which leads to enhanced smooth cross-border business, easy investment, and legal issues.
- Informing international organizations: The comparative study can facilitate the formation of standard guidelines on the international stage. This will develop a better and standard international guideline that can be applicable throughout the globe.
COMPARATIVE STUDY BETWEEN REGULATORY SYSTEM-USA & INDIA
Table 1. Comparative study between regulatory system-USA & India [6]


A Site Master File is well-prepared documentation containing factual information and specification about the product and manufacturing control process [11, 12]. These two major countries have their own GMP guidelines for Site Master File. The comparison of similarity and dissimilarity are as follows-
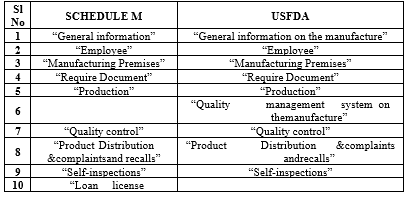
Table 2.General compares between Schedule M (INDIA) and USFDA guidelines[8]
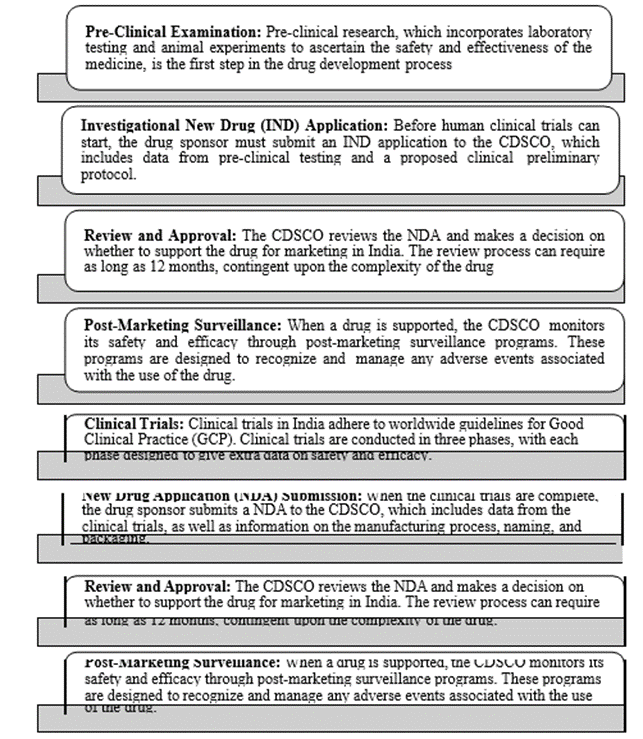
Drug Approval process by respective regulatory bodies (USFDA &CDSCO)
The FDA's Current Good Manufacturing Practices (cGMP) requirements mandate process approval. It is a formalized methodology for ensuring that a manufacturing process consistently results in an item that satisfies established requirements and standards for quality [13,14]. The FDA's process approval guidelines are momentarily described below:
Table 4.Drug approval process by USFDA[11]

The need of taking on a risk-backed strategy for process approval is emphasized in the FDA's process approval recommendations. This implies that the amount of approval required for a specific strategy should be determined by its possible impact on persistent safety and item quality. The recommendations emphasize the benefit of documenting during the process of process approval, including the production of test plans, reports, and protocol development[15, 16].
Drug Approval Process by CDSCO:
The Central Drugs Standard Control Organization (CDSCO), India's public regulatory organization for pharmaceuticals and medical devices, oversees the licensing process for drugs [17, 18]. Here is a fast once-over of India's medication approval methodology:
Table 5.Drug approval process by CDSCO [12]
It is important to take note that the drug approval process in India is persistently advancing, with efforts being made to streamline the process and make it more productive. Moreover, India has as of late implemented a system for sped-up review of specific drugs, such as those for uncommon diseases, to assist with ensuring that patients have timely access to inventive treatments [19, 20].
India and USA pharmaceutical market improvement up to the current situation
The pharmaceutical industries in the two India and the US have seen progress and development as yet [21]. With a fixation on research and development as well as a developing emphasis on quality control and regulatory compliance, the pharmaceutical sector in India has been contributing significantly to the development of the country's economy [22]. The Indian pharmaceutical industry, which developed at a CAGR of 14.5% from 2015 to 2020, was estimated to be worth $41 billion in 2020, according to research by the India Brand
Value Establishment[23].
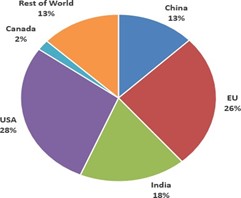
Fig 1. Percentage of API manufacturing facilitates for all drugs Region [13]
By 2030, the market is projected to increase at a CAGR of 15% to $130 billion, impelled by factors including rising healthcare awareness, rising demand for generic medications, and government measures to overhaul the healthcare system. [24,25] The pharmaceutical industry in the US has also continued to expand, with an emphasis on innovation and legitimate compliance. According to a Statistic analysis, the market was estimated to be worth $445.6 billion in 2020 and is expected to rise at a CAGR of 5.6% from 2021 to 2025 to reach $625.4 billion. With programs like the Affordable Consideration Act and the current drive to reduce prescription prices, the market has seen a rising emphasis on accessibility and affordability of healthcare.[26,27,28] The COVID-19 pandemic has also impacted the two sectors, driving rising demand for pharmaceuticals and medical equipment [29]. With multiple Indian firms working in partnership with foreign businesses to research and deliver vaccinations, the Indian pharmaceutical sector has been actively engaged in the manufacturing of COVID-19 medications and vaccines [30,31]. Also, the US pharmaceutical sector has created COVID- 19 medications and vaccinations, with many businesses getting FDA emergency use authorizations.[32]

Fig 2. Business growth for Indian Pharmaceutical market [19]
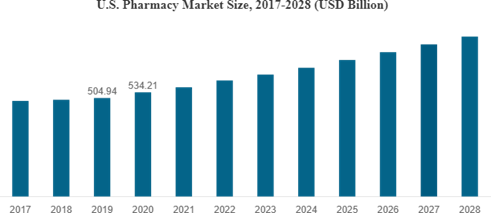
Fig 3.Marketing Growth analysis of US
In conclusion, the pharmaceutical markets in two India and the US have continued to expand and create up to the present, driven by elements like innovation, regulatory compliance, and a developing focus on healthcare accessibility and cost[34]. The two markets have been affected by the COVID-19 epidemic, which has raised demand for pharmaceuticals and medical supplies [35].
REFERENCES
- TGA/Pics 2018. Describes the Production in a guide to good manufacturing Practice for medicinal products. Part I. Chapter 5 – Production. Describes the Production in a guide to good manufacturing. Pages: 54. [Accessed On 1 July 2018]
- Barton, P. 2007. The Great Quinine Fraud”: Legality Issues in the “Non-Narcotic” Drug Trade in British India. The Social History of Alcohol and Drugs 22(1):6–25.
- Harper, I. 2007. Good Manufacturing Practice in the Pharmaceutical Industry–Working Paper 3, prepared for Workshop on 'Tracing Pharmaceuticals in South Asia', 2–3 July. The University of Edinburgh. The Centre for International Public Health Policy. http://www.csas.ed.ac.uk/ data/assets/pdf_file/0011/38828/GMPinPharmaIndust ry.pdf Pages: 02-35
- Part 211— USFDA 2016. Current Good Manufacturing Practice for Finished Pharmaceuticals -e-CFR data is current as of January 12,2016. https://www.fda.gov/drugs/pharmaceutical-quality-resources/current-good-manufacturing-practice-cgmp-regulations [Accessed on 21 September 2020]
- Part 1 – Schedule M 2019. Good Manufacturing Practices for Premises and Materials of Good Manufacturing Practices and Requirements of Premises. https://ipapharma.org/wp- content/uploads/2019/02/schedule-m-1.pdf Plant and Equipment for Pharmaceutical Products. [Accessed on February 2019]
- MHRA 2020. Manufacturers. https://assets.publishing.service.gov.uk/government/upload s/system/uploads/attachment_data/file/400232/Guidance_for specials manufacturers.p df Medicines and Healthcare Products Regulatory Agency. Pages: 41. [Accessed on 15 January 2015]
- EU GMP guide part II 2006. Guidance on Good Manufacturing Practice (GMP)- Production.Humaregulatory. https://www.ema.europa.eu/en/human-regulatory/research- development/compliance/good-manufacturing-practice/guidance-good-manufacturing- practice-good-distribution-practice-questions-answers European Medicines Agency. [Accessed on 15 July 2006].
- Singh, S H. 2005. Government of India Ministry of Health and family welfare department of Health and family welfare Lok Sabha unstarred question no. 655.
- Haleem, RehemM. 2015. Quality in the pharmaceutical industr – A literature review. Saudi Pharmaceutical Journal 23(5):463–469.
- Food and Drug Administration - Sterile Drug Process Inspection. Accessed 26 September 2013.2.
- Schedule M, Part I-A, Specific Requirements for Manufacture of Sterile Products,Parenteral Preparations and Sterile Ophthalmic preparations.Accessed 26 September 2013.3.
- Guidance for Industry for Sterile Drug Products Produced by Aseptic Processing and accessed 1 November 2013.4.
- 21 CFR 211 - Current Good Manufacturing Practice In Manufacturing, Processing, Packing or Holding of Drugs;Current Good Manufacturing Practice For. Accessed 27 September 2013.5.
- J. E. Enders. Quality assurance and control, In Gennaro A. R. (Eds), Remington: The Science and Practice of Pharmacy, Vol. I, Lippincott Williams &Wilkins, New York, 2000, pp. 980-985.
- L. Lachman, S. A. Hanna, and K. Lin. Quality control and assurance, In Lachman L, Lieberman H. A, Kanig J. L. (Eds.), The Theory and practice of industrial pharmacy, Verghese Publishing House, Bombay, 1976, pp. 804-855.
- Rose and H. Kenneth. Project Quality Management: Why, What and How, J. Ross Publishing, Fort Lauderdale, Florida, 2005, pp. 41.
- S. K. Mandal. Introduction to TQM: In TQM, Principles and practice, Vikas publishing house, New Delhi, Second reprint, pp. 1-18.
- Lakshmana Rao, M. Bharani, Y. Madhu and G. G. Sankar. TQM: The Need of New Era, Indian J. Pharm. Educ. Res. 39(4): 203-206 (2005).
- Hoyle and David. Quality Management Essentials, Oxford, United Kingdom: Butterworth-Heinemann, 2007, pp. 200.
- Pfeifer and Tilo. Quality Management: Strategies, Methods, Techniques, Munich, Germany: Carl Hanser Verlag, 2002, pp. 5.
- L. Rao. TQM: The need fora new era, Indian Journal of Pharmaceutical Education 39(4): 203 (2005).
- M. Bhaskar, B. Sanjib and Y. Abhishek. TQM in pharmaceuticals: A review, Int. J. Pharm Tech Res.
- De Feo, A. Joseph and W. Barnard. Juran Institute’s six sigma breakthrough and beyond - quality performance breakthrough methods, Tata McGraw-Hill Publishing Company Limited, 2005.
- J. F. Krafcik. Triumph of the lean production system, Sloan Management Review, 30 (1): 41–52 (1988).
- T. Ohno. Toyota Production System, Productivity
- B. Zhou, "Lean principles, practices, and impacts: a study on small and medium-sized enterprises (SMEs)," Annals of Operations Research, pp. 457-474, 2012.
- M. Demirbag, E. Tatoglu, M. Tekinkus and S. Zaim, "An analysis of the relationship between TQM implementation and organizational performance:Evidence from Turkish SMEs," Journal of Manufacturing Technology Management 17(6), pp. 829-847, 2006.
- M. Trahan and V. Kapoor, "TQM journey of an Indian milk-producing cooperative," The TQM Journal 23(4), pp. 423-434, 2011.
- M. Hizballah, L. Gutiérrez-Gutiérrez and J. F. M. Rosas, "Total quality management practices, competitive strategies, and financial performance: the case of the Palestinian industrial SMEs," Total Quality Management & Business Excellence 25(5-6), pp. 635- 649, 2014.
- V. R. Kannan and K. C. Tan, "Just in time, total quality management, and supply chain management: understanding their linkages and impact on business performance," Omega 33(2), pp. 153-162, 2005.
- Sila, "Examining the effects of contextual factors on TQM and performance through the lens of organizational theories: an empirical study," Journal of Operations Management 25(1), pp. 83-109, 2007.
- R. Sousa and C. Voss, "Contingency research in operations management practices," Journal of Operations Management 26(6), pp. 697-713, 2008.
- Yang, C. W. Y. Wong, K. -H. Lai and A. N. Ntoko, "The antecedents of dyadic quality performance and its effect on buyer-supplier relationship improvement," International Journal of Production Economics 120(1), pp. 243-251, 2009.
- M. Kumar and J. Antony, "Comparing the quality management practices in UK SMEs," Industrial Management & Data Systems 108(9), pp. 1153-1166, 2008.


 souvik kundu*
souvik kundu*
 Rahul Patra
Rahul Patra







 10.5281/zenodo.13774364
10.5281/zenodo.13774364