Abstract
Microcystin (MC-LR) is the most hazardous cyanotoxin present in water bodies, originating as by-products of cyanobacterial metabolism. Present study was planned to assess the role of coenzyme Q10 on aspartate aminotransferase and alanine aminotransferase during MC-LR induced toxicity. The animals of normal control group (N) received water and normal diet ad libitum and (MC-LR as well as MC-LR+Q) group received MC-LR (10 ?g/kg bw/day, ip) for 14 days. MC-LR treated mice were co-administered with coenzyme Q10 (MC-LR+Q) (10 mg/kg bw/day, im) for 14 days. The findings of the study indicated that the administration of coenzyme Q10 resulted in a decrease in the levels of aspartate aminotransferase and alanine aminotransferase in the liver, kidney and heart of MC-LR mice, indicating that it has the potential to fulfil therapeutic purposes.
Keywords
Microcystin-LR, coenzyme Q10, transaminases, liver, kidney, heart
Introduction
Algal blooms have emerged as a significant ecological concern on a global scale, leading to water pollution, eutrophication, and the discharge of various algal pollutants. These phenomena present risks to both human health and other organisms [1]. The existence of these bacteria has the potential to generate an assortment of toxins, including cyanotoxins, which pose a concern for public health and the environment. Through the food chain, cyanotoxins can accumulate in aquatic organisms and be transferred to higher trophic levels [2]. Microcystins (MCs) are the most common form of cell endotoxin among cyanobacterial toxins, displaying a broad spectrum of biological toxicities and having more than 100 isomers [3-5]. A well-known and very hazardous MC isomer, microcystin-LR (MC-LR), is chemically stable, difficult to break down, and may linger in natural water for months or even years [6, 7]. MC-LR has the potential to infiltrate the human body via various routes, including ingestion of aquatic products contaminated with MC-LR [8]. The liver is the main organ affected by MC-LR, causing significant hepatotoxicity. Additionally, MC-LR also demonstrates nephrotoxicity and carcinogenic activities [9]. As a result, MC-LR was categorized as an unconventional test substance in the updated Chinese Code of Hygienic Practice for Drinking Water, which was put into effect in 2001. This code set a safety limit of 1 ?g/L for the presence of MC-LR in drinking water [10, 11]. On the other hand, the concentration of MC-LR in natural water often surpasses 13 ?g/L [12]. At present, there are no efficient strategies to manage Microcystis blooms or to mitigate and address the risks associated with MCs. Hence, it is of paramount practical importance to carry out a comprehensive examination of the harmful processes of MC-LR in order to protect human and ecological well-being. Coenzyme Q10 (CoQ10) is an essential vitamin-like molecule that is fat-soluble and is found naturally in all cellular membranes [13]. Coenzyme Q10 is a kind of antioxidant that functions as a substance that may eliminate harmful free radicals [14]. Coenzyme Q10 also plays a role in the production of adenosine triphosphate in the mitochondrial respiratory chain, which is crucial for organs that need a lot of energy, such as skeletal muscle [15, 16]. CoQ10 is present in all tissues of the human body in both its reduced and oxidised forms. While the human body can synthesise it, the production of this substance declines as one ages. It is important to note that a lack of CoQ10 is strongly associated with several health issues such as diabetes, cardiovascular disease, and neurodegenerative disorders [17]. Aminotransferases, namely ALT and AST, are a class of enzymes that facilitate the conversion of amino acids and alpha-oxoacids by transferring amino groups (Figure 1) [18]. It has been proposed that these enzymes possess the most substantial clinical relevance. The intricate isoenzyme pattern of aspartate aminotransferase (AST), one of the most active enzymes in the cell, is tissue-specific and exists in both cytosolic and mitochondrial forms [19]. In addition to being present in liver cells, AST is also highly expressed in several non-hepatic tissues such as the heart, skeletal muscle, blood, kidney, pancreas, spleen, lung and erythrocytes [20]. The enzyme alanine aminotransferase (ALT) facilitates the transfer of amino groups in order to produce oxaloacetate, a hepatic metabolite [21]. The protein comprises 496 amino acids, the encoding of which is facilitated by a gene situated on chromosome 8's long arm. ALT is present in both cytosolic and mitochondrial forms. The liver has high levels of ALT, whereas the heart, muscle and kidney have comparatively lower levels [22]. This research aims to demonstrate the preventive and therapeutic benefits of coenzyme Q10 on MC-LR-induced damage in mice, specifically in relation to the liver, kidney and heart.

Figure 1. Aminotransferase: an overview
MATERIALS AND METHODS
Chemicals
Coenzyme Q10 was purchased from Sanofi India Limited and MC-LR was obtained from Sigma-Aldrich Co., USA. ALT and AST kits were purchased from ERBA Diagnostics Pvt Ltd.
Experimental design
BALB/c strain mice of 12-14 weeks’ age weighing 25 to 30 g were used for the experiments. Mice were randomly divided into 3 groups (Normal, MC-LR and MC-LR+Q) with 3 mice in each group. The second group of mice (MC-LR) was treated with MC-LR (10 ?g/kg bw/day, ip) for 14 days and the third group (MC-LR+Q) was co-treated with MC-LR (10 ?g/kg bw/day, ip) and Coenzyme Q10 (10 mg/kg bw/day, im) for 14 days. Simultaneously normal control group mice were also treated with same volume of normal saline. This work was approved by Institutional Animal Ethics Committee (Approval No:379/CPCSEA/IAEC/202l/ll).
Preparation of homogenate
Following the conclusion of the treatment, mice were sacrificed from each group via cervical dislocation, and the intended organs were removed. The tissues were promptly cleaned using ice-cold normal saline and kept at -80°C until they were needed for biochemical analysis. The 10% tissue homogenate was produced by homogenizing it in ice-cold 20 mM Tris buffer (pH 7.4). The supernatant was collected by centrifugation at 20,000 x g for 40 minutes. The concentration of protein was ascertained by employing BSA as the standard [23].
Analysis of aspartate aminotransferase and alanine aminotransferase
In accordance with the International Federation of Clinical Chemistry (1976), the activity of aspartate aminotransferase (AST) was determined using a commercially available reagent from ERBA Diagnostics Mannheim, Germany [24, 25]. The tissue extract was appropriately diluted and then combined with the AST reagent, which consists of L-aspartate, malate dehydrogenase, 2-oxoglutarate, LDH, NADH, ethylenediaminetetraacetic acid, and Tris-buffer at pH 7.5. The mixture was well mixed in a cuvette and incubated at a temperature of 37 °C for a duration of 5 minutes. Using a UV spectrophotometer, the decrease in absorbance was measured at 340 nm against a blank at intervals of 60 seconds for 5 minutes. The activity of AST was represented as IU/L. In addition, the measurement of alanine aminotransferase (ALT) was conducted following the guidelines set by the International Federation of Clinical Chemistry in 1976 [24, 26]. The tissue extract, diluted to the appropriate concentration, was combined with the ALT reagent, which consists of lactate dehydrogenase, nicotinamide adenine dinucleotide (NADH), 2 oxoglutarate, L ?alanine, and Tris-buffer at pH 7.5. The mixture in the cuvette was well mixed and then incubated at a temperature of 37 °C for a duration of 5 minutes. Using a UV spectrophotometer, the absorbance decrease against blank was measured at 340 nm for 5 minutes at intervals of 60 seconds. ALT activity was represented as IU/L.
Statistical analysis
The experimental results were presented as mean ± standard deviation. The student's t test was performed to determine the degree of significance between the control and experimental groups. p-values < 0>
RESULTS
Coenzyme Q10 restored the declined level of AST and ALT in liver, kidney and heart of MC-LR mice
Transaminases are enzymes that participate in the breakdown of amino acids and facilitate the conversion of a pair of amino acids and a pair of ?-keto acids via catalytic processes. Aspartate aminotransferase (AST) serves as an interface between carbohydrate and protein metabolism. Aspartate aminotransferase (AST) is an enzyme present in several tissues of the body, with particularly high levels in liver, heart, and skeletal muscle cells. The level of AST increased considerably in the MC-LR group during toxicity induced by MC-LR, in comparison to the normal control mice (p<0>
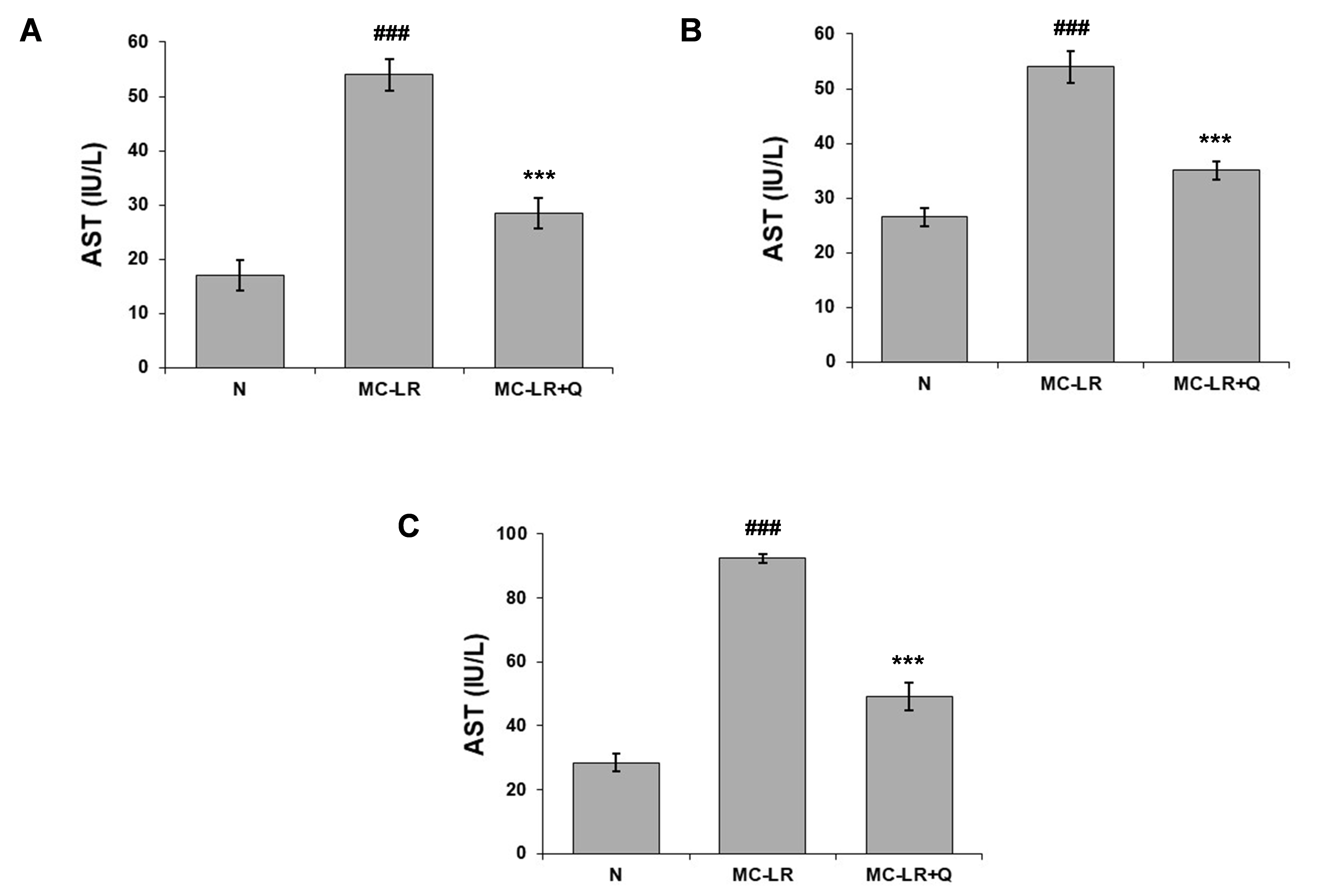
Figure 2. Aspartate aminotransferase level in the (A) liver, (B) kidney and (C) heart of normal (N), MC-LR, MC-LR mice treated with coenzyme Q10 (MC-LR + Q). Values represent mean ± SD, where n = 3. ###p < 0>???p < 0>
Similarly, alanine aminotransferase (ALT) enzyme facilitates the transfer of amino groups from L-alanine to ?-ketoglutarate, resulting in the production of L-glutamate and pyruvate. ALT is widely distributed throughout the human body, being present in several organs such as the kidney, heart, skeletal muscle, brain, pancreas, spleen and lung. The level of ALT increased considerably in the MC-LR group during MC-LR toxicity, in comparison to the normal control mice (p<0>
DISCUSSION
Aminotransferases facilitate the transfer of nitrogen atoms between amino acids and their corresponding oxoacids, which play a role in protein metabolism and gluconeogenesis. The transamination process is the first step for both the breakdown of amino acids and the production of these compounds. An amino group is moved from an acceptor ?-keto acid to a donor amino acid. Reversible transamination reactions take place within hepatic cells and serve as clinical indicators of hepatocyte injury when the liver is exposed to pathological conditions [27]. Amino transferases are enzymes that are reliant on pyridoxal phosphate and are responsible for its catalytic activity in transamination processes. As a result of the ?-amino nitrogen that is produced from the breakdown of amino acids being transported by glutamate, glutamate is an essential source of nitrogen for both biosynthesis and excretion [28].
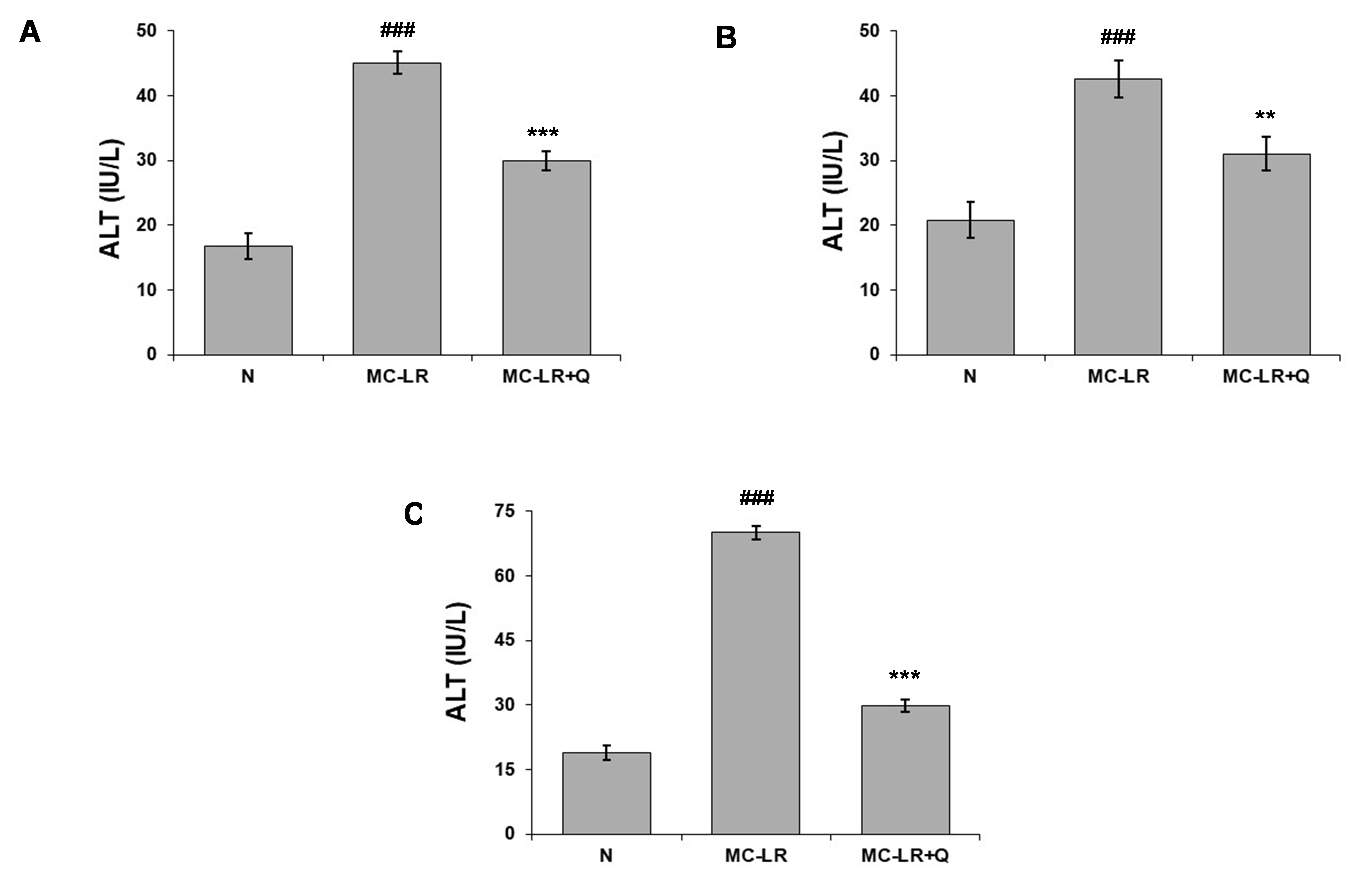
Figure 3. Alanine aminotransferase level in the (A) liver, (B) kidney and (C) heart of normal (N), MC-LR, MC-LR mice treated with coenzyme Q10 (MC-LR + Q). Values represent mean ± SD, where n = 3. ###p < 0>???p < 0> ??p < 0>
A less common kind of oxidative deamination occurs when an amino acid is converted into its equivalent ?-keto acid. The assessment of liver damage is based on the measurement of hepatic transaminase enzyme levels. Enzymes such as glutamate pyruvate transaminase (GPT/ALT), glutamate oxaloacetate transaminase (GOT/AST), lactate dehydrogenase, arginase and ?-glutamyl transaminase discharge out of the cell following damage to liver parenchymal cells or membrane permeability. This leads to an increase in the number of enzymes in the blood compared to normal [25, 29]. Since aminotransaminases have long been used to indicate liver damage and it has also been shown that elevated aminotransferases (ALT and AST) are linked to systemic control of metabolic processes and human illnesses [30]. Coenzyme Q10 supplementation effectively improved liver function, as seen by the reduction in AST and ALT levels [31, 32]. Analysis of the heart and kidney tissue revealed an increase in the levels of ALT and AST enzymes. This elevation can be attributed to the AST/ALT ratio (Figure 4), which serves as a reliable biomarker for non-liver diseases such as cardiovascular disease, different types of cancer and type 2 diabetes mellitus (T2DM) [33]. Modulation of AST/ALT ratio may reflect a markedly impaired kidney and cardiac function, which may be associated with acute kidney injury (AKI) and coronary artery disease (CAD) [34-36]. As seen by our results Coenzyme Q10 quite effectively ameliorated the level of AST and ALT in the kidney and heart of mice.

Figure 4. AST/ALT ratio in the (A) liver, (B) kidney and (C) heart of normal (N), MC-LR, MC-LR mice treated with coenzyme Q10 (MC-LR + Q). Values represent mean ± SD, where n = 3. ##p < 0>#p < 0>???p < 0> ??p < 0>
CONCLUSION
Notably, this is the first study to emphasize the function of coenzyme Q10 in maintaining a balance between the elevated levels of alanine aminotransferase and aspartate aminotransferase observed in MC-LR-induced toxicity in liver, kidney and heart. Since coenzyme Q10 inhibits aspartate aminotransferase and alanine aminotransferase, which are known to be important in mobilizing amino acids for gluconeogenesis and to act as a link between protein and carbohydrate metabolism under altered MC-LR metabolism, these enzymes may be targeted as potential therapeutic targets to treat MC-LR-induced toxicity.
ACKNOWLEDGMENTS
RR expresses gratitude to the Indian Council of Medical Research (ICMR), New Delhi, India for providing the fellowship (45/17/2022/ TOXI/BMS). The funding for this study was provided by a project from DST-SERB (CRG/2018/003780) and DBT-BUILDER (BT/INF/22/SP47619/2022), which was allocated to RKK. The authors express gratitude to the Department of Zoology at Dr. Harisingh Gour Vishwavidyalaya Sagar and the DST-FIST project (SR/FST/ LS-1/2018/176(C)) for providing the necessary infrastructure to the Department of Zoology.
CONFLICTS OF INTEREST
The authors state that they have no competing interest in this article.
REFERENCES
- Cao L, Shao N, Du J, Zhu H, Gao J, Li Q, Sun Y, Hu J, Yin G and Xu G: Involvement of reactive oxygen species (ROS) in the hepatopancreatic cytotoxicity, oxidative stress, and apoptosis induced by microcystin-LR in Eriocheir sinensis. Comparative Biochemistry and Physiology Part C: Toxicology & Pharmacology. 2024; 276:109801.
- Xu S, Yi X, Liu W, Zhang C, Massey IY, Yang F and Tian L: A review of nephrotoxicity of microcystins. Toxins. 2020; 12:693.
- Merel S, Walker D, Chicana R, Snyder S, Baures E and Thomas O: State of knowledge and concerns on cyanobacterial blooms and cyanotoxins. Environment International. 2013; 59:303-327.
- Sharma NK, Tiwari SP, Tripathi K and Rai AK: Sustainability and cyanobacteria (blue-green algae): facts and challenges. Journal of Applied Phycology. 2011; 23:1059-1081.
- Bouhaddada R, Nelieu S, Nasri H, Delarue G and Bouaicha N: High diversity of microcystins in a microcystis bloom from an Algerian lake. Environmental Pollution. 2016; 216:836-844.
- Kaasalainen U, Fewer DP, Jokela J, Wahlsten M, Sivonen K and Rikkinen J: Cyanobacteria produce a high variety of hepatotoxic peptides in lichen symbiosis. Proceedings of the National Academy of Sciences. 2012; 109:5886-5891.
- A de la Cruz A, G Antoniou M, Hiskia A, Pelaez M, Song W, E O'Shea K, He X and Dionysiou D: Can we effectively degrade microcystins?-Implications on human health. Anti-Cancer Agents in Medicinal Chemistry. 2011; 11:19-37.
- Ni W, Zhang J and Luo Y: Microcystin accumulation in bighead carp (Aristichthys nobilis) during a Microcystis-dominated bloom and risk assessment of the dietary intake in a fish pond in China. Environmental Science and Pollution Research. 2017; 24:8894-8902.
- Li T, Fan X, Cai M, Jiang Y, Wang Y, He P, Ni J, Mo A, Peng C and Liu J: Advances in investigating microcystin-induced liver toxicity and underlying mechanisms. Science of The Total Environment. 2023;167167.
- Lone Y, Bhide M and Koiri RK: Amelioratory effect of coenzyme Q10 on potential human carcinogen microcystin-LR induced toxicity in mice. Food and Chemical Toxicology. 2017; 102:176-185.
- Rajpoot R and Koiri RK: Profoundal effects of Microcystin-LR induced cardiotoxicity in mammals. The Ulutas Medical Journal. 2023; 9:92-105.
- Amrani A, Nasri H, Azzouz A, Kadi Y and Bouaicha N: Variation in cyanobacterial hepatotoxin (microcystin) content of water samples and two species of fishes collected from a shallow lake in Algeria. Archives of Environmental Contamination and Toxicology. 2014; 66:379-389.
- Zghair A: Hepatoprotective effect of coenzyme Q10 in rats with diclofenac toxicity. Archives of Razi Institute. 2022; 77:599.
- Molyneux SL, Young JM, Florkowski CM, Lever M and George PM: Coenzyme Q10: is there a clinical role and a case for measurement? The Clinical Biochemist Reviews. 2008; 29:71.
- Quinzii CM, Emmanuele V and Hirano M: Clinical presentations of coenzyme Q10 deficiency syndrome. Molecular Syndromology. 2014; 5:141-146.
- Chang PS, Yen CH, Huang YY, Chiu CJ and Lin PT: Associations between coenzyme Q10 status, oxidative stress, and muscle strength and endurance in patients with osteoarthritis. Antioxidants. 2020; 9:1275.
- Renke G, Pereira MB, Renke A. Coenzyme Q10 for diabetes and cardiovascular disease: useful or useless? Current Diabetes Reviews. 2023; 19:67-72.
- Cahyono SB: Evaluation of increased serum aminotransferase level in asymptomatic patient. The Indonesian Journal of Gastroenterology, Hepatology, and Digestive Endoscopy. 2011; 12:109-115.
- Ndrepepa G: Aspartate aminotransferase and cardiovascular disease-a narrative review. Journal of Laboratory and Precision Medicine. 2021;6.
- Kobayashi A, Suzuki Y and Sugai S: Specificity of transaminase activities in the prediction of drug-induced hepatotoxicity. Journal of Toxicological Sciences. 2020; 45:515-537.
- Kim WR, Flamm SL, Di Bisceglie AM and Bodenheimer HC: Serum activity of alanine aminotransferase (ALT) as an indicator of health and disease. Hepatology. 2008; 47:1363-1370.
- Sookoian S and Pirola CJ: Alanine and aspartate aminotransferase and glutamine-cycling pathway: their roles in pathogenesis of metabolic syndrome. World Journal of Gastroenterology. 2012; 18:3775.
- Lowry O, Rosebrough N, Farr AL and Randall R: Protein measurement with the Folin phenol reagent. Journal of Biological Chemistry. 1951; 193:265-275.
- Yamanaka T, Ido K, Kimura K and Saito T: Serum levels of thyroid hormones in liver diseases. Clinica Chimica Acta. 1980; 101:45-55.
- Rawat D and Koiri RK: Tadalafil inhibits elevated glutamic oxaloacetic transaminase during alcohol aflatoxin induced hepatocellular carcinoma in rats. International Journal of Immunotherapy and Cancer Research. 2020; 6:10-13.
- Naik RA, Rawat D, Ahi JD and Koiri RK: Ameliorative effect of piracetam on emamectin benzoate induced perturbations in the activity of lactate dehydrogenase in murine system. Advances in Redox Research. 2021; 3:100019.
- Ling ZN, Jiang YF, Ru JN, Lu JH, Ding B and Wu J: Amino acid metabolism in health and disease. Signal Transduction and Targeted Therapy. 2023; 8:345.
- Koper K, Han SW, Pastor DC, Yoshikuni Y and Maeda HA: Evolutionary origin and functional diversification of aminotransferases. Journal of Biological Chemistry. 2022; 298:102122.
- Al-Rikabi ZGK, Al-Saffar MA and Abbas AH: The accumulative effect of heavy metals on liver and kidney functions. Medico Legal Update. 2021; 21:1114-1119.
- Sookoian S and Pirola CJ: Liver enzymes, metabolomics and genome-wide association studies: from systems biology to the personalized medicine. World Journal of Gastroenterology. 2015; 21:711.
- Saleh AAS, Shahin MI and Kelada NA: Hepatoprotective effect of taurine and coenzyme Q10 and their combination against acrylamide-induced oxidative stress in rats. Tropical Journal of Pharmaceutical Research. 2017; 16:1849-1855.
- Soleimani Damaneh M, Fatahi S, Aryaeian N and Bavi Behbahani H: The effect of coenzyme Q10 supplementation on liver enzymes: A systematic review and meta-analysis of randomized clinical trials. Food Science & Nutrition 2023; 11:4912-4925.
- Chen W, Wang W, Zhou L, Zhou J, He L, Li J, Xu X, Wang J and Wang L: Elevated AST/ALT ratio is associated with all?cause mortality and cancer incident. Journal of Clinical Laboratory Analysis. 2022;36: e24356.
- Bezan A, Mrsic E, Krieger D, Stojakovic T, Pummer K, Zigeuner R, Hutterer GC and Pichler M: The preoperative AST/ALT (De Ritis) ratio represents a poor prognostic factor in a cohort of patients with nonmetastatic renal cell carcinoma. Journal of Urology. 2015; 194:30-35.
- He HM, He C, Zhang SC, You ZB, Lin XQ, Luo MQ, Lin MQ, Guo YS, Zheng WP and Lin KY: Predictive value of aspartate aminotransferase-to-alanine aminotransferase ratio for contrast-associated acute kidney injury in patients undergoing elective percutaneous coronary intervention. Journal of Cardiology. 2022; 79:618.
- Liu X and Liu P: Elevated AST/ALT ratio is associated with all-cause mortality in patients with stable coronary artery disease: a secondary analysis based on a retrospective cohort study. Scientific Report. 2022; 12:9231


 Dr Raj Kumar Koiri*
Dr Raj Kumar Koiri*
 Roshni Rajpoot
Roshni Rajpoot




 10.5281/zenodo.14867054
10.5281/zenodo.14867054