Abstract
The oral mouth dissolving drug delivery system is regarded as an essential alternative to the peroral route for systemic drug administration since it is considered the most convenient, simple, and safest mode of administration. In terms of flexibility and comfort, mouth dissolving patches may be preferable than mouth dissolving tablets. The goal of this study is to design and characterize a liquorice oral mouth dissolving patch. Oral films made of Xyloglucan, PEG, and glycerine were created by solvent casting. The mechanical characteristics, swelling index, and in vitro drug release were all investigated. For instance, the use of adhesive mucosal preparations has been suggested for the oral delivery of drugs that would prevent swallowing of the dose due to various needs, which include adhesive tablets, adhesive gels, adhesive patches, and various other dosage forms with many combinations of polymers and enhancers of absorption. There is also an increasing interest in natural polymers, and mucoadhesive polymers benefit drug delivery through extended contact time and residence time with mucous membranes.
Keywords
Mouth dissolving patch, Mouth ulcer, Liquorice, Solvent casting method, Xyloglucan.
Introduction
Rapid dissolving medication administration is gaining popularity in the pharmaceutical industry since it is easy to administer and leads to improved patient compliance. These systems melt or dissolve in just a minute and do not require water or chewing. This technique has emerged as a convenient way to provide unit quantities of medication to adult, paediatric, and geriatric patients who may have difficulty swallowing traditional tablets, capsules, liquid orals, or syrup. Tablets, films/strips, and microspheres are examples of rapidly disintegrating dose forms. It's also known as oral wafers or strips. In terms of flexibility and comfort, mouth dissolving film may be preferred over mouth dissolving tablets. MDF strips are generally designed for oral administration, with the user putting the strip on or beneath the tongue (sublingual) or along the inside of the cheek (buccal). These drug delivery systems allow the medicine to evade first-pass metabolism, which improves bioavailability. Liquorice is a hardy plant or shrub that grows to a height of about 2 metres. The roots are long, cylindrical, thick, and have many branches. The plant's root and rhizome are used. Liquorice has a water-soluble, physiologically active component that makes up 40-50 percent of the total dry material weight. This complex includes triterpene saponins, flavonoids, polysaccharides, pectin, simple sugars, amino acids, mineral salts, and other components. Glycyrrhizin, a triterpenoid molecule, is responsible for the sweet taste of liquorice root. Glycyrrhizin acid is a natural saponin made up of a hydrophilic component, two molecules of glucuronic acid, and a hydrophobic fragment, glycyrrhetic acid.
Buccal Drug Delivery System [2,8]
The oral mucosa differs greatly from the mucosa of the rest of the gastrointestinal system and resembles skin more morphologically. While it is often acknowledged that skin permeability is low, less people are aware that the mouth mucosa does not have the same level of permeability as the intestine. The organization of the epithelia, which have somewhat distinct roles, is primarily responsible for these variations within the gastrointestinal system. In the colon, small intestine, and stomach, a straightforward, single-layered epithelium allows for a minimal absorbent transit distance. For many years, medications have been applied topically to the oral mucosa. Nonetheless, there has been a renewed focus on using the mouth as a route for medication delivery to the bloodstream. This mode of delivery has several benefits despite the epithelium's comparatively low permeability features. The ability to escape first-pass metabolism, the convenience of delivery site access, and the possibility of continuous drug delivery, mostly through the buccal tissues, are the most important of these.
ADVANTAGES
The blood supply to the oral mucosa is abundant. Medications enter the bloodstream through the brachiocephalic, internal jugular, and deep lingual or face veins after being absorbed from the mouth cavity through the oral mucosa. Bypassing the first pass effect, the medication enters the systemic circulation directly after buccal delivery.
- It is easier to administer and remove dose forms from because of its extensive vascularization.
- No first-pass hepatic impact.
- The digestive tract has no pre-systemic metabolism.
- Administration ease.
- A region of mostly immobile mucosa and smooth muscle that is appropriate for the delivery of retentive dose forms.
- Prevent the medications from coming into contact with the digestive juices.
- Faster cellular recuperation and localized site development on the buccal mucosa's smooth surface.
- Low enzyme activity, making them suitable for medications and excipients that irritate or harm the mucosa in a moderately reversible manner. The oral mucosa is frequently subjected to a wide variety of foreign substances. It has therefore developed a strong membrane that is less vulnerable to irreparable harm from the medication, dose form, or chemicals employed in it.
- Non-invasive medication delivery system.
- Ease of incorporation of an enzyme inhibitor, pH modifier, or permeability enhancer into the formulation.
DISADVANTAGES
- Small surface area (170 cm2); low permeability of the buccal membrane in particular when compared to the sublingual membrane.
- The oral cavity continuously secretes 0.5–2 L of saliva per day, which dilutes medications at the site of absorption and lowers drug concentrations at the absorbing membrane's surface.
Buccal Dosage Form [17]
- Matrix type: A combination of drug, adhesive, and additives is included in a buccal patch built in a matrix format.
- Reservoir type: The medicine and additives are kept apart from the adhesive in a cavity in the buccal patch that is designed with a reservoir system. In order to avoid drug loss, minimize distortion and disintegration of the patch while in the mouth, and regulate the route of drug distribution, an impermeable backing is placed.
Table No. 1 Instruments.
|
Sr no
|
Instruments
|
Model Names
|
|
1
|
UV spectrophotometer
|
Shimadzu 1900
|
|
2
|
Centrifuge
|
Remi Centrifugation
|
|
3
|
Franz Diffusion
|
Borosil
|
|
4
|
Screw gauge
|
Bexco Micrometer
|
|
5
|
Hot air oven
|
Bio Technics India (Model No. Bti 30)
|
|
6
|
Electronic Balance
|
Wensar Digital Balance
|
|
7
|
Digital pH Meter
|
Equiptronics
|
|
8
|
Magnetic Stirrer
|
Remi 1mlh
|
|
Sr no
|
Materials
|
Manufacturers
|
Uses
|
|
1
|
Ethanol 95%
|
Sar Senapati santaji ghorpade sugar factory and distillery PVT LTD
|
Solvent
|
|
2
|
Liquorice
|
Local Market
|
Drug (API)
|
|
3
|
Citric acid
|
Molychem
|
Preservative, Permeation Enhancer
|
|
4
|
Starch
|
Lobachemie
|
Mechanical strength, Biopolymer
|
|
5
|
Propylene Glycol
|
Lobachemie
|
Plasticizer, Permeation Enhancer
|
|
6
|
Carbopol
|
Lobachemie
|
Additional Polymer
|
|
7
|
Tamarind Kernels
|
Local Market
|
Main Polymer
|
|
8
|
Mannitol
|
Molychem
|
Sweeting agent
|
|
9
|
Glycerine
|
Lobachemie
|
Plasticizer, Self-preservative
|
Collection And Authentication [3]

Fig No. 1 Tamarind Kernels
- The plant species of Tamarindus indica as shown in fig.1 were identified and the seeds of tamarind were collected. Collected seed material were washed and kept for drying in open sunlight for 2 days.
- After drying the seeds, it was grinded in the grinder to make the seeds into the powder form.
- After making it into powder form the xyloglucan mucilage is extracted from seeds and used it for preparation of patches.
METHODS [4,5,6,11]
Extraction of xyloglucan
Step 1
- 20 gm of tamarind kernel powder is added into 200ml of cold distilled water to prepare a slurry.
- Then slurry poured into 800ml of boiled distilled water.
- Solution which is made was boiled for 20 mins in stirring condition.
- Then the clear solution occurred and it was kept for overnight.
Step 2
- Next day the solution is centrifuged at 2000 rpm for 20 mins.
- Supernatant liquid appeared and separated out and collected into a beaker.
- Then this liquid is poured into excess absolute alcohol and stirred for 10 mins.
- Precipitate was formed.
- Then it is washed with 200 ml of absolute ethanol (95%). And then it is dried at 50oC.
- Xyloglucan is extracted.
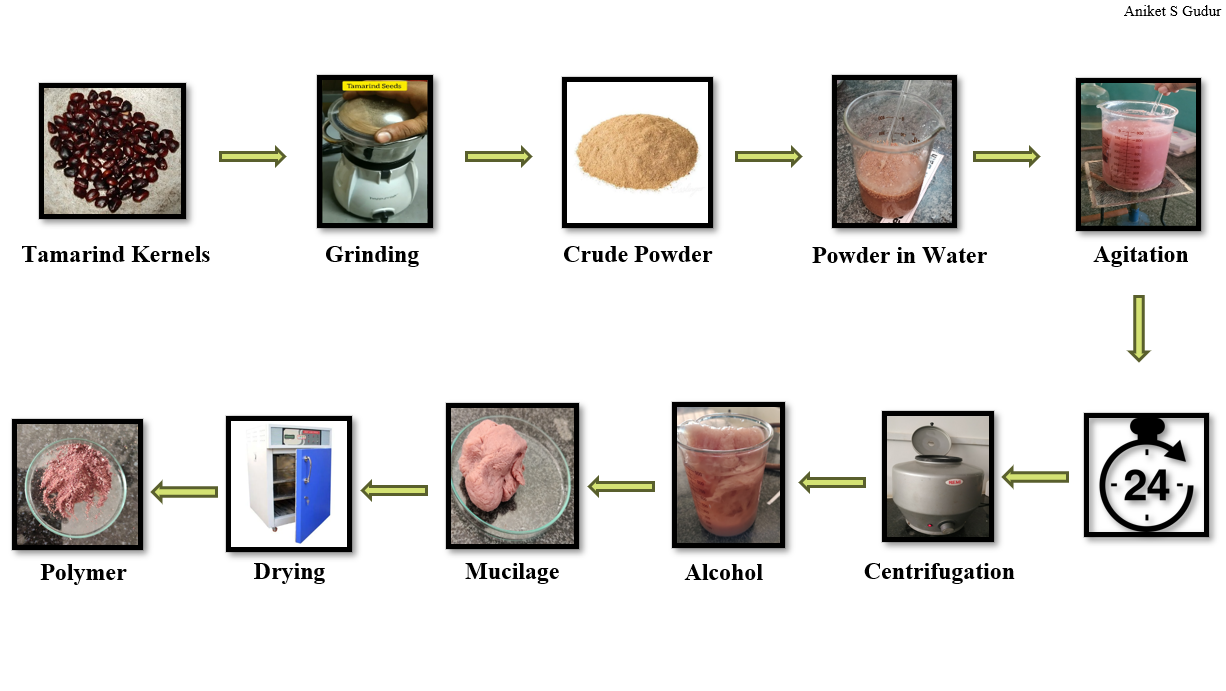
Figure No. 2 Extraction of Xyloglucan
Extraction of liquorice
For extraction two methods were used
1) Maceration
2) Decoction
Maceration:
30 gm of liquorice was weighed
1000 ml distilled water was boiled and kept aside
The liquorice powder was then mixed with warm water and left for 12 hrs undisturbed
After 12 hrs the solution was then filtered and reduced by heating
Decoction:
30 gm of liquorice was weighed and mixed with 1000 ml of distilled water
The solution was boiled for 2hrs
The resultant solution was filtered and then reduced by heating.

Figure No. 3 Extraction of Liquorice
Drug Characterization [10]
- Identification of glycyrrhizin
Glycyrrhizin is basically triterpenoids and saponin glycoside which are conformed by foam test and haemolytic test.
- Solubility Studies
A solubility study was carried out to find out the solubility of drug in different solvents. According to this method, the pure drug was added to the solvent medium and shaken. The saturation was confirmed by observation of presence of un-dissolved material.
- UV-Visible Spectrophotometric Analysis for standard concentration
10mg of glycyrrhizin was dissolved in 10 ml of distilled water and volume was made up to 100ml in a volumetric flask. And then pipette out this solution to prepared the serial dilution, solutions with concentration 10µg/ML, 20µg/ml, 30µg/ml, 40µg/ml and 50µg/ml were prepared was measured on a Shimadzu Double Beam Spectrophotometer at 254nm.
Formulation Design [2,7]
|
Sr no
|
Ingredients
|
F1
|
F2
|
F3
|
F4
|
|
1
|
Liquorice
|
3ml
|
3ml
|
3ml
|
3ml
|
|
2
|
Xyloglucan
|
8ml
|
-
|
10ml
|
10ml
|
|
3
|
Carbopol
|
-
|
8ml
|
-
|
-
|
|
4
|
Glycerine/ PG
|
0.3%w/v
|
0.3%w/v
|
0.5%w/v
|
-
|
|
5
|
Mannitol
|
-
|
-
|
1ml
|
0.5ml
|
|
6
|
Citric Acid
|
-
|
-
|
0.5ml
|
0.5ml
|
|
7
|
Starch
|
-
|
-
|
-
|
1ml
|
|
8
|
Water
|
qs
|
qs
|
qs
|
qs
|
Preparation of Xyloglucan mucilage [12,13]
The xyloglucan mucilage was prepared by adding 2g of xyloglucan extract powder to 100ml of cold water and letting it sit for 3–4 hours to swell. 10 minutes were spent stirring the solution.
Preparation of Buccal Patches [15]
- By using solvent Casting Method
Buccal patch formulation is prepared using the solvent casting method. The oral fast-dissolving patches were made by mixing xyloglucan, propylene glycol/glycerine, and liquorice in varying concentrations. To release all of the trapped air bubbles, xyloglucan mucilage was distributed in distilled water, continuously stirred for up to an hour using a magnetic stirrer, and then left for 30 minutes. This was mixed with propylene glycol, a plasticizer. A liquorice extract solution was made in a different container. After combining the two solutions, the mixture was let to rest for 15 to 30 minutes to allow the foams to subside. This mixture was then stirred for at least fifteen minutes using a magnetic stirrer. The resultant solution was placed in an appropriate petri dish of a hot air oven with the patch former's temperature set between 50° and 55°C. The amount of the solution was cast, which was determined based on the batch size. The patch was removed from the patch former after it had dried. After that, the patch was examined for flaws and trimmed to the 1 x 1 inch size needed for testing. The specimens were preserved in a glass jar covered with aluminium foil, kept at the proper temperature, and kept in a desiccator in anticipation of additional assessment research.
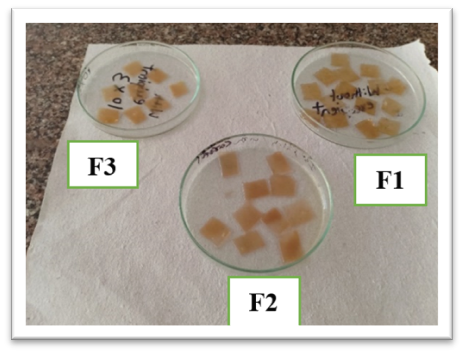
Figure No. 4 Casted Patches
Evaluation [1,16]
1)Visual appearance
On visual appearance it was observed that all the patch formed by using glycerine and xyloglucan was partially transparent in appearance.

Figure No. 5 Visual Appearance Test
Weight Uniformity Test
Ten 1 cm2 patches were weighed individually, and the average of those patches was calculated.

Figure No. 6 Weight Uniformity Test
- Patch Thickness
The thickness of 10 patches was measured using a screw gauge and the average was computed.

Figure No. 7 Thickness Test
- Folding Endurance
One patch was folded continuously at one spot until it broke, or up to 300 manual folds, which was thought to be sufficient to demonstrate good patch attributes, was used to evaluate the folding durability of the patches. The number of times a patch could be folded in the same spot without breaking was used to calculate folding endurance. Five patches were used for this test.
- Surface pH
The buccal mucosa may become irritated by an acidic or alkaline pH, so the surface pH of the patches was assessed to determine the possibility of any negative consequences resulting from a pH shift in vivo. The test patch was put in a petri dish, filled with 0.5 cc of distilled water, and allowed to sit for 30 seconds. After putting the electrode of a pH meter up against the formulation's surface and giving it a minute to acclimate, the pH was determined.
- Swelling Index
The swelling index method was used to ascertain the patch's overall swelling characteristics. An 1x1 cm2 drug-loaded patch was weighed using a cover slip that had been previously pre-weighed. It was kept in a Petri dish and mixed with 50 millilitres of pH 6.8 phosphate buffer. Up to thirty minutes, the cover slip was taken off and weighed every five minutes. The weight difference is the amount of weight gained as a result of patch swelling and water absorption. The following formula was used to calculate the swelling index.

Figure No. 8 Swelling Index Test
- In-vitro Permeation study [14,18]
Utilizing a modified Franz diffusion cell across an egg shell membrane, an in vitro diffusion investigation was conducted. Concentrated HCL is used to assist separate the membrane of the egg shell. First, the egg's contents are taken out, and then it is cleansed using distilled water. In a beaker, add 15 millilitres of pure water to 30 millilitres of strong hydrochloric acid. Let the empty egg shell sit in this mixture for an hour. With the aid of distilled water, remove it using a glass rod and clean the membrane. The membrane is then left in phosphate buffer for 12 hours to activate its pores. PBS, or phosphate buffer solution, with a pH of 6.8 served as the diffusion study's medium. Patches measuring one square centimetre were positioned on the membrane that separated the donor and receptor compartments of the Franz diffusion cell. The membrane of the egg shell was exposed to PBS with a pH of 6.8 that contained a receptor compartment. Using a magnetic bead stirrer set at 50 rpm, the temperature was kept at 37 °C. At predetermined intervals, 1 ml of the sample was removed from the receptor compartment and replaced with brand-new, pH 6.8 PBS. Samples were tested for absorbance using a UV-Visible spectrophotometer set at 254 nm with an appropriate dilution.

Figure No. 9 In-Vitro Permeation Test
RESULTS & DISCUSSION
- Phytochemical Test
Table No. 4
|
Test
|
Observation
|
Inference
|
|
Saponin
|
Foam was produced
|
Saponin Confirmed
|
|
Flavonoids
|
Yellow Colour Produced
|
Flavonoids Confirmed
|
|
Terpenoids
|
Reddish Brown colour produced
|
Terpenoids Confirmed
|

Figure No. 10 Phytochemical Test
- Calibration Curve of Standard Liquorice
Table No. 5
|
Concentration (Microgram)
|
Absorbance
|
|
10
|
0.1407
|
|
20
|
0.1673
|
|
30
|
0.2011
|
|
40
|
0.2403
|
|
50
|
0.2565
|
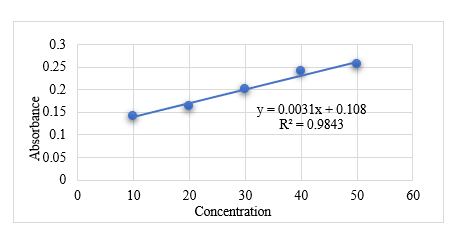
Graph No. 1 Calibration Curve Analysis
Table No. 6 Results of Patch
|
Patches
|
Visual Appearance
|
Weight Uniformity
|
Thickness
|
Folding Endurance
|
Surface pH
|
Swelling Index
|
|
F1
|
Yellow and partially transparent
|
0.104g + 0.05g
|
0.035mm + 0.002mm
|
225 + 35
|
6.82
|
66 + 5
|
|
F2
|
Yellow and partially transparent
|
0.120g + 0.07g
|
0.046mm + 0.015mm
|
230 + 20
|
6.73
|
103 + 2
|
|
F3
|
Yellow and partially transparent
|
0.135g + 0.032g
|
0.038mm + 0.005mm
|
260 + 35
|
6.60
|
- + 7
|
- In – Vitro Permeation Study
Drug + Xyloglucan Patch (F1)
|
Time
|
Absorbance
|
X
|
% Drug Release
|
|
0
|
0
|
0
|
0
|
|
5
|
0.0124
|
8
|
26%
|
|
10
|
0.0189
|
16.1
|
53.6%
|
|
15
|
0.0205
|
18.1
|
60.3%
|
|
20
|
0.021
|
18.75
|
62.3%
|
|
25
|
0.0219
|
19.8
|
66%
|

Graph No. 2 Graph of F1
Table No. 8
|
Time
|
Absorbance
|
X
|
% Drug Release
|
|
0
|
0
|
0
|
0
|
|
5
|
0.0012
|
0.65
|
2.1%
|
|
10
|
0.0016
|
0.75
|
2.5%
|
|
15
|
0.0017
|
0.77
|
2.5%
|
|
20
|
0.0017
|
0.77
|
2.5%
|
|
25
|
0.0124
|
3.4
|
11.3%
|
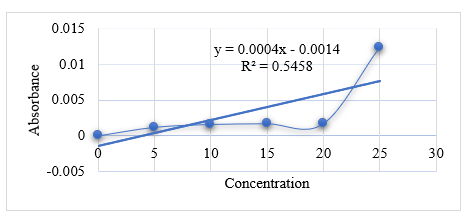
Graph No. 3 Graph of F2
Drug + Xyloglucan + Other Excipients (F3)
Table No. 9
|
Time
|
Absorbance
|
X
|
% Drug Release
|
|
0
|
0
|
0
|
0
|
|
5
|
0.0125
|
9.5
|
31.6%
|
|
10
|
0.0178
|
17.1
|
57.1%
|
|
15
|
0.0189
|
18.1
|
62.3%
|
|
20
|
0.0202
|
20.5
|
68.2%
|
|
25
|
0.0210
|
21.7
|
72.4%
|
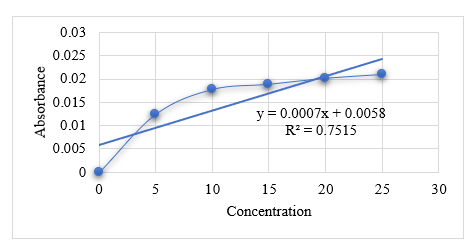
Graph No. 4 Graph of F3
CONCLUSION: The research study on evaluating xyloglucan for exploring its matrix forming potential in buccal patches of Liquorice. The patches were prepared by solvent casting method. Pure liquorice shows a good linearity in phosphate buffer 7.4. Liquorice solubility was determined in various solvent systems. The maximum solubility is obtained in purified water. The folding endurance for all the patches was found to be good. The increase in the polymer content was directly proportional to the increase in folding endurance. The weights varied from 100 to 200 mg. The method used, i.e. solvent casting method, is found satisfactory for the preparation of Liquorice Buccal Patches. However, it was noted that Formulation F3 showed good drug release properties, as it contained xyloglucan as the sole polymer.
REFERENCES
- Parse S, Umekar M. International Journal of Modern Pharmaceutical Research.
- Rao NR, Shravani B, Reddy MS. Overview on buccal drug delivery systems. Journal of pharmaceutical sciences and research. 2013 Apr 1;5(4):80.
- Kumar P, Gupta RK. Formulation and Characterization of Mouth Dissolving Films of Amlodipine using Natural Polymer. Research Journal of Pharmacy and Technology. 2022;15(8):3651-5.
- Kumar R, Govardhan UV. Extraction and Evaluation of Tamarind Kernel Mucilage powder for Hydrocolloidal Properties. International Journal of Advanced Engineering Research and Science.;5(11):268243.
- Mahavarkar RV, Ahirrao S, Kshirsagar S, Rayate V. Formulation and evaluation of tamarind seed polysaccharide matrix tablet. Pharm Biol Eva. 2016 Apr 22;3:241-55.
- Chauhan S, Gulati N, Nagaich U. Glycyrrhizic acid: extraction, screening and evaluation of anti–inflammatory property. Ars Pharmaceutica (Internet). 2018 Jun;59(2):61-7.
- Shojaei AH. Buccal mucosa as a route for systemic drug delivery: a review. J Pharm Pharm Sci. 1998 Jan 1;1(1):15-30.
- Hoogstraate JA, Wertz PW. Drug delivery via the buccal mucosa. Pharmaceutical Science & Technology Today. 1998 Oct 1;1(7):309-16.
- Olukoga A, Donaldson D. Liquorice and its health implications. The journal of the Royal Society for the Promotion of Health. 2000 Jun;120(2):83-9.
- Wahab S, Annadurai S, Abullais SS, Das G, Ahmad W, Ahmad MF, Kandasamy G, Vasudevan R, Ali MS, Amir M. Glycyrrhiza glabra (Licorice): A comprehensive review on its phytochemistry, biological activities, clinical evidence and toxicology. Plants. 2021 Dec 14;10(12):2751.
- Piqué N, Gómez-Guillén MD, Montero MP. Xyloglucan, a plant polymer with barrier protective properties over the mucous membranes: an overview. International Journal of Molecular Sciences. 2018 Feb 27;19(3):673.
- Fry SC. The structure and functions of xyloglucan. Journal of Experimental Botany. 1989 Jan 1;40(1):1-1.
- Mahajan HS, Deshmukh SR. Development and evaluation of gel-forming ocular films based on xyloglucan. Carbohydrate polymers. 2015 May 20;122:243-7.
- Nair AB, Kumria R, Harsha S, Attimarad M, Al-Dhubiab BE, Alhaider IA. In vitro techniques to evaluate buccal films. Journal of Controlled Release. 2013 Feb 28;166(1):10-21.
- Thakur N, Bansal M, Sharma N, Yadav G, Khare P. Overview “a novel approach of fast dissolving films and their patients”. Advances in biological research. 2013 Aug;7(2):50-8.
- Bhyan B, Jangra S, Kaur M, Singh H. Orally fast dissolving films: innovations in formulation and technology. Int J Pharm Sci Rev Res. 2011 Jul;9(2):9-15.
- Dinge A, Nagarsenker M. Formulation and evaluation of fast dissolving films for delivery of triclosan to the oral cavity. Aaps Pharmscitech. 2008 Jun;9:349-56.
- Fonseca-Santos B, Chorilli M. An overview of polymeric dosage forms in buccal drug delivery: State of art, design of formulations and their in vivo performance evaluation. Materials Science and Engineering: C. 2018 May 1;86:129-43


 Aniket Gudur*
Aniket Gudur*
 Rishi Ramesan
Rishi Ramesan














 10.5281/zenodo.14511652
10.5281/zenodo.14511652