Abstract
Computer System Validation (CSV) is a crucial process established in the 1970s to ensure the reliable performance of a computerized system's components, encompassing hardware, software, peripherals, and networks. Advocated by Ted Byers and Bud Loftus, CSV has become indispensable for creating high-quality pharmaceutical products in adherence to global Good Manufacturing Practice (GMP) regulations. Enforced by authorities worldwide, particularly the Food and Drug Administration (FDA), CSV involves gathering and analyzing data to scientifically prove that equipment, utilities, or facilities consistently produce high-quality goods. Systems must comply with predetermined requirements, ensuring predictability and adherence to standards. As a distinctive method in the pharmaceutical sector, CSV maximizes efficiency, saves time, and ensures product quality. Any computerized system crucial to proper operation falls under the purview of computer system validation, evaluated against GMP and GAMP standards. CSV is used by the pharmaceutical industry to guarantee product safety before it is sold or distributed. It addresses complex electronic devices that are important to technical, commercial, and healthcare operations. It is an essential part of the quality management system. As regulatory agencies like the FDA and the European Community Guide to Good Manufacturing Practice for Medicinal Products have noted, the benefits of computer systems in guaranteeing data consistency, dependability, security, and correctness are realized through predictable and repeatable performance.
Keywords
Computer System Validation (CSV), Validation, GMP, GAMP, FDA.
Introduction
To demonstrate that the system's hardware, software, peripherals, and network will consistently and reliably perform their intended activities, be "fit-for-purpose," and comply with all applicable laws and regulations, Computer System Validation (CSV) provides documented proof. Because regulators won't believe you if you tell them the system operates predictably by their criteria, CSV must show that this conclusion is backed up by official, verifiable evidence.
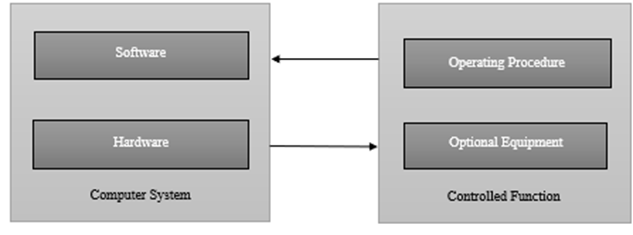
Fig.1 Computerised System
Ted Byers and Bud Loftus first proposed the concept of validation in the mid-1970s as a way to improve the quality of pharmaceutical products. Validation is currently necessary in the pharmaceutical manufacturing industry to produce high-quality pharmaceutical products that follow good manufacturing practice (GMP) guidelines. Global authorities impose validation as an essential requirement to regulate the production of medications and medical supplies. Subpar outputs could be produced by unvalidated equipment, utilities, or infrastructure. Validation is required by the Food and Drug Administration (FDA) and is the process of collecting and evaluating data to produce scientific evidence that a facility, piece of machinery, or service can reliably produce high-quality products. Validation, on the one hand, comprises confirming via inquiry and objective evidence that the specific conditions for a certain intended use are satisfied. Computer system validation is the process of making sure a computer-based system will generate information or data that complies with a set of established requirements. verifying computer programs that help improve system functionality and solve problems in the medical device and pharmaceutical sectors. Computer system evidence is primarily used to ensure that the system performs delicately, consistently, responsibly, and consistently by defined standards. Computer system evidence plays an important part in pharmaceutical sedulity to meliorate product quality, accelerate the performance of processes, and support high-quality products. The major benefit of an evidence-based computer system is that supporting quality controls to ensure that the process is followed correctly, to reduce manual error. The pharmaceutical, cosmetic, food, and beverage industries are among the many sectors that the Food and Drug Administration (FDA) supervises. Data security and product safety are two things that these industries need to ensure. In the US, 21 CFR Part 11, which addresses electronic records and signatures, is the Code of Federal Regulations (CFR) that governs computer system validation. Any computerized system that includes software, hardware, and other components that are crucial to the system's proper operation falls under the umbrella of computer system validation. Computer systems should be evaluated against GMP and GAMP standards and principles since they directly affect the standard of pharmaceutical and medical device products. If there is a system flaw, it could affect data integrity. Computer system verification identifies errors occasionally imperfections and errors (software bugs). The pharmaceutical sector requires computer system validation to ensure that products are safe for sale and distribution. One of these observation criteria is computer system validation, which is also a component of the quality management system used in the production of pharmaceuticals. More and more sophisticated electronic gadgets that regulate or assist technical or business operations are made up mostly of software items. Software now plays a big role in business, science, and technology as well as the healthcare industry, making it a key economic determinant (Schonberger, 2014). Computers are frequently employed in the creation and production of pharmaceuticals and medical equipment. Consistency, dependability, security, and accuracy of the data are largely dependent on the software and computer system's proper operation and performance. By reducing the likelihood of human error and providing reliable and safe functioning, computer system applications are expected to serve the essential criterion of limiting risk to product identification, purity, strength, and effectiveness. The benefits of using computer systems from a regulatory and corporate perspective can only be realized by making sure that each system performs its intended function in a dependable and repeatable manner. The EU's Annex 11 to the GMP guide for medicinal products is inspected and enforced by the Food and Drug Administration (FDA), which also oversees compliance with the GMP regulations found in the U.S. Code of Federal Regulations (CFRs).
Regulatory background
- 21 CFR section 211.68, the current good manufacturing practice for finished medical products, details. “A medicine product may be produced, reused, packaged, and held using automated, mechanical, electronic, or other forms of outfit, including computers, or related systems that will satisfactorily carry out a task. However, it must regularly be calibrated, and examined, if a similar outfit is used in this way. These estimation tests and examinations must be proved in jotting.”
- The definition of medical bias is good manufacturing practice (21 CFR, part 820). “The capability to produce dependable results is a demand for all product and quality assurance dimension outfit, including mechanical, automated, and electronic bias. The computer software programs must be validated by sufficient and proven testing when computers are employed as part of an automated product or quality assurance system.”
- According to 21 CFR part 58, "good laboratory practice" applies to non-clinical laboratory research, “The person in charge of direct data input in an automated data collection system must be linked at the moment of data entry. Any revision to an automatic entry must be made to hide the original entry, state the explanation for the revision, be dated, and name the person who made the change.”
Need of validation:
Many facets of a pharmaceutical company's operations, including research and development, laboratory testing and analysis, product inspection and acceptance, production and process control, environmental controls, packaging, labeling, traceability, document control, complaint management, and many more, mainly depend on computers and automated machinery. Embedded systems, such as robotics, statistical process control, programmable logic, and digital function controllers, may be heavily utilized in automated plant floor activities.
Processes Needed for CSV
Qualification Conditioning should be described in a master plan. The best instrument for internalizing this strategy is the qualification master plan. However, also a design plan is the prosecution stage If a master plan defines the figure. Depending on the project's complexity, CSV can be broadly divided into the following procedures:

Fig.2 Master plan and project plan relationship
Master plan:
This verifies that the specs adhere to user needs. Teams that will oversee the entire process are also formed during this phase. Also established is the list of tasks that must be completed during validation. Essentially, this is the process of creating the CSV's full blueprint. This procedure serves as the center of a validation program since it validates all aspects of the setup, including the physical hardware, software, and sites as well as procedures like risk mitigation and redundancy techniques.
Project plan:
The actions needed to bring a certain system into compliance are outlined in the project plan. It gives inspectors a first sense of the degree of control a laboratory has over a certain instrument or system and an initial evaluation of the qualification quality. This process, which is a subset of the master validation plan, outlines the SOPs for every process in a program for validation evaluation. What's more, it gives an end date for the CSV. Standard operating procedures (SOPs) are meticulously written and thoroughly trained on throughout this process.
Model of Life Cycle:
Software development is done using an SDLC, or life-cycle development paradigm. Every phase of the software development life cycle has a corresponding computer validation stage. This model may therefore be used to illustrate each stage of the computer system validation process. The development process can be effectively documented as a result. In brief, Augsburg et al. (1994) describe the model.
Steps of the life cycle model:
Coding: Starting this step with the validation plan completed is a good idea. Installation procedures, the validation test environment, and the system's assumptions, exclusions, and restrictions should all be understood well. In this stage, both the software and hardware development are addressed.
Testing:
This intricate stage consists of several phases. It is necessary to test every module, whether it is software or hardware. The next step is to conduct an integration test. Although the developers perform unit, system, and integration testing, the Validation Committee can specify the test cases and data utilized in the acceptance test. Functional specifications, system design documents, and requirement documents should all be available for review and revision.
Installation Testing:
With his or her information and methods, the end user completes the system's task. Failure of the system necessitates new documentation and redesign.
Production Maintenance:
Production on the program is approved. Software and hardware changes to the system need to be monitored and controlled carefully. The design qualification that was previously described can be linked to steps one and two of the previously mentioned life-cycle model; installation qualification will validate stages three and four; and the performance job will be linked to step five.
V-model:
Boehm (1979) presented this V-model in Figure. All levels of the V-model have semantic equivalents to life-cycle model steps. Even though the V-model overlooks several important steps, such as vendor evaluation, it is still very helpful if software development is involved in the validation process. It looks fairly complicated as well, for a truly commercial off-the-shelf solution that doesn't require bespoke development. The design specification, code development, and code testing steps are not required. This methodology includes user requirement specification (URS), design qualification (DQ), installation qualification (IQ), operational qualification (OQ), and performance qualification (PQ).
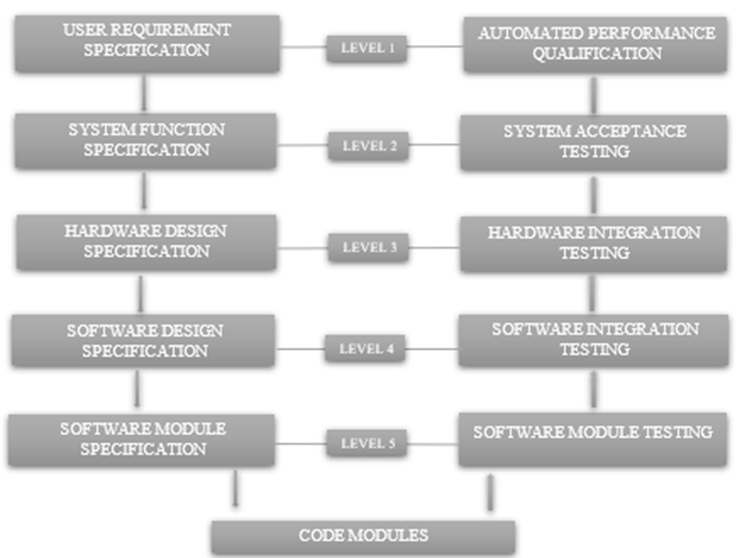
Fig.3 V-model
CONCLUSION
By implementing various measures to mitigate or eliminate the inadequacies associated with the computer system validation challenges outlined above, pharmaceutical businesses can enhance their validation initiatives. Validation activities may benefit greatly from cooperation, priority, planning, monitoring, and a distinct sense of purpose. It is important to research the existing validation frameworks to find any potential advantages that might help to avoid most of the problems mentioned in this paper. The computer system needs to be validated at the time of installation. A qualified individual with thorough knowledge of the system and the project in question must conduct a validation before beginning any project or making changes to the computer system. A knowledgeable individual with complete information is required for efficient validation. In addition to improving GMP compliance and raising 21 CFR part 11 compliance—which affects the quality, safety, identity, or efficacy of products subject to Gape standards—good computer system validations also raise quality assurance and save time and money for subsequent validations. Validation of computer software is required under FDA regulations, and these regulations have legal consequences. Failure in an FDA audit may lead to inspectional observations and warning letters from the FDA. In addition, consent judgments, heavy financial penalties, and the closure of industrial facilities might arise from not taking corrective measures at a given moment. The certification of computer software is therefore a necessary consideration for pharmaceutical companies and laboratories.
REFERENCES
- Singh, Asheesh, Pradeep Singour, and Parul Singh. "Computer system validation in the perspective of the pharmaceutical industry." *Journal of Drug Delivery and Therapeutics* 8, no. 6-s (2018): 359-365.
- Ragini Dilip Patil et al. *Ijppr Human* 23, no. 4 (2022): 329-336.
- Patil, Yogesh, Mali Kamlesh, Bodhane Mohini, Ram Phad, Shaikh Ismail, and Lale Shivam. "Computer system validation." *Volume 4, Issue 09* (2015).
- Hoffmann, Andreas, et al. "Computer system validation: An overview of official requirements and standards." *Pharmaceutica Acta Helvetiae* 72, no. 6 (1998): 317-325.
- Hoffmann, Andreas, Jacqueline IGihny-Simonius, Marcel Plattner, Vanja SchmidliVckovski, and Christian Kronseder. "Computer system validation: An overview of official requirements and standards." *Pharmaceutica Acta Helvetiae* 72 (1998): 317-325.
- EC. *Guide to Good Manufacturing Practice (GMP).* In: The Rules Governing Medicinal Products in the European Community, vol. 4. Amended, Good Manufacturing Practice for Medicinal Products in the European Community. January, pp. 13-165, 1989.
- CFR. *21 CFR, Part 211: Current Good Manufacturing Practice for Finished Pharmaceuticals, sections 21 I.22: Proposed Rules.* Vol. 61 Federal Register, pp., 1996: 20104-20115.
- Strause, Sharon. "Computer System Validation—Definition and Requirements." *Journal of Validation Technology* Spring 2009, pp. 1-5.
- Nash, R. A., and A. H. Wachter. *Pharmaceutical Process Validation, An International 3rd Edition, Revised and Expanded.* Marcel Dekker, New York, March 2003: 31-57.
- International Conference on Harmonization (ICH) of Technical Requirements for the Registration of Pharmaceuticals for Human Use. *Validation of Analytical Procedures: Definitions and Terminology.* Geneva, 1996: 748-832.
- U.S. FDA. *Guidance for Industry (Draft) Analytical Procedures and Methods Validation: Chemistry, Manufacturing, and Controls and Documentation.* 2000: 223-243.
- General Principles of Validation. Food and Drug Administration, Drug Evaluation and Research; Rockville, Maryland, 1987: 345-378.
- IEEE Standard of Software Verification and Validation. Institute of Electrical and Electronics Engineers (IEEE), New York, 1998: 1034-1065.
- Ali, Jawed, Alka Abuja, and R. K. Char. *A Textbook of DOSAGE FORM DESIGN.* Birla Publication Pvt. Ltd., 4th edition, 2008-2009: 52-83.
- US FDA. *Guideline on General Principles of Process Validation.* 1987: 52-67.
- US Food & Drug Administration. *General Principles of Software Validation; Final Guidance for Industry and FDA Staff.* January 2002.
- GAMP Forum. *GAMP Guide, A Risk-Based Approach to Complaint GxP Computerized Systems.* Ver. 5.0.
- *ComputerValidation.com Web site.* http://www.computervalidation.com.
- Agalloco, JP, and FJ Carleton. *Validation of Pharmaceutical Processes.* 3rd Edn; Informa Healthcare USA Inc, New York, 2008: 1-4.
- Huber, L. *Validation and Qualification in Analytical Laboratories.* 2nd Edn; Informa Healthcare USA Inc, New York, 2007: 25-3.
- EMEA Guideline. "Note for the Guideline on Process Validation." European Agency for the Evaluation of Medicinal Products, London; CPMP/QWP/848/96, EMEA/CVMP/598/99, 2001.
- ICH. *Validation of Analytical Procedures; Methodology, Q7 A, “Process Validation” Good Manufacturing Practice Guide for Active Pharmaceutical Ingredients.* International Conference on Harmonization, IFPMA, Geneva.
- *Supplementary Guidelines on Good Manufacturing Practices Validation.* Annex 4.
- Chao, AY, FJ Forbes, RF Johnson, and PV Doehren. *Pharmaceutical Process Validation.* 3rd Edn; Marcel Dekker, Inc, New York, 2003: 7-9.
- Trubinski, CJ. *Pharmaceutical Process Validation.* 3rd Edn; Marcel Dekker Inc, New York, 2003: 31-35.


 Meenal Katre*
Meenal Katre*
 Diksha Ghyar
Diksha Ghyar
 Saurabh Raghute
Saurabh Raghute
 Chandak Kamala
Chandak Kamala



 10.5281/zenodo.12739502
10.5281/zenodo.12739502