Abstract
Lamotrigine is a versatile antiepileptic medication first approved in 1994. It was initially authorized for use as an additional treatment for partial seizures in adults and for generalized seizures associated with Lennox-Gastaut syndrome in both children (over 2 years old) and adults. It has beneficial pharmacokinetic characteristics and is usually well tolerated. Beyond its primary uses, Lamotrigine has shown promise in treating additional Neurological conditions, such as Migraines and Neuropathic pain, with preliminary research yielding positive results. Lamotrigine’s efficacy in managing Bipolar Disorder Type I is also well-documented and widely recognized. Lamotrigine has proven to be particularly effective in treating Epilepsy in women and older adults, offering a valuable treatment option for these groups. A significant proportion of patients (63.5%) achieved seizure control with lamotrigine monotherapy, with female patients showing a slightly higher response rate (64%) than their male counterparts (61.6%). Lamotrigine stands out as a preferred treatment option for elderly patients due to its favourable tolerability and minimal risk of drug interactions, providing a safer alternative to other antiepileptic drugs. Healthcare providers should carefully evaluate the risk of adverse reactions and drug interactions when prescribing lamotrigine, particularly in combination with other medications like antiepileptic drugs, psychiatric medications, oestrogens, and alternative therapies.
Keywords
Lamotrigine, Epilepsy, Neurological Disorders, special populations, safety and efficiency, tolerability, adverse drug reactions, drug interactions.
Introduction
Lamotrigine, marketed as Lamictal by GlaxoSmithKline, is a mild inhibitor of dihydrofolate reductase. Its development was initially driven by the hypothesis that folate played a role in triggering Epileptic activity, suggesting that modulating folate levels could help control Seizures. (1) In the field of Psychiatry, Lamotrigine is FDA-approved as a maintenance treatment for bipolar disorder, with a focus on preventing Depressive episodes. (2) Lamotrigine was initially approved in Ireland (1990) and the UK (1991), followed by US FDA approval in 1994. Its indications include Adjunctive therapy for partial seizures, primary generalized tonic-clonic seizures, and generalized seizures associated with Lennox-Gastaut syndrome in adults and children over 2 years old and above, Monotherapy conversion in patients aged 16 years and above with partial seizures, Maintenance treatment of bipolar I disorder in adults aged 18 years and above.(3) Lamotrigine has been used off-label for various conditions, including Rapid-cycling bipolar depression management, Maintenance treatment of bipolar disorder type I, Preventing basilar migraines with aura, Treating panic disorder, Managing binge eating disorder.(4,5,6) Several older antiepileptic drugs (AEDs), including Carbamazepine, Phenytoin, and Phenobarbital, have been found to possess antifolate properties. However, no conclusive evidence suggests a direct link between antifolate activity and antiepileptic efficacy.(7) Lamotrigine is indicated in 55 countries to maintain bipolar disorder treatment, delaying the onset of mood episodes (Depression, Mania, Hypomania, mixed) in patients stabilized with standard therapy.(8)

Figur 1: Structure of Lamotrigine
Effect of Lamotrigine on epilepsy
Epilepsy, a Neurological Disorder, has a prevalence of around 1% worldwide.9 A comparative analysis reveals that epilepsy prevalence in 23 Asian countries parallels that of the United States and Europe. The mainstay of Epilepsy treatment involves pharmacotherapy with antiepileptic drugs, which modulate neuronal activity by concomitantly decreasing excitatory and inhibitory neurotransmission, thereby reducing seizure frequency. (10) Recent years have seen a surge in innovative epilepsy treatments, including introduction of multiple new pharmacological entities. Lamotrigine, a well-established antiepileptic drug with over two decades of clinical experience, exemplifies this progress. (11) The past decade has witnessed substantial progress in epilepsy pharmacotherapy. Lamotrigine, an antiepileptic drug with an extensive clinical history (over 20 years), exhibits a broad spectrum of efficacy Partial-onset seizures, secondarily generalized tonic-clonic seizures, Primary generalized seizures (absence, tonic-clonic), Atypical absence seizures, Tonic/atonic seizures, Lennox-Gastaut syndrome. (12) Lamotrigine has various side effects, with allergic reactions being the most significant concern. The gradual introduction helps minimize these reactions. While skin reactions are common, severe cases (like erythema multiform, Stevens-Johnson syndrome, and toxic epidermal necrolysis) are rare. The pharmacodynamic properties of lamotrigine confer a significant advantage: reduced cognitive impairment and diminished sedative effects, relative to comparable therapeutic agents. (11) A recent investigation revealed lamotrigine’s potent anti-aging properties in an animal model, characterized by diminished mortality rates and enhanced lifespan, underscoring its potential therapeutic utility. This discussion assessed the efficacy of lamotrigine in managing Epilepsy. All patients presenting to our clinic with Epilepsy were enrolled. Treatment was initiated with low-dose Lamotrigine (25-50 mg/day), gradually increasing until Seizure freedom or adverse effects occurred. Initially, once-daily administration was used; for doses exceeding 150 mg/day, twice-daily administration was employed. Patients achieving ?6 months of Seizure freedom were included in the control group. Those experiencing intolerable side effects discontinued treatment. Patients with comorbid non-neurological conditions were excluded. Pre-treatment evaluations included laboratory tests (CBC, blood sugar, urea, calcium) and brain MRI/EEG. Patients with abnormal lab results or normal EEGs were excluded. Follow-up MRI/EEG assessments occurred every 3-6 months. The efficacy of lamotrigine, administered as monotherapy or combination therapy, was evaluated across various epilepsy subtypes. Notable response rates were observed Primary generalized tonic-clonic epilepsy: ?88%, Secondary generalized tonic-clonic epilepsy: 71%, Complex partial epilepsy: 72%, Juvenile myoclonic epilepsy: 81.5%, Familial epilepsy: 96.7%, Epilepsy with known focal lesions: 94% Discussion: Efficacy of Lamotrigine in Epileptic Patients. A total of 905 epileptic patients (502 males, 403 females) received lamotrigine treatment. Of these, 505 patients (282 males, 223 females) completed the study, with treatment duration exceeding 6 months (mostly over 2 years). Monotherapy seizure control: 63.5% (61.6% males, 64?males), Females showed significantly better seizure control than males Combined Therapy with Sodium Valproate, 107 patients required combination therapy, Overall seizure control rate: 60% (45% males, 77.5?males). Efficacy of Lamotrigine in Lamotrigine monotherapy achieved seizure control in 63.5% of patients (61.6% males, 64?males), with significantly better outcomes observed in females. Combination therapy with sodium valproate was required for 107 patients, yielding an overall seizure control rate of 60% (45% males, 77.5?males). Lamotrigine demonstrated effective seizure control in epileptic patients, particularly in females. Combination therapy with sodium valproate showed promising results, especially in female patients.(13)
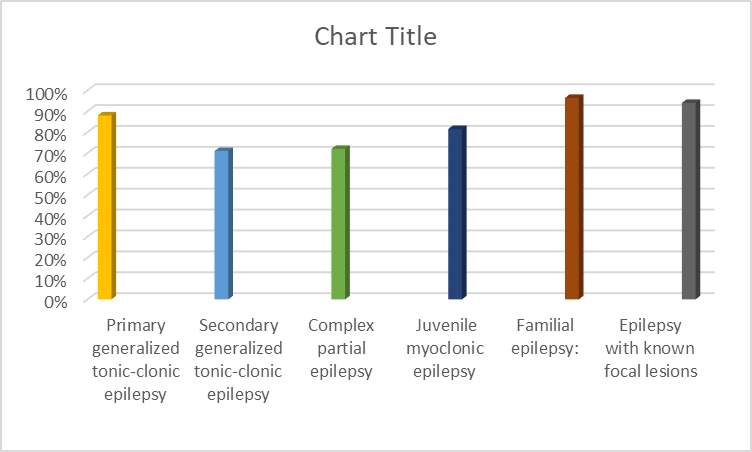
Figure 2: Graphical representation of efficacy of Lamotrigine in epileptic patients (13)
Use of lamotrigine in other psychiatric diseases
Lamotrigine in mood disorders:
Lamotrigine (LAM) is FDA-approved for preventing depressive episodes in bipolar disorder. This indication is supported by two identical 18-month randomized controlled trials (RCTs) involving 638 participants who responded well to initial LAM treatment. A pooled analysis revealed that both LAM and lithium (Li) significantly outperformed placebo in delaying the onset of mood episodes. (14) LAM monotherapy improves clinical response rates in acute bipolar depression. Meta-analysis supports its efficacy, Widespread clinical use for this indication, First-line treatment option, Significant enhancement in clinical outcomes. (8) Lamotrigine (LAM) demonstrated significant superiority over placebo on the Clinical Global Impressions (CGI) scale at the endpoint, with notable efficacy observed in both unipolar Major Depressive Disorder (MDD) and bipolar II disorder. (15) LAM is not superior to placebo in acute mania. Multiple-treatment meta-analysis, Consistent with expectations, Limited efficacy in acute mania, No significant benefit over placebo.(16) Multiple-treatment meta-analysis found no statistically significant difference between LAM and placebo in acute mania efficacy. (17)
Lamotrigine in schizophrenia:
A systematic review and meta-analysis of five randomized controlled trials examined the efficacy of adjunctive lamotrigine (LAM) in treatment-resistant schizophrenia (TRS). The results demonstrated statistically significant reductions in positive, negative, and general psychopathology subscale scores, as assessed by the Positive and Negative Syndrome Scale (PANSS), supporting the therapeutic potential of adjunctive LAM in TRS.(18) A statistically significant difference was observed in the incidence of nausea, favoring the placebo group (p < 0>(19) Lamotrigine (LAM) has demonstrated neuroprotective effects in chronic schizophrenia, slowing functional decline and reducing cognitive and functional impairment progression. These findings support LAM’s potential as a therapeutic option for managing chronic schizophrenia. (20)
Lamotrigine in obsessive-compulsive disorder:
Obsessive-compulsive disorder (OCD) is characterized by abnormally high glutamatergic levels in the caudate nucleus, as evidenced by genetic, behavioral, and neuroimaging studies. (21)The combination of LAM and SRI may attenuate glutamatergic transmission through serotonin-mediated inhibition of corticostriatal glutamate release.(22)This randomized controlled trial evaluated LAM augmentation (up to 100 mg/d) in patients receiving SSRIs, revealing substantial benefits. LAM significantly improved obsessive-compulsive and affective symptoms, with modest cognitive gains in semantic fluency. These findings support LAM augmentation as a well-tolerated, effective strategy for treatment-resistant OCD. (23)
Lamotrigine in posttraumatic stress disorder:
A pilot randomized controlled trial (RCT) of 15 PTSD patients suggested the potential benefits of adjunctive LAM therapy when added to antidepressant treatment. (24)
Lamotrigine in borderline personality disorder:
Our RCT findings suggest that LAM is effective in mitigating impulsivity and anger in female borderline personality disorder patients, with a favorable tolerability profile and no significant impact on body weight.(25) Researchers found LAM effective and safe for managing aggression in women with borderline personality disorder over the long term.(26)
Lamotrigine in depersonalization:
Depersonalization is a complex phenomenon that manifests across a range of neurologic and psychiatric disorders, with depersonalization-derealization disorder (DPRD) being a distinct primary condition. (27) Our double-blind RCT demonstrated LAM’s efficacy over placebo in treating depersonalization disorder without psychiatric comorbidity, with a statistically significant response rate (50% reduction on Camden Depersonalization Scale) observed in the LAM group. (28)
Lamotrigine in autism and intellectual disabilities:
Our study found LAM to be effective in achieving seizure control in children and adolescents with intractable epilepsy, with a significant reduction in autistic symptoms observed in 61.5% of ASD patients. (29) A randomized controlled trial (RCT) investigating LAM’s efficacy in autism spectrum disorder (ASD) revealed no significant differences between LAM and placebo in reducing irritability, hyperactivity, attention deficits, or enhancing adaptive outcomes. (30)
Lamotrigine in traumatic brain injury:
This preliminary research suggests LAM may have therapeutic potential in mitigating problem behaviors and enhancing neurobehavioral recovery post-TBI. (31)
Lamotrigine in dementia:
The use of atypical antipsychotics to alleviate behavioral and psychological symptoms of dementia persists, notwithstanding FDA warnings highlighting elevated mortality risks in geriatric patients.(32) In a 20-patient case series, LAM demonstrated acceptable tolerability and modest clinical improvement in 90% (18/20) of patients, primarily nursing home residents with complex psychiatric and medical profiles.(33)
Lamotrigine in elderly patients
Psychiatric and behavioral disturbances are common and refractory to treatment among geriatric nursing home populations. (34) Few studies have explored the effectiveness of lamotrigine in elderly patients with behavioral disturbances and psychiatric conditions. Although research on lamotrigine’s efficacy and safety in geriatric mental health is scarce, our clinical observations suggest its potential utility in managing challenging behavioral and psychiatric presentations. (35) Research evidence supports the effectiveness of LTG as a treatment option for patients with bipolar spectrum disorders. (36) Research shows LTG helps manage epilepsy in elderly patients. (37) Lamotrigine demonstrated efficacy in managing the patient’s aggressive behavioral symptoms, including verbal and physical outbursts.38 A retrospective chart review of 20 nursing home patients examined the tolerability and efficacy of lamotrigine in managing agitated and aggressive behaviors across various psychiatric and medical diagnoses, yielding favorable outcomes. (34)
Safety of lamotrigine in elderly patients
Treating epilepsy in the elderly is complicated by comorbidities and polypharmacy, increasing the risk of drug interactions and adverse effects that may lead to treatment discontinuation.(39)Brodie et al.’s study of 150 elderly individuals with epilepsy revealed that carbamazepine was associated with a substantially higher incidence of adverse drug reactions (42%) compared to lamotrigine (18%). Notably, lamotrigine demonstrated superior efficacy, with 39% of patients remaining seizure-free at 16 weeks, compared to 21% of those receiving carbamazepine.(40) A 12-month study of 593 elderly patients found withdrawal rates due to ADRs: lamotrigine (44%), gabapentin (51%), and carbamazepine (64%).(41) Research by Maggs et al. demonstrated in vivo that lamotrigine is susceptible to oxidative biotransformation in the absence of saturation or blockade of competing metabolic pathways.(42) This study demonstrated superior treatment retention with lamotrigine versus carbamazepine (71% vs 42%; p < 0>(40)
Efficacy of lamotrigine in elderly
Lamotrigine is a novel antiepileptic medication that demonstrates efficacy as monotherapy or adjunctive treatment, exerting its effects by modulating sodium channels, stabilizing neuronal membranes, and inhibiting excitatory amino acid release.(43,44) Studies in adults have found that lamotrigine is effective and has a good safety profile, with few patients experiencing serious side effects.(45,46)Our research aimed to investigate the tolerability of lamotrigine in elderly patients (>65 years) by leveraging a comprehensive global epilepsy database and a large-scale comparative clinical trial.(47)The relatively straightforward administration of lamotrigine in elderly patients, combined with its favorable tolerability profile, suggests that treatment responses in this age group are comparable to those in younger adults, facilitating the development of effective treatment strategies.(48)
Lamotrigine in pediatric patients
Epilepsy affects about 1 in 100 children, making effective treatment essential to support their growth and development.(49) Managing childhood epilepsy poses significant challenges due to the complexity of multiple seizure types, co-existing cognitive or behavioral issues, and distinct pharmacokinetic and pharmacodynamic profiles compared to adults.(50) Globally, lamotrigine has been approved for epilepsy management in 93 countries, including over 70 countries that have cleared its use in pediatric populations, backed by rigorous clinical trial results.51 Emerging evidence suggests that lamotrigine, a medication with a long history of safe and effective use in adults, is also a valuable treatment option for controlling seizures in children with various types of epilepsy.(50, 52)
Safety of lamotrigine in pediatric patients
Studies and clinical experience have shown that lamotrigine has a comparable adverse event profile in pediatric patients as in adults, highlighting its safety and tolerability in this younger population. Lamotrigine demonstrates a favorable safety profile with respect to neurologic adverse events, including asthenia, dizziness, and somnolence, which are common with older medications like carbamazepine and phenytoin, and it may even have a beneficial effect on cognitive function.53 Similar to other antiepileptic drugs, lamotrigine has been associated with a low incidence of severe rash, and adhering to recommended dosing guidelines can help minimize this risk.(53, 54)
Efficacy of lamotrigine in pediatric patients
Pediatric clinical trials have established lamotrigine’s effectiveness in managing generalized seizures associated with Lennox-Gastaut syndrome, partial seizures, and absence seizures, both as an adjunctive and monotherapy treatment option. (55,56,57) In addition to the rigorous clinical trials, numerous open-label studies and case reports provide further evidence of lamotrigine’s effectiveness in managing various seizure types. Results from rigorous clinical trials and open-label studies show that lamotrigine is effective in treating a wide range of seizure disorders and syndromes in children, both as monotherapy and adjunctive therapy. Lamotrigine’s efficacy in pediatric patients with partial and generalized seizures is similar to that seen in adults, and its therapeutic benefits extend beyond seizure control, with promising psychotropic and behavioral effects that deserve further exploration in prospective research.(51)

Figure 3: Graphical representation of Lamotrigine for the treatment of epilepsy in childhood
Lamotrigine in pregnant women
Research has revealed that infants of mothers with epilepsy are more likely to have birth defects, with a risk that is 2-3 times higher than that of the general population, according to multiple prospective and retrospective studies.(58,59,60,61,62,63,64) The emergence of new antiepileptic drugs (AEDs) over the past decade, including oxcarbazepine, vigabatrin, lamotrigine, gabapentin, tiagabine, zonisamide, topiramate, and levetiracetam, has added complexity to treatment decisions for women of childbearing age, as the teratogenic risks associated with these medications are not yet fully understood. Lamotrigine and oxcarbazepine have gained popularity as initial therapies of choice for a large proportion of epilepsy patients in Denmark. (65)
Safety of lamotrigine in pregnant women
As of now, lamotrigine has not been associated with any pregnancy-related adverse events, and it is classified as a category C medication in pregnancy; however, several pregnancy registries are collecting and analyzing data on the potential effects of lamotrigine exposure during pregnancy. According to the registry data, the incidence of major birth defects following first-trimester exposure to lamotrigine ranged from approximately 1% to 5.6%, which is comparable to the 2-3% rate observed in the general population.(69) .The majority of the reported data indicated that the rates of major malformations were comparable to the expected baseline rates, but the limited sample size precluded any definitive conclusions about specific patterns of abnormalities.(70) Among the patients enrolled in the controlled lamotrigine trials for bipolar disorder, 7 became pregnant, and 2 of these patients were receiving lamotrigine. Of these 2 pregnancies, one resulted in a normal delivery, while the other was terminated by choice.(71)
Efficacy of lamotrigine in pregnant women
The limitations of this pilot study include not only its small sample size but also the lack of randomization, inconsistent LTG dosing, and uncontrolled discontinuation of mood stabilizers, which may impact the validity of the findings. Notably, the findings reveal a strong connection between sustained lamotrigine treatment and a lower incidence of bipolar disorder recurrences, which occur later in pregnancy, suggesting a potential therapeutic benefit.(66,67) Considering the high risk of depressive relapses in bipolar disorder patients early in pregnancy when mood stabilizers are discontinued, and the significant rate of unplanned pregnancies, lamotrigine appears to be a viable treatment option for women with bipolar disorder during their childbearing years, particularly if its potential for lower teratogenic risks compared to other mood-stabilizing anticonvulsants is confirmed, and its risk profile relative to lithium and modern antipsychotics is better understood.(68)
Pharmacokinetics and pharmacodynamics of lamotrigine Pharmacokinetics: The pharmacokinetic profile of lamotrigine was assessed in 12 elderly individuals (65-76 years old) after a single dose of 150 mg. The mean lamotrigine half-life was found to be 31.2 hours, with a range of 24.5-43.4 hours, and the mean clearance was 0.40 ml/min/kg, ranging from 0.26 to 0.48 ml/min/kg. Population pharmacokinetic analyses in pediatric patients (2-18 years old) revealed that lamotrigine clearance is primarily affected by total body weight and concurrent antiepileptic drug (AED) therapy. Notably, children exhibited higher oral clearance rates of lamotrigine on a body weight basis compared to adults. Furthermore, weight-normalized clearance was greater in children weighing less than 30 kg versus those weighing more than 30 kg, and age did not significantly impact clearance after adjusting for body weight.(72) Research on 11 pregnant women who were taking lamotrigine as a single medication showed that the ratio of plasma lamotrigine levels to the prescribed dose decreased by 65% during the second trimester and 66% during the third trimester, compared to levels before pregnancy.(73) Researchers examined the pharmacokinetics of lamotrigine in 9 pregnant women and their 10 babies during three critical periods: childbirth, the newborn period, and while breastfeeding. Results showed that lamotrigine levels in maternal plasma and umbilical cord plasma were similar at delivery. By 72 hours postpartum, infant plasma concentrations had decreased to 75% of cord levels. The median milk-to-plasma concentration ratio 2-3 weeks after delivery was 0.61, confirming that lamotrigine is transferred across the placenta and excreted into breast milk. (74)
Pharmacodynamics: Although the precise mechanism of lamotrigine’s therapeutic effect is not fully understood, it is believed to involve the antagonism of Type 2 voltage-gated sodium channels (VGSCs), which is similar to the mechanism of action of older antiepileptic drugs such as phenytoin and carbamazepine.(75,76,77,78) Lamotrigine exhibits a preferential binding affinity for the inactivated state of voltage-gated sodium channels (VGSCs), thereby acting as an antagonist to modulate channel activity.(79,80) Lamotrigine’s binding site is situated on the alpha subunit’s extracellular surface, where it overlaps with the binding sites of carbamazepine and phenytoin.(81) Lamotrigine’s pharmacological profile also includes antagonism of high-voltage-activated calcium channels, including N-, P-, and Q-type channels, which may play a role in its antiseizure effects, complementing its sodium channel-blocking activity.(75,82,83) The modulation of neurotransmitter release by lamotrigine, resulting from its blockade of voltage-gated sodium and calcium channels, is characterized by a decrease in glutamate release, as consistently demonstrated in studies.(84,85) Lamotrigine’s impact on GABA release is complex, with some studies indicating an increase in the release of this inhibitory neurotransmitter, while others suggest a decrease.(75,86,87,88,89) Lamotrigine’s impact on ion flux may also influence intracellular signaling pathways, including those mediated by calcium as a second messenger. An in-vitro study demonstrated that lamotrigine altered CaM kinase II activity in mouse neuronal cultures, resulting in changes to intracellular calcium concentrations. (90)
Adverse drug reactions of lamotrigine
Compared to patients receiving carbamazepine monotherapy, those taking lamotrigine generally experienced lower rates of somnolence, rash, and headache, with incidence rates approximately half those reported for carbamazepine. The incidence of severe AEs was as follows: 13% (3/24) in patients receiving lamotrigine add-on therapy, 11% (13/122) in those on lamotrigine monotherapy, 15% (8/53) in carbamazepine-treated patients, and 22% (2/9) in patients receiving phenytoin. The severe AEs experienced by patients in each treatment group were as follows Lamotrigine add-on therapy: dizziness/vertigo, rash, and eye disorders, Lamotrigine monotherapy: various AEs, including gastrointestinal symptoms, neurological events, and psychiatric reactions, Carbamazepine monotherapy: AEs such as asthenia, hallucinations, rash, and gastrointestinal symptoms, Phenytoin: severe AEs including abdominal pain, myocardial infarction, and somnolence. Seven patients (5%, 7/146) in the lamotrigine group reported SAEs that were considered to be related to the study drug, specifically Depression, Grand mal convulsion, Hematemesis/stomach ulcer, Abdominal pain/nausea/vomiting, Fractured femur, Paranoid reaction, Jaundice.(48) Lamotrigine’s adverse event profile in children with epilepsy is consistent with that observed in adults. In pediatric clinical trials, the most frequently reported treatment-emergent adverse events were infection (20% vs 17% with placebo), vomiting (20% vs 16% with placebo), and somnolence (17% vs 15% with placebo).(72) During the monotherapy phases of controlled trials in adults, lamotrigine was associated with a higher incidence of certain adverse events (? 5%) compared to the control group, specifically Vomiting, Coordination abnormality, Dyspepsia, Nausea, Dizziness, Rhinitis, Anxiety, Insomnia, Infection, Pain, Weight decrease, Chest pain, Dysmenorrhoea.(91)
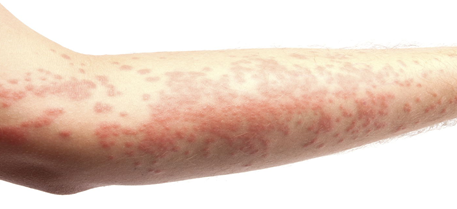
Figure 4: Rash and Inflammation while on Lamictal
Drug interaction
A modeling approach was used to assess the potential DDIs between lamotrigine and two strong enzyme inducers, rifampin and ritonavir, which increase the activity of UGT1A4 and other UGT and CYP enzymes. Established models for these compounds in the Simcyp simulator were utilized, with assigned IndC50 values of 5 µM
for rifampin and 0.2 µM for ritonavir. (92,93) The assigned IndC50 values were chosen to match observed induction effects in clinical studies and were consistent with in vivo plasma levels resulting from standard dosing. The maximum induction capacity (Indmax) of UGT 1A4 was set at 18-fold for rifampin and 17-fold for ritonavir, reflecting their potent enzyme-inducing properties. (94) Valproic acid exhibits inhibitory activity against a range of enzymes, specifically UGT1A4 and UGT2B7, CYP2C9, CYP2C19 (weak inhibition), CYP3A4 (weak inhibition. (92 ,95) The clearance of lamotrigine, which is mainly metabolized by glucuronic acid conjugation, can be influenced by drugs that either induce or inhibit glucuronidation, leading to changes in its apparent clearance rate.(72)The pharmacokinetics of lamotrigine are affected by concomitant AEDs Enzyme-inducing AEDs (carbamazepine, phenytoin, phenobarbital, and primidone) increase lamotrigine’s apparent clearance, Valproate decreases lamotrigine’s apparent clearance and more than doubles its elimination half-life.(96) The use of ethinyl estradiol-containing contraceptives was associated with a reduced lamotrigine serum concentration-to-dose ratio, whereas progestogen-only contraceptives did not have a significant impact on this ratio.(97)
CONCLUSION
We recommend lamotrigine as a primary treatment option for various epilepsy types, including:
- Secondary generalized epilepsy
- Primary generalized epilepsy
- Juvenile myoclonic epilepsy
Due to its excellent tolerability, low adverse event rate, and convenient dosing schedule. The pharmacological portfolio of lamotrigine has been illuminated in this overview, demonstrating its potential as a pluripotent agent with a range of off-label uses, especially in the treatment of challenging psychiatric disorders. Its unique anti-glutamate activity distinguishes it from other psychotropic medications. This case series suggests that lamotrigine may offer a modest therapeutic benefit in elderly patients with challenging-to-treat conditions, while also demonstrating a favorable safety profile. Lamotrigine may provide a safe and effective treatment approach for patients with bipolar disorder, helping to regulate mood symptoms over an extended period. Lamotrigine offers a safer treatment option for pediatric patients, as it is less likely to cause neurologic and metabolic side effects that are often associated with traditional antiepileptic medications is a beneficial AED that does not induce sedation or impact cognitive function, making it a desirable treatment option. Nevertheless, its potential for severe CADRs poses a significant concern, potentially jeopardizing patient health and safety. The pharmacokinetic profile of lamotrigine is characterized by high absorption, minimal binding to plasma proteins, and dose-proportional (linear) kinetics. While lamotrigine shares the risk of serious rash with other AEDs, such as carbamazepine and phenytoin, it has a favorable profile concerning drug-drug interactions, which is crucial for ensuring patient safety, especially in those receiving polytherapy.
REFERENCES
- Hommes, O.R. and Obbens, E.A.M.T. (1972) ‘The epileptogenic action of na-folate in the rat’, Journal of the Neurological Sciences, 16(3), pp. 271–281. Doi:10.1016/0022-510x(72)90192-x.
- Nolen, W.A. and Kupka, R.W. (2000) ‘The efficacy of valproate, lamotrigine and gabapentin in bipolar disorder; review of double-blind controlled studies’, Acta Neuropsychiatrica, 12(3), pp. 122–127. Doi:10.1017/s0924270800035572.
- PA, B. et al. (2022) ‘Dosage optimization of Lamotrigine in pregnancy: A pharmacometric approach using modeling and Simulation’, The Journal of Clinical Pharmacology, 62(12), pp. 1557–1565. Doi:10.1002/jcph.2111.
- Köhler-Forsberg, O. et al. (2022b) ‘Lithium plus antipsychotics or anticonvulsants for bipolar disorder: Comparing clinical response and metabolic changes’, Australian & New Zealand Journal of Psychiatry, 57(1), pp. 93–103. Doi:10.1177/00048674221077619.
- Ranganathan, L.N., Ramamurthy, G. and Kanthimathinathan, S. (2021) ‘Preventive oral treatment of episodic migraine’, Neurology India, 69(Suppl 1). Doi:10.4103/0028-3886.315985.
- Besag, F.M.C. et al. (2021) ‘Efficacy and safety of lamotrigine in the treatment of bipolar disorder across the lifespan: A systematic review’, Therapeutic Advances in Psychopharmacology, 11. Doi:10.1177/20451253211045870.
- Cusin, C. et al. (2000) ‘Impact of clinical variables on illness time course in mood disorders’, Psychiatry Research, 97(2–3), pp. 217–227. Doi:10.1016/s0165-1781(00)00233-x.
- Bowden, C.L. (2003) ‘A placebo-controlled 18-month trial of lamotrigine and lithium maintenance treatment in recently manic or hypomanic patients with bipolar I disorder’, Archives of General Psychiatry, 60(4), p. 392. Doi:10.1001/archpsyc.60.4.392.
- Ropper, A.H. and Brown, R.H. (2005) Cerebrovascular diseases. In: Adams and Victor’s Principles of Neurology, 8th Edition, McGraw-Hill Co., Inc., New York, 34, 682-684
- Greenhill, S.D. and Jones, R.S.G. (2010) ‘Diverse antiepileptic drugs increase the ratio of background synaptic inhibition to excitation and decrease neuronal excitability in neurones of the rat entorhinal cortex in vitro’, Neuroscience, 167(2), pp. 456–474. Doi:10.1016/j.neuroscience.2010.02.021.
- Mockenhaupt, M. et al. (2005) ‘Risk of stevens–Johnson syndrome and toxic epidermal necrolysis in new users of Antiepileptics’, Neurology, 64(7), pp. 1134–1138. Doi:10.1212/01.wnl.0000156354.20227.f0.
- Dichter, M.A. and Brodie, M.J. (1996) ‘New Antiepileptic Drugs’, New England Journal of Medicine, 334(24), pp. 1583–1590. Doi:10.1056/nejm199606133342407
- Ebrahimi HA, Ebrahimi F. The effect of lamotrigine on epilepsy. Iran J Neurol. 2012;11(4):162-3. PMID: 24250888; PMCID: PMC3829260.
- Naguy, A. and Al-Enezi, N. (2019) ‘Lamotrigine uses in psychiatric practice’, American Journal of Therapeutics, 26(1). Doi:10.1097/mjt.0000000000000535.
- Bowden, C.L. (2003) ‘A placebo-controlled 18-month trial of lamotrigine and lithium maintenance treatment in recently manic or hypomanic patients with bipolar I disorder’, Archives of General Psychiatry, 60(4), p. 392. Doi:10.1001/archpsyc.60.4.392.
- Goodwin, G.M. et al. (2004) ‘A pooled analysis of 2 placebo-controlled 18-month trials of lamotrigine and lithium maintenance in bipolar I disorder’, The Journal of Clinical Psychiatry, 65(3), pp. 432–441. Doi:10.4088/jcp.v65n0321.
- Geddes, J.R., Calabrese, J.R. and Goodwin, G.M. (2009) ‘Lamotrigine for treatment of Bipolar Depression: Independent meta-analysis and meta-regression of individual patient data from five randomised trials’, British Journal of Psychiatry, 194(1), pp. 4–9. Doi:10.1192/bjp.bp.107.048504.
- Barbosa, L., Berk, M. and Vorster, M. (2003) ‘A double-blind, randomized, placebo-controlled trial of augmentation with lamotrigine or placebo in patients concomitantly treated with fluoxetine for resistant major depressive episodes’, The Journal of Clinical Psychiatry, 64(4), pp. 403–407. Doi:10.4088/jcp.v64n0407.
- Jain, S. (2011) ‘Faculty opinions recommendation of comparative efficacy and acceptability of antimanic drugs in Acute Mania: A multiple-treatments meta-analysis.’, Faculty Opinions – Post-Publication Peer Review of the Biomedical Literature [Preprint]. Doi:10.3410/f.13357407.14726827.
- Premkumar TS, Pick J. Lamotrigine for schizophrenia. Cochrane Database Syst Rev. 2006 Oct 18;(4):CD005962. Doi: 10.1002/14651858.CD005962.pub2. PMID: 17054266.
- Ohnuma, T. et al. (2013) ‘Low-dose lamotrigine augmentation therapy improves residual symptoms in treatment-resistant schizophrenia: A report of five cases’, Asia-Pacific Psychiatry, 5(4), pp. 336–343. Doi:10.1111/j.1758-5872.2012.00225.x.
- Stewart, E. and Kuan, A.J. (2012) ‘Faculty opinions recommendation of lamotrigine augmentation of serotonin reuptake inhibitors in treatment-resistant obsessive-compulsive disorder: A double-blind, placebo-controlled study.’, Faculty Opinions – Post-Publication Peer Review of the Biomedical Literature [Preprint]. Doi:10.3410/f.715498009.791052959.
- Hertzberg, M.A. et al. (1999a) ‘A preliminary study of lamotrigine for the treatment of posttraumatic stress disorder’, Biological Psychiatry, 45(9), pp. 1226–1229. Doi:10.1016/s0006-3223(99)00011-6.
- Kozari?-Kova?i?, D. and Eterovi?, M. (2013a) ‘Lamotrigine abolished aggression in a patient with treatment-resistant posttraumatic stress disorder’, Clinical Neuropharmacology, 36(3), pp. 94–95. Doi:10.1097/wnf.0b013e318288a7d3.
- Lieb, K. et al. (2010) ‘Pharmacotherapy for borderline personality disorder: Cochrane systematic review of Randomised Trials’, British Journal of Psychiatry, 196(1), pp. 4–12. Doi:10.1192/bjp.bp.108.062984.
- Tritt, K. et al. (2005) ‘Lamotrigine treatment of aggression in female borderline-patients: A randomized, double-blind, placebo-controlled study’, Journal of Psychopharmacology, 19(3), pp. 287–291. Doi:10.1177/0269881105051540.
- Leiberich, P. et al. (2008) ‘Lamotrigine treatment of aggression in female borderline patients, part II: An 18-month follow-up’, Journal of Psychopharmacology, 22(7), pp. 805–808. Doi:10.1177/0269881107084004.
- Aliyev, N.A. and Aliyev, Z.N. (2011) ‘Lamotrigine in the immediate treatment of outpatients with depersonalization disorder without psychiatric comorbidity’, Journal of Clinical Psychopharmacology, 31(1), pp. 61–65. Doi:10.1097/jcp.0b013e31820428e1.
- Uvebrant, P. and Bauzienè, R. (1994) ‘Intractable epilepsy in children. The efficacy of lamotrigine treatment, including non-seizure-related benefits’, Neuropediatrics, 25(06), pp. 284–289. Doi:10.1055/s-2008-1073041.
- Belsito KM, Law PA, Kirk KS, Landa RJ, Zimmerman AW. Lamotrigine therapy for autistic disorder: a randomized, double-blind, placebo-controlled trial. J Autism Dev Disord. 2001 Apr;31(2):175-81. Doi: 10.1023/a:1010799115457. PMID: 11450816.
- Besag FM. Lamotrigine in the treatment of epilepsy in people with intellectual disability. J Intellect Disabil Res. 1998 Dec;42 Suppl 1:50-6. PMID: 10030433.
- Pachet A, Friesen S, Winkelaar D, Gray S. Beneficial behavioural effects of lamotrigine in traumatic brain injury. Brain Inj. 2003 Aug;17(8):715-22. Doi: 10.1080/0269905031000110445. PMID: 12850956.
- Showalter PE, Kimmel DN. Stimulating consciousness and cognition following severe brain injury: a new potential clinical use for lamotrigine. Brain Inj. 2000 Nov;14(11):997-1001. Doi: 10.1080/02699050050191931. PMID: 11104139.
- Cohen-Mansfield J, Werner P, Marx MS. An observational study of agitation in agitated nursing home residents. Int Psychogeriatr. 1989 Fall;1(2):153-65. Doi: 10.1017/s1041610289000165. PMID: 2491142.
- Aulakh JS, Hawkins JW, Athwal HS, Sheikh JI, Yesavage J, Tinklenberg JR. Tolerability and effectiveness of lamotrigine in complex elderly patients. J Geriatr Psychiatry Neurol. 2005 Mar;18(1):8-11. Doi: 10.1177/0891988704271762. PMID: 15681622.
- Calabrese JR, Bowden CL, Sachs GS, Ascher JA, Monaghan E, Rudd GD. A double-blind placebo-controlled study of lamotrigine monotherapy in outpatients with bipolar I depression. Lamictal 602 Study Group. J Clin Psychiatry. 1999 Feb;60(2):79-88. Doi: 10.4088/jcp.v60n0203. PMID: 10084633.
- Giorgi L, Gomez G, O’Neill F, Hammer AE, Risner M. The tolerability of lamotrigine in elderly patients with epilepsy. Drugs Aging. 2001;18(8):621-30. Doi: 10.2165/00002512-200118080-00006. PMID: 11587248.
- Devarajan S, Dursun SM. Aggression in dementia with lamotrigine treatment. Am J Psychiatry. 2000 Jul;157(7):1178. Doi: 10.1176/appi.ajp.157.7.1178. PMID: 10873935.
- Malik S, Arif H, Hirsch LJ. Lamotrigine and its applications in the treatment of epilepsy and other neurological and psychiatric disorders. Expert Rev Neurother. 2006 Nov;6(11):1609-27. Doi: 10.1586/14737175.6.11.1609. PMID: 17144777.
- Brodie MJ, Overstall PW, Giorgi L. Multicentre, double-blind, randomised comparison between lamotrigine and carbamazepine in elderly patients with newly diagnosed epilepsy. The UK Lamotrigine Elderly Study Group. Epilepsy Res. 1999 Oct;37(1):81-7. Doi: 10.1016/s0920-1211(99)00039-x. PMID: 10515178.
- Rowan AJ, Ramsay RE, Collins JF, Pryor F, Boardman KD, Uthman BM, Spitz M, Frederick T, Towne A, Carter GS, Marks W, Felicetta J, Tomyanovich ML; VA Cooperative Study 428 Group. New onset geriatric epilepsy: a randomized study of gabapentin, lamotrigine, and carbamazepine. Neurology. 2005 Jun 14;64(11):1868-73. Doi: 10.1212/01.WNL.0000167384.68207.3E. PMID: 15955935.
- Maggs JL, Naisbitt DJ, Tettey JN, Pirmohamed M, Park BK. Metabolism of lamotrigine to a reactive arene oxide intermediate. Chem Res Toxicol. 2000 Nov;13(11):1075-81. Doi: 10.1021/tx0000825. PMID: 11087428
- Goa KL, Ross SR, Chrisp P. Lamotrigine. A review of its pharmacological properties and clinical efficacy in epilepsy. Drugs. 1993 Jul;46(1):152-76. Doi: 10.2165/00003495-199346010-00009. PMID: 7691504.
- Maggs JL, Naisbitt DJ, Tettey JN, Pirmohamed M, Park BK. Metabolism of lamotrigine to a reactive arene oxide intermediate. Chem Res Toxicol. 2000 Nov;13(11):1075-81. Doi: 10.1021/tx0000825. PMID: 11087428.
- Schapel GJ, Beran RG, Vajda FJ, Berkovic SF, Mashford ML, Dunagan FM, Yuen WC, Davies G. Double-blind, placebo controlled, crossover study of lamotrigine in treatment resistant partial seizures. J Neurol Neurosurg Psychiatry. 1993 May;56(5):448-53. Doi: 10.1136/jnnp.56.5.448. PMID: 8505632; PMCID: PMC1014998.
- Messenheimer J, Mullens EL, Giorgi L, Young F. Safety review of adult clinical trial experience with lamotrigine. Drug Saf. 1998 Apr;18(4):281-96. Doi: 10.2165/00002018-199818040-00004. PMID: 9565739.
- Brodie MJ, Overstall PW, Giorgi L. Multicentre, double-blind, randomised comparison between lamotrigine and carbamazepine in elderly patients with newly diagnosed epilepsy. The UK Lamotrigine Elderly Study Group. Epilepsy Res. 1999 Oct;37(1):81-7. Doi: 10.1016/s0920-1211(99)00039-x. PMID: 10515178.
- Giorgi L, Gomez G, O’Neill F, Hammer AE, Risner M. The tolerability of lamotrigine in elderly patients with epilepsy. Drugs Aging. 2001;18(8):621-30. Doi: 10.2165/00002512-200118080-00006. PMID: 11587248.
- Holmes GL, Ben-Ari Y. The neurobiology and consequences of epilepsy in the developing brain. Pediatr Res. 2001 Mar;49(3):320-5. Doi: 10.1203/00006450-200103000-00004. PMID: 11228256.
- Culy CR, Goa KL. Lamotrigine. A review of its use in childhood epilepsy. Paediatr Drugs. 2000 Jul-Aug;2(4):299-330. Doi: 10.2165/00128072-200002040-00006. PMID: 10946418.
- Messenheimer J. Efficacy and safety of lamotrigine in pediatric patients. J Child Neurol. 2002 Feb;17 Suppl 2:2S34-2S42. Doi: 10.1177/08830738020170021001. PMID: 11952035.
- Messenheimer JA, Giorgi L, Risner ME. The tolerability of lamotrigine in children. Drug Saf. 2000 Apr;22(4):303-12. Doi: 10.2165/00002018-200022040-00003. PMID: 10789824.
- Brodie MJ, Richens A, Yuen AW. Double-blind comparison of lamotrigine and carbamazepine in newly diagnosed epilepsy. UK Lamotrigine/Carbamazepine Monotherapy Trial Group. Lancet. 1995 Feb 25;345(8948):476-9. Doi: 10.1016/s0140-6736(95)90581-2. Erratum in: Lancet 1995 Mar 11;345(8950):662. PMID: 7710545.
- Steiner TJ, Dellaportas CI, Findley LJ, Gross M, Gibberd FB, Perkin GD, Park DM, Abbott R. Lamotrigine monotherapy in newly diagnosed untreated epilepsy: a double-blind comparison with phenytoin. Epilepsia. 1999 May;40(5):601-7. Doi: 10.1111/j.1528-1157.1999.tb05562.x. PMID: 10386529.
- Motte J, Trevathan E, Arvidsson JF, Barrera MN, Mullens EL, Manasco P. Lamotrigine for generalized seizures associated with the Lennox-Gastaut syndrome. Lamictal Lennox-Gastaut Study Group. N Engl J Med. 1997 Dec 18;337(25):1807-12. Doi: 10.1056/NEJM199712183372504. Erratum in: N Engl J Med 1998 Sep 17;339(12):851-2. PMID: 9400037.
- Duchowny M, Pellock JM, Graf WD, Billard C, Gilman J, Casale E, Womble G, Risner M, Manasco P. A placebo-controlled trial of lamotrigine add-on therapy for partial seizures in children. Lamictal Pediatric Partial Seizure Study Group. Neurology. 1999 Nov 10;53(8):1724-31. Doi: 10.1212/wnl.53.8.1724. PMID: 10563619.
- Frank LM, Enlow T, Holmes GL, Manasco P, Concannon S, Chen C, Womble G, Casale EJ. Lamictal (lamotrigine) monotherapy for typical absence seizures in children. Epilepsia. 1999 Jul;40(7):973-9. Doi: 10.1111/j.1528-1157.1999.tb00805.x. PMID: 10403222.
- Duchowny M, Pellock JM, Graf WD, Billard C, Gilman J, Casale E, Womble G, Risner M, Manasco P. A placebo-controlled trial of lamotrigine add-on therapy for partial seizures in children. Lamictal Pediatric Partial Seizure Study Group. Neurology. 1999 Nov 10;53(8):1724-31. Doi: 10.1212/wnl.53.8.1724. PMID: 10563619.
- Friis ML. Facial clefts and congenital heart defects in children of parents with epilepsy: genetic and environmental etiologic factors. Acta Neurol Scand. 1989 Jun;79(6):433-59. Doi: 10.1111/j.1600-0404.1989.tb03814.x. PMID: 2675529.
- Lindhout D, Meinardi H, Meijer JW, Nau H. Antiepileptic drugs and teratogenesis in two consecutive cohorts: changes in prescription policy paralleled by changes in pattern of malformations. Neurology. 1992 Apr;42(4 Suppl 5):94-110. PMID: 1574185.
- Sabers A, aRogvi-Hansen B, Dam M, Fischer-Rasmussen W, Gram L, Hansen M, Møller A, Winkel H. Pregnancy and epilepsy: a retrospective study of 151 pregnancies. Acta Neurol Scand. 1998 Mar;97(3):164-70. Doi: 10.1111/j.1600-0404.1998.tb00631.x. PMID: 9531432.
- Arpino C, Brescianini S, Robert E, Castilla EE, Cocchi G, Cornel MC, de Vigan C, Lancaster PA, Merlob P, Sumiyoshi Y, Zampino G, Renzi C, Rosano A, Mastroiacovo P. Teratogenic effects of antiepileptic drugs: use of an International Database on Malformations and Drug Exposure (MADRE). Epilepsia. 2000 Nov;41(11):1436-43. Doi: 10.1111/j.1528-1157.2000.tb00119.x. PMID: 11077457.
- Diav-Citrin O, Shechtman S, Arnon J, Ornoy A. Is carbamazepine teratogenic? A prospective controlled study of 210 pregnancies. Neurology. 2001 Jul 24;57(2):321-4. Doi: 10.1212/wnl.57.2.321. PMID: 11468320.
- Samrén EB, van Duijn CM, Christiaens GC, Hofman A, Lindhout D. Antiepileptic drug regimens and major congenital abnormalities in the offspring. Ann Neurol. 1999 Nov;46(5):739-46. PMID: 10553991.
- Tomson T, Battino D, Bonizzoni E, Craig J, Lindhout D, Perucca E, Sabers A, Vajda F; Collaborative EURAP Study Group. EURAP: an International registry of antiepileptic drugs and pregnancy. Epilepsia. 2004 Nov;45(11):1463-4. Doi: 10.1111/j.0013-9580.2004.451101.x. PMID: 15509250.
- Bowden CL, Calabrese JR, Sachs G, Yatham LN, Asghar SA, Hompland M, Montgomery P, Earl N, Smoot TM, DeVeaugh-Geiss J; Lamictal 606 Study Group. A placebo-controlled 18-month trial of lamotrigine and lithium maintenance treatment in recently manic or hypomanic patients with bipolar I disorder. Arch Gen Psychiatry. 2003 Apr;60(4):392-400. Doi: 10.1001/archpsyc.60.4.392. Erratum in: Arch Gen Psychiatry. 2004 Jul;61(7):680. PMID: 12695317.
- Calabrese JR, Bowden CL, Sachs G, Yatham LN, Behnke K, Mehtonen OP, Montgomery P, Ascher J, Paska W, Earl N, DeVeaugh-Geiss J; Lamictal 605 Study Group. A placebo-controlled 18-month trial of lamotrigine and lithium maintenance treatment in recently depressed patients with bipolar I disorder. J Clin Psychiatry. 2003 Sep;64(9):1013-24. Doi: 10.4088/jcp.v64n0906. PMID: 14628976.
- Newport DJ, Stowe ZN, Viguera AC, Calamaras MR, Juric S, Knight B, Pennell PB, Baldessarini RJ. Lamotrigine in bipolar disorder: efficacy during pregnancy. Bipolar Disord. 2008 May;10(3):432-6. Doi: 10.1111/j.1399-5618.2007.00565.x. PMID: 18402631.
- Nguyen HT, Sharma V, McIntyre RS. Teratogenesis associated with antibipolar agents. Adv Ther. 2009 Mar;26(3):281-94. Doi: 10.1007/s12325-009-0011-z. Epub 2009 Mar 28. PMID: 19330496.
- Viguera AC, Koukopoulos A, Muzina DJ, Baldessarini RJ. Teratogenicity and anticonvulsants: lessons from neurology to psychiatry. J Clin Psychiatry. 2007;68 Suppl 9:29-33. Erratum in: J Clin Psychiatry. 2007 Dec;68(12):1989. PMID: 17764382.
- Bowden CL, Asnis GM, Ginsberg LD, Bentley B, Leadbetter R, White R. Safety and tolerability of lamotrigine for bipolar disorder. Drug Saf. 2004;27(3):173-84. Doi: 10.2165/00002018-200427030-00002. PMID: 14756579.
- Viguera AC, Koukopoulos A, Muzina DJ, Baldessarini RJ. Teratogenicity and anticonvulsants: lessons from neurology to psychiatry. J Clin Psychiatry. 2007;68 Suppl 9:29-33. Erratum in: J Clin Psychiatry. 2007 Dec;68(12):1989. PMID: 17764382.
- Petrenaite V, Sabers A, Hansen-Schwartz J. Individual changes in lamotrigine plasma concentrations during pregnancy. Epilepsy Res. 2005 Jul;65(3):185-8. Doi: 10.1016/j.eplepsyres.2005.06.004. PMID: 16084694.
- Ohman I, Vitols S, Tomson T. Lamotrigine in pregnancy: pharmacokinetics during delivery, in the neonate, and during lactation. Epilepsia. 2000 Jun;41(6):709-13. Doi: 10.1111/j.1528-1157.2000.tb00232.x. PMID: 10840403.
- Lees G, Leach MJ. Studies on the mechanism of action of the novel anticonvulsant lamotrigine (Lamictal) using primary neurological cultures from rat cortex. Brain Res. 1993 May 28;612(1-2):190-9. Doi: 10.1016/0006-8993(93)91660-k. PMID: 7687190.
- Qiao X, Sun G, Clare JJ, Werkman TR, Wadman WJ. Properties of human brain sodium channel ?-subunits expressed in HEK293 cells and their modulation by carbamazepine, phenytoin and lamotrigine. Br J Pharmacol. 2014 Feb;171(4):1054-67. Doi: 10.1111/bph.12534. PMID: 24283699; PMCID: PMC3925043.
- Nakatani Y, Masuko H, Amano T. Effect of lamotrigine on Na(v)1.4 voltage-gated sodium channels. J Pharmacol Sci. 2013;123(2):203-6. Doi: 10.1254/jphs.13116sc. Epub 2013 Oct 4. PMID: 24096830.
- Czapi?ski P, Blaszczyk B, Czuczwar SJ. Mechanisms of action of antiepileptic drugs. Curr Top Med Chem. 2005;5(1):3-14. Doi: 10.2174/1568026053386962. PMID: 15638774.
- Kuo CC, Lu L. Characterization of lamotrigine inhibition of Na+ channels in rat hippocampal neurones. Br J Pharmacol. 1997 Jul;121(6):1231-8. Doi: 10.1038/sj.bjp.0701221. PMID: 9249262; PMCID: PMC1564785.
- Xie X, Lancaster B, Peakman T, Garthwaite J. Interaction of the antiepileptic drug lamotrigine with recombinant rat brain type IIA Na+ channels and with native Na+ channels in rat hippocampal neurones. Pflugers Arch. 1995 Jul;430(3):437-46. Doi: 10.1007/BF00373920. PMID: 7491269.
- Kuo CC. A common anticonvulsant binding site for phenytoin, carbamazepine, and lamotrigine in neuronal Na+ channels. Mol Pharmacol. 1998 Oct;54(4):712-21. PMID: 9765515.
- Stefani A, Spadoni F, Siniscalchi A, Bernardi G. Lamotrigine inhibits Ca2+ currents in cortical neurons: functional implications. Eur J Pharmacol. 1996 Jun 20;307(1):113-6. Doi: 10.1016/0014-2999(96)00265-8. PMID: 8831112.
- Grunze H, von Wegerer J, Greene RW, Walden J. Modulation of calcium and potassium currents by lamotrigine. Neuropsychobiology. 1998 Oct;38(3):131-8. Doi: 10.1159/000026528. PMID: 9778600.
- Stefani A, Spadoni F, Bernardi G. Voltage-activated calcium channels: targets of antiepileptic drug therapy? Epilepsia. 1997 Sep;38(9):959-65. Doi: 10.1111/j.1528-1157.1997.tb01477.x. PMID: 9579933.
- Cunningham MO, Jones RS. The anticonvulsant, lamotrigine decreases spontaneous glutamate release but increases spontaneous GABA release in the rat entorhinal cortex in vitro. Neuropharmacology. 2000 Aug 23;39(11):2139-46. Doi: 10.1016/s0028-3908(00)00051-4. PMID: 10963757.
- Wang SJ, Huang CC, Hsu KS, Tsai JJ, Gean PW. Presynaptic inhibition of excitatory neurotransmission by lamotrigine in the rat amygdalar neurons. Synapse. 1996 Nov;24(3):248-55. Doi: 10.1002/(SICI)1098-2396(199611)24:3<248>3.0.CO;2-E. PMID: 8923665.
- Leach MJ, Marden CM, Miller AA. Pharmacological studies on lamotrigine, a novel potential antiepileptic drug: II. Neurochemical studies on the mechanism of action. Epilepsia. 1986 Sep-Oct;27(5):490-7. Doi: 10.1111/j.1528-1157.1986.tb03573.x. PMID: 3757936.
- Teoh H, Fowler LJ, Bowery NG. Effect of lamotrigine on the electrically-evoked release of endogenous amino acids from slices of dorsal horn of the rat spinal cord. Neuropharmacology. 1995 Oct;34(10):1273-8. Doi: 10.1016/0028-3908(95)00104-e. PMID: 8570024.
- Kuzniecky R, Ho S, Pan J, Martin R, Gilliam F, Faught E, Hetherington H. Modulation of cerebral GABA by topiramate, lamotrigine, and gabapentin in healthy adults. Neurology. 2002 Feb 12;58(3):368-72. Doi: 10.1212/wnl.58.3.368. PMID: 11839834.
- Lee ES, Ryu JH, Kim EJ, Kim GT, Cho YW, Park HJ, Tak HM, Han J, Kang D. Lamotrigine increases intracellular Ca(2+) levels and Ca(2+)/calmodulin-dependent kinase II activation in mouse dorsal root ganglion neurones. Acta Physiol (Oxf). 2013 Feb;207(2):397-404. Doi: 10.1111/apha.12034. Epub 2012 Dec 11. PMID: 23227957.
- Biton V, Sackellares JC, Vuong A, Hammer AE, Barrett PS, Messenheimer JA. Double-blind, placebo-controlled study of lamotrigine in primary generalized tonic-clonic seizures. Neurology. 2005 Dec 13;65(11):1737-43. Doi: 10.1212/01.wnl.0000187118.19221.e4. PMID: 16344515.
- Zaccara G, Perucca E. Interactions between antiepileptic drugs, and between antiepileptic drugs and other drugs. Epileptic Disord. 2014 Dec;16(4):409-31. Doi: 10.1684/epd.2014.0714. PMID: 25515681.
- Patsalos PN. Drug interactions with the newer antiepileptic drugs (AEDs)—Part 2: pharmacokinetic and pharmacodynamic interactions between AEDs and drugs used to treat non-epilepsy disorders. Clin Pharmacokinet. 2013 Dec;52(12):1045-61. Doi: 10.1007/s40262-013-0088-z. PMID: 23794036.
- Smith CM, Faucette SR, Wang H, LeCluyse EL. Modulation of UDP- glucuronosyltransferase 1A1 in primary human hepatocytes by prototypical inducers. J Biochem Mol Toxicol. 2005;19(2):96-108. Doi: 10.1002/jbt.20058. PMID: 15849716.
- Zhou SF, Xue CC, Yu XQ, Li C, Wang G. Clinically important drug interactions potentially involving mechanism-based inhibition of cytochrome P450 3A4 and the role of therapeutic drug monitoring. Ther Drug Monit. 2007 Dec;29(6):687-710. Doi: 10.1097/FTD.0b013e31815c16f5. PMID: 18043468.
- Weintraub D, Buchsbaum R, Resor SR Jr, Hirsch LJ. Effect of antiepileptic drug comedication on lamotrigine clearance. Arch Neurol. 2005 Sep;62(9):1432-6. Doi: 10.1001/archneur.62.9.1432. PMID: 16157751.
- Biton V. Pharmacokinetics, toxicology and safety of lamotrigine in epilepsy. Expert Opin Drug Metab Toxicol. 2006 Dec;2(6):1009-18. Doi: 10.1517/17425255.2.6.1009. PMID: 17125413.


 Kalla Vasavi*
Kalla Vasavi*
 Noupada Sravanthi
Noupada Sravanthi
 Jaladanki Sandhya
Jaladanki Sandhya
 Dr Paila Bhanuji Rao
Dr Paila Bhanuji Rao




 10.5281/zenodo.14823785
10.5281/zenodo.14823785