Abstract
Antioxidant therapy has emerged as a promising approach for managing unexplained male infertility, a condition in which no specific cause is identified despite thorough evaluation. Oxidative stress occurs when there is an imbalance between reactive oxygen species (ROS) and the body’s ability to detoxify them using antioxidants. In men, excessive ROS can lead to sperm damage, reducing sperm quality and motility, which are critical factors for successful fertilization. Oxidative stress is increasingly recognized as a contributor to male infertility, affecting sperm quality, motility, and DNA integrity. This detailed overview explores the role of oxidative stress in male infertility, how antioxidants work to mitigate this issue, commonly used antioxidants, and considerations for their therapeutic use.
Keywords
Antioxidant therapy, Unexplained male infertility, Oxidative stress, Reactive oxygen species (ROS).
Introduction
UNDERSTANDING OXIDATIVE STRESS AND MALE INFERTILITY
Oxidative stress arises when there is an imbalance between reactive oxygen species (ROS) and the antioxidant defenses of the body. While a certain level of ROS is necessary for normal sperm functions, excessive ROS can cause lipid peroxidation, protein damage, and DNA fragmentation in sperm cells[1,2,3,4]. Sperm cells are particularly susceptible to oxidative stress due to their limited cytoplasmic content and high levels of polyunsaturated fatty acids, which are prone to oxidation. In cases of unexplained male infertility, oxidative stress is a critical factor that can negatively impact sperm function, reducing fertility potential[5,6,7].
HOW OXIDATIVE STRESS AFFECTS SPERM
Sperm cells are particularly vulnerable to oxidative damage due to their unique structure and function:
- High Content of Polyunsaturated Fatty Acids: Sperm membranes are rich in polyunsaturated fatty acids, which are susceptible to lipid peroxidation caused by ROS. This oxidative damage can compromise membrane fluidity, essential for sperm motility and the ability to fuse with the egg.
- Limited Antioxidant Defenses: Sperm cells have minimal cytoplasm, which reduces their capacity to house antioxidant enzymes. As a result, they rely on antioxidants in seminal fluid for protection against oxidative stress.
- DNA Damage: Excessive ROS can lead to single- and double-strand breaks in sperm DNA, which can affect fertility by reducing the sperm's viability and increasing the risk of genetic abnormalities in offspring. DNA fragmentation in sperm has been linked to increased rates of miscarriage and decreased success rates in assisted reproductive technologies (ART).
- Reduced Sperm Motility: Oxidative stress damages proteins and enzymes critical for sperm motility, which is essential for the sperm to navigate the female reproductive tract and reach the egg.
- Impaired Sperm Morphology: Oxidative damage can also affect the structural integrity of sperm, leading to abnormal morphology that can impede fertilization.[8,9,10,11,12,15,18]
SOURCES OF OXIDATIVE STRESS IN MEN
Oxidative stress in men, particularly related to infertility, can result from a combination of lifestyle, environmental, and physiological factors. These sources contribute to an excess of reactive oxygen species (ROS), which can lead to sperm damage and impaired reproductive function. Here are the primary sources of oxidative stress in men:
1. Lifestyle Factors
Smoking:
Tobacco smoke contains numerous harmful chemicals that increase ROS production in the body. Smoking is strongly associated with reduced sperm count, motility, and abnormal sperm morphology due to oxidative damage.
Excessive Alcohol Consumption:
Alcohol metabolism generates free radicals, which can overwhelm the body’s antioxidant defenses. Chronic alcohol use is linked to lower testosterone levels, decreased sperm quality, and increased oxidative stress.
Poor Diet:
Diets high in processed foods, trans fats, and sugars but low in fruits, vegetables, and antioxidants can lead to oxidative stress. Nutrient deficiencies, particularly in antioxidants like vitamins C and E, zinc, and selenium, further exacerbate oxidative damage in sperm cells.
Lack of Physical Activity or Obesity:
While moderate exercise enhances antioxidant defenses, a sedentary lifestyle or excessive exercise can increase oxidative stress. Obesity, often related to poor lifestyle choices, is also associated with increased ROS production due to chronic inflammation and insulin resistance[19,21,22,35,38].
2. Environmental Toxins and Pollutants
Air Pollution:
Exposure to pollutants such as particulate matter, heavy metals, and other environmental contaminants can increase ROS production. Studies have shown that air pollution exposure correlates with lower sperm quality.
Pesticides and Chemicals:
Occupational and environmental exposure to pesticides, industrial chemicals, and endocrine disruptors (like bisphenol A) can lead to oxidative stress. These chemicals can be absorbed through the skin, inhalation, or ingestion and are linked to impaired sperm function.
Heavy Metals:
Lead, cadmium, and mercury, which can be found in industrial areas or contaminated water, are known to induce oxidative stress. These metals accumulate in the body and can damage sperm DNA and reduce fertility.
Radiation and UV Exposure:
High levels of radiation, including UV exposure, can cause oxidative damage to sperm DNA. This is particularly relevant for men working in occupations involving radiation exposure or extensive time outdoors without sun protection[23,24,25,28,61,65].
3. Medical Conditions and Infections
Chronic Diseases:
Conditions such as diabetes, hypertension, and cardiovascular disease are associated with elevated oxidative stress due to chronic inflammation. Diabetes, for example, causes increased ROS production, which can impair sperm quality and function.
Varicocele:
This condition involves the enlargement of veins within the scrotum, leading to increased testicular temperature and reduced antioxidant capacity. Varicocele is a well-known risk factor for male infertility and is linked to oxidative stress-induced sperm damage.
Infections:
Urogenital infections, such as prostatitis, epididymitis, and sexually transmitted infections, can lead to increased ROS in the reproductive tract. These infections may directly damage sperm cells or lead to inflammation, which increases oxidative stress[29,30,31,32].
4. Age-Related Factors
Natural Decline in Antioxidant Defenses:
As men age, the body’s natural antioxidant defenses tend to weaken, which can increase oxidative stress. Older men also have a higher likelihood of accumulated environmental and lifestyle exposures, compounding their risk of oxidative damage.
Hormonal Changes:
Testosterone levels decline with age, which can impact sperm production and quality. This hormonal change can indirectly increase oxidative stress and its effects on sperm[36,38,51,59].
5. Psychological Stress
Chronic Stress:
Prolonged psychological stress is linked to higher cortisol levels, which can disrupt hormonal balance and lead to increased ROS production. Chronic stress has been shown to reduce sperm quality and motility, likely through oxidative stress pathways.
Sleep Deprivation:
Poor sleep quality or chronic sleep deprivation can contribute to oxidative stress. Lack of sleep affects the body’s ability to repair and regenerate, exacerbating the effects of ROS on sperm and overall health[39,41,53,68,75]. Addressing oxidative stress in men requires a holistic approach, considering lifestyle adjustments, environmental factors, and medical management where necessary. Limiting exposure to environmental toxins, adopting a healthier lifestyle, and using antioxidant-rich foods or supplements can help manage oxidative stress and support better reproductive health.
ANTIOXIDANTS AS A DEFENSE MECHANISM
The body utilizes antioxidants to neutralize ROS and protect cells from oxidative damage. In the male reproductive system, both enzymatic antioxidants (such as superoxide dismutase, catalase, and glutathione peroxidase) and non-enzymatic antioxidants (such as vitamins C and E, selenium, and zinc) play critical roles in safeguarding sperm from oxidative stress. Antioxidant therapy has gained interest as a treatment for unexplained male infertility, given its potential to reduce oxidative damage and improve sperm quality. By bolstering the body's antioxidant defenses, antioxidant supplementation aims to restore the balance between ROS production and neutralization, supporting overall sperm health and fertility potential[43,52,56,57].
HOW ANTIOXIDANTS COUNTERACT OXIDATIVE STRESS
Antioxidants are molecules that neutralize ROS, preventing or mitigating oxidative damage. In sperm cells, antioxidants work by:
Neutralizing ROS:
Antioxidants scavenge and deactivate ROS, thus protecting sperm from oxidative damage.
Protecting Lipids and Proteins:
Antioxidants prevent lipid peroxidation in the sperm membrane, which is crucial for maintaining membrane fluidity and integrity. They also protect sperm proteins that are essential for motility and function.
Maintaining DNA Integrity:
Excessive ROS can lead to DNA fragmentation, affecting sperm viability and the genetic quality of the sperm. Antioxidants help safeguard DNA by preventing ROS-induced breaks and mutations.
Enhancing Sperm Function:
By reducing oxidative damage, antioxidants contribute to improved sperm motility, morphology, and concentration, all of which are essential for successful fertilization[59,60,61,62,63,65].
COMMON ANTIOXIDANTS USED IN MALE INFERTILITY TREATMENT
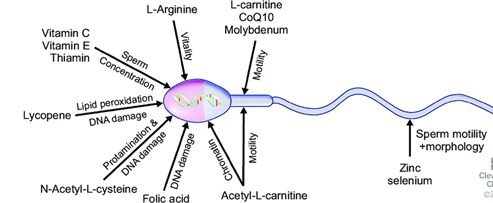
Fig.1 The antioxidant substances that significantly impact the functioning of the sperm
1. Vitamin C (Ascorbic Acid)
Role:
Acts as a primary antioxidant, neutralizing free radicals in seminal plasma and protecting sperm DNA.
Benefits:
Vitamin C can enhance sperm count and reduce DNA fragmentation.
Dosage:
Commonly used doses range from 500 to 1000 mg per day.
2. Vitamin E (Tocopherol)
Role:
A lipid-soluble antioxidant that protects sperm membranes from lipid peroxidation.
Benefits:
Vitamin E has been shown to improve sperm motility and reduce sperm DNA damage.
Dosage:
Typically administered in doses of 200 to 400 IU per day.
3. Coenzyme Q10 (CoQ10)
Role:
Functions in mitochondrial energy production and as an antioxidant that protects sperm cells from oxidative damage.
Benefits:
CoQ10 supplementation is associated with improved sperm motility and density.
Dosage:
Common doses range from 100 to 300 mg per day.
4. Selenium
Role:
A trace mineral that supports the antioxidant enzyme glutathione peroxidase, which protects against oxidative damage.
Benefits:
Selenium has been linked to enhanced sperm motility and reduced sperm DNA fragmentation.
Dosage:
Typically 55 to 100 mcg per day.
5. Zinc
Role:
Supports testosterone synthesis and sperm development, with antioxidant properties that protect sperm DNA and proteins.
Benefits:
Zinc deficiency is often associated with reduced sperm quality, so supplementation can improve overall sperm health.
Dosage:
Standard doses are around 15 to 30 mg per day.
6. L-Carnitine and Acetyl-L-Carnitine
Role:
Involved in fatty acid metabolism and energy production, helping to support sperm motility.
Benefits:
Research indicates improvements in sperm motility and concentration with L-Carnitine supplementation.
Dosage:
Doses vary, commonly 1-3 g per day.
7. N-Acetylcysteine (NAC)
Role:
Acts as a precursor to glutathione, a powerful antioxidant, and supports detoxification processes.
Benefits:
NAC can improve sperm concentration and reduce oxidative stress in seminal fluid.
Dosage:
Generally around 600 mg per day.[1,2,3,4,5]
CONCLUSION:
In conclusion, antioxidant therapy offers a promising avenue for managing unexplained male infertility, especially in cases where oxidative stress is suspected to impair sperm function. By neutralizing reactive oxygen species (ROS), antioxidants can help protect sperm from oxidative damage, improving parameters such as motility, vitality, count, concentration, morphology, and DNA integrity. Common antioxidants used in therapy, including vitamins C and E, Coenzyme Q10, selenium, zinc, and L-carnitine, have shown potential benefits in various clinical studies. While antioxidant supplementation is generally safe and accessible, its effectiveness can vary based on individual factors, such as lifestyle, medical history, and baseline oxidative stress levels. Therefore, it is essential for men considering antioxidant therapy to consult with healthcare providers to tailor their approach. Additionally, incorporating lifestyle changes that reduce oxidative stress—such as a balanced diet, regular exercise, and avoiding environmental toxins—can further support fertility outcomes. Further research, particularly large-scale, well-designed clinical trials, is needed to refine antioxidant treatment protocols and establish standardized guidelines for their use in male infertility. As our understanding of the complex role of oxidative stress in fertility evolves, antioxidant therapy, combined with personalized medicine, holds great potential for improving fertility outcomes for men with unexplained infertility.
REFERENCE:
- Dr. Lokesh. K, Dr. Borus Purushothaman, Dr. Yashmi Agwina Xavier, Veerammal, Dr. Suman Sharma, The Impact of Oxidative Stress in Male Infertility; Dr. Borus Andro Lan and Research Center, Chennai, 2024. Volume 9, Issue 5 Sep - Oct 2024, pp: 177-185.
- Dr. Lokesh. K, Dr. Borus Purushothaman, Dr. Harini. V, veerammal, Dr. Suman sharma, Antioxidant Supplementation and Duration of Antioxidant in Male Infertility – A Systemic Review; Dr. Borus Andro Lan and Research Center, Chennai, 2024.
- Dr. Lokesh. K, Dr. Borus Purushothaman, Dr. Harini. V, veerammal, Dr. Suman sharma, A Comprehensive Approach and Critical Evaluation of Clinical Practice Guidelines for Sperm DNA Fragmentation; Dr. Borus Andro Lan and Research Center, Chennai, 2024. Volume 9, Issue 3 May-June 2024, pp: 844-848.
- Dr. Lokesh. K, Dr. Borus Purushothaman, Dr. Yashmi Agwina Xavier, Veerammal, Dr. Suman Sharma, Antioxidants and Idiopathic Male Infertility: Their Impact on Sperm Quality Parameters and Pregnancy Rates; Dr. Borus Andro Lan and Research Center, Chennai, 2024. Volume 9, Issue 5 Sep - Oct 2024, pp: 335-340 www.ijprajournal.com
- Agarwal, A., & Sekhon, L. H. (2010). "The role of antioxidant therapy in the treatment of male infertility." Human Fertility, 13(4), 217-225.
- Showell, M. G., Brown, J., Yazdani, A., Stankiewicz, M. T., & Hart, R. J. (2014). "Antioxidants for male subfertility." Cochrane Database of Systematic Reviews, Issue 12.
- Agarwal, A., & Majzoub, A. (2017). "Role of Antioxidants in Assisted Reproductive Techniques." World Journal of Men's Health, 35(2), 77-93.
- Gharagozloo, P., & Aitken, R. J. (2011). "The role of sperm oxidative stress in male infertility and the significance of oral antioxidant therapy." Human Reproduction, 26(7), 1628-1640.
- Tremellen, K. (2008). "Oxidative stress and male infertility—a clinical perspective." Human Reproduction Update, 14(3), 243-258.
- Agarwal A, Mulgund A, Hamada A, Chyatte MR. A unique view on male infertility around the globe. Reprod Biol Endocrinol. 2015;13:37.
- Agarwal A, Parekh N, Panner Selvam MK, Henkel R, Shah R, Homa ST, et al. Male oxidative stress infertility (MOSI): proposed terminology and clinical practice guidelines for management of idiopathic male infertility. World J Mens Health. 2019;37:296–312.
- Hamada A, Esteves SC, Nizza M, Agarwal A. Unexplained male infertility: diagnosis and management. Int Braz J Urol. 2012;38:576–594.
- Leisegang K, Dutta S. Do lifestyle practices impede male fertility? Andrologia. 2020 doi: 10.1111/and.13595.
- Leisegang K, Henkel R. Oxidative stress: relevance, evaluation, and management. In: Rizk B, Agarwal A, Sabanegh ES, editors. Male infertility in reproductive medicine: diagnosis and management. Boca Raton: CRC Press; 2019. pp. 119–128.
- Cardoso JP, Cocuzza M, Elterman D. Optimizing male fertility: oxidative stress and the use of antioxidants. World J Urol. 2019;37:1029–1034.
- Kuchakulla M, Soni Y, Patel P, Parekh N, Ramasamy R. A systematic review and evidence-based analysis of ingredients in popular male fertility supplements. Urology. 2020;136:133–141.
- Showell MG, Brown J, Yazdani A, Stankiewicz MT, Hart RJ. Antioxidants for male subfertility. Cochrane Database Syst Rev. 2011;(1):CD007411.
- Showell MG, Mackenzie-Proctor R, Brown J, Yazdani A, Stankiewicz MT, Hart RJ. Antioxidants for male subfertility. Cochrane Database Syst Rev. 2014;(12):CD007411.
- Majzoub A, Agarwal A. Systematic review of antioxidant types and doses in male infertility: benefits on semen parameters, advanced sperm function, assisted reproduction and live-birth rate. Arab J Urol. 2018;16:113–124.
- Smits RM, Mackenzie-Proctor R, Yazdani A, Stankiewicz MT, Jordan V, Showell MG. Antioxidants for male subfertility. Cochrane Database Syst Rev. 2019;3:CD007411
- Adewoyin M, Ibrahim M, Roszaman R, Isa MLM, Alewi NAM, Rafa AAA, et al. Male infertility: the effect of natural antioxidants and phytocompounds on seminal oxidative stress. Diseases. 2017;5:9.
- Buhling K, Schumacher A, Eulenburg CZ, Laakmann E. Influence of oral vitamin and mineral supplementation on male infertility: a meta-analysis and systematic review. Reprod Biomed Online. 2019;39:269–279.
- McPherson NO, Shehadeh H, Fullston T, Zander-Fox DL, Lane M. Dietary micronutrient supplementation for 12 days in obese male mice restores sperm oxidative stress. Nutrients. 2019;11:2196
- Salas-Huetos A, Bulló M, Salas-Salvadó J. Dietary patterns, foods and nutrients in male fertility parameters and fecundability: a systematic review of observational studies. Hum Reprod Update. 2017;23:371–389.
- Chattopadhyay R, Yasmin S, Chakravarty B. Effect of continuous 6 months oral antioxidant combination with universally recommended dosage in idiopathic male infertility. IJIFM. 2016;7:1–6.
- da Silva TM, Maia MCS, Arruda JT, Approbato FC, Mendonça CR, Approbato MS. Folic acid does not improve semen parametrs in subfertile men: a double-blin, randomized, placebo-controlled study. JBRA Assist Reprod. 2013;17:152–157.
- Keskes-Ammar L, Feki-Chakroun N, Rebai T, Sahnoun Z, Ghozzi H, Hammami S, et al. Sperm oxidative stress and the effect of an oral vitamin E and selenium supplement on semen quality in infertile men. Arch Androl. 2003;49:83–94.
- Kessopoulou E, Powers HJ, Sharma KK, Pearson MJ, Russell JM, Cooke ID, et al. A double-blind randomized placebo cross-over controlled trial using the antioxidant vitamin E to treat reactive oxygen species associated male infertility. Fertil Steril. 1995;64:825–831.
- Ménézo YJ, Hazout A, Panteix G, Robert F, Rollet J, Cohen-Bacrie P, et al. Antioxidants to reduce sperm DNA fragmentation: an unexpected adverse effect. Reprod Biomed Online. 2007;14:418–421.
- Halliwell B. Free radicals and antioxidants - quo vadis? Trends Pharmacol Sci. 2011;32:125–130.
- Castagné V, Lefèvre K, Natero R, Clarke PG, Bedker DA. An optimal redox status for the survival of axotomized ganglion cells in the developing retina. Neuroscience. 1999;93:313–320.
- Henkel R, Sandhu IS, Agarwal A. The excessive use of antioxidant therapy: a possible cause of male infertility? Andrologia. 2019;51:e13162.
- Panner Selvam MK, Agarwal A, Henkel R, Finelli R, Robert KA, Iovine C, et al. The effect of oxidative and reductive stress on semen parameters and functions of physiologically normal human spermatozoa. Free Radic Biol Med. 2020;152:375–385.
- Arafa M, Agarwal A, Majzoub A, Panner Selvam MK, Baskaran S, Henkel R, et al. Efficacy of antioxidant supplementation on conventional and advanced sperm function tests in patients with idiopathic male infertility. Antioxidants (Basel) 2020;9:219
- Busetto GM, Agarwal A, Virmani A, Antonini G, Ragonesi G, Del Giudice F, et al. Effect of metabolic and antioxidant supplementation on sperm parameters in oligo-astheno-teratozoospermia, with and without varicocele: a double-blind placebo-controlled study. Andrologia. 2018;50:e12927
- Moher D, Liberati A, Tetzlaff J, Altman DG PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6:e1000097.
- Murray J, Farrington DP, Eisner MP. Drawing conclusions about causes from systematic reviews of risk factors: the Cambridge Quality Checklists. J Exp Criminol. 2009;5:1–23.
- Higgins JP, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, et al. Cochrane Bias Methods Group; Cochrane Statistical Methods Group. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928.
- Jadad AR, Moore RA, Carroll D, Jenkinson C, Reynolds DJ, Gavaghan DJ, et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials. 1996;17:1–12.
- Schulz KF, Altman DG, Moher D CONSORT Group. CONSORT 2010 statement: updated guidelines for reporting parallel group randomised trials. BMJ. 2010;340:c332.
- Roseff SJ. Improvement in sperm quality and function with French maritime pine tree bark extract. J Reprod Med. 2002;47:821–824.
- Tremellen K, Miari G, Froiland D, Thompson J. A randomised control trial examining the effect of an antioxidant (Menevit) on pregnancy outcome during IVF-ICSI treatment. Aust N Z J Obstet Gynaecol. 2007;47:216–221.
- Tunc O, Thompson J, Tremellen K. Improvement in sperm DNA quality using an oral antioxidant therapy. Reprod Biomed Online. 2009;18:761–768.
- Shukla KK, Mahdi AA, Ahmad MK, Jaiswar SP, Shankwar SN, Tiwari SC. Mucuna pruriens reduces stress and improves the quality of semen in infertile men. Evid Based Complement Alternat Med. 2010;7:137–144.
- Bejarano I, Monllor F, Marchena AM, Ortiz A, Lozano G, Jiménez MI, et al. Exogenous melatonin supplementation prevents oxidative stress-evoked DNA damage in human spermatozoa. J Pineal Res. 2014;57:333–339.
- Martínez-Soto JC, Domingo JC, Cordobilla B, Nicolás M, Fernández L, Albero P, et al. Dietary supplementation with docosahexaenoic acid (DHA) improves seminal antioxidant status and decreases sperm DNA fragmentation. Syst Biol Reprod Med. 2016;62:387–395.
- Hosseini J, Mardi Mamaghani A, Hosseinifar H, Sadighi Gilani MA, Dadkhah F, Sepidarkish M. The influence of ginger (Zingiber officinale) on human sperm quality and DNA fragmentation: a double-blind randomized clinical trial. Int J Reprod Biomed. 2016;14:533–540.
- Stenqvist A, Oleszczuk K, Leijonhufvud I, Giwercman A. Impact of antioxidant treatment on DNA fragmentation index: a double-blind placebo-controlled randomized trial. Andrology. 2018;6:811–816.
- Ahmad MK, Mahdi AA, Shukla KK, Islam N, Jaiswar SP, Ahmad S. Effect of Mucuna pruriens on semen profile and biochemical parameters in seminal plasma of infertile men. Fertil Steril. 2008;90:627–635.
- Alizadeh F, Javadi M, Karami AA, Gholaminejad F, Kavianpour M, Haghighian HK. Curcumin nanomicelle improves semen parameters, oxidative stress, inflammatory biomarkers, and reproductive hormones in infertile men: a randomized clinical trial. Phytother Res. 2018;32:514–521.
- Salehi P, Zahra Shahrokhi S, Kamran T, Ajami A, Taghiyar S, Reza Deemeh M. Effect of antioxidant therapy on the sperm DNA integrity improvement; a longitudinal cohort study. Int J Reprod Biomed. 2019;17:99–106.
- Hasoon MA. Using of the L-arginine and co-enzyme Q10 shows improvement of the male subfertility. IJDDT. 2019;9:544–551.
- Nurmawati D, Hinting A, Sudjarwo Astaxanthin improves erythrocyte sedimentation rate (ESR), Malondialdehyde (MDA), 8-hydroxydeoxyguanosine (8-OH-Dg) levels, and semen quality in human sperm. IJSTR. 2020;9:6896–6903.
- Hadi AM, Abbass YI, Yadgar MA. The impact of L-carnitine supplement on semen variables and the levels of sexual hormones (serum LH, FSH, testosterone, and inhibin) in males with infertility. Medico Leg Update. 2020;20:772–776.
- Schisterman EF, Sjaarda LA, Clemons T, Carrell DT, Perkins NJ, Johnstone E, et al. Effect of folic acid and zinc supplementation in men on semen quality and live birth among couples undergoing infertility treatment: a randomized clinical trial. JAMA. 2020;323:35–48.
- Comhaire FH, Christophe AB, Zalata AA, Dhooge WS, Mahmoud AM, Depuydt CE. The effects of combined conventional treatment, oral antioxidants and essential fatty acids on sperm biology in subfertile men. Prostaglandins Leukot Essent Fatty Acids. 2000;63:159–165.
- Paradiso Galatioto G, Gravina GL, Angelozzi G, Sacchetti A, Innominato PF, Pace G, et al. May antioxidant therapy improve sperm parameters of men with persistent oligospermia after retrograde embolization for varicocele? World J Urol. 2008;26:97–102.
- Oliva A, Dotta A, Multigner L. Pentoxifylline and antioxidants improve sperm quality in male patients with varicocele. Fertil Steril. 2009;91(4 Suppl):1536–1539.
- Festa R, Giacchi E, Raimondo S, Tiano L, Zuccarelli P, Silvestrini A, et al. Coenzyme Q10 supplementation in infertile men with low-grade varicocele: an open, uncontrolled pilot study. Andrologia. 2014;46:805–807.
- Pourmand G, Movahedin M, Dehghani S, Mehrsai A, Ahmadi A, Pourhosein M, et al. Does L-carnitine therapy add any extra benefit to standard inguinal varicocelectomy in terms of deoxyribonucleic acid damage or sperm quality factor indices: a randomized study. Urology. 2014;84:821–825.
- Nematollahi-Mahani SN, Azizollahi GH, Baneshi MR, Safari Z, Azizollahi S. Effect of folic acid and zinc sulphate on endocrine parameters and seminal antioxidant level after varicocelectomy. Andrologia. 2014;46:240–245.
- Cyrus A, Kabir A, Goodarzi D, Moghimi M. The effect of adjuvant vitamin C after varicocele surgery on sperm quality and quantity in infertile men: a double blind placebo controlled clinical trial. Int Braz J Urol. 2015;41:230–238.
- Gual-Frau J, Abad C, Amengual MJ, Hannaoui N, Checa MA, Ribas-Maynou J, et al. Oral antioxidant treatment partly improves integrity of human sperm DNA in infertile grade I varicocele patients. Hum Fertil (Camb) 2015;18:225–229.
- Barekat F, Tavalaee M, Deemeh MR, Bahreinian M, Azadi L, Abbasi H, et al. A preliminary study: N-acetyl-L-cysteine improves semen quality following varicocelectomy. Int J Fertil Steril. 2016;10:120–126.
- K?z?lay F, Altay B. Evaluation of the effects of antioxidant treatment on sperm parameters and pregnancy rates in infertile patients after varicocelectomy: a randomized controlled trial. Int J Impot Res. 2019;31:424–431.
- Ardestani Zadeh A, Arab D, Kia NS, Heshmati S, Amirkhalili SN. The role of vitamin E - selenium - folic acid supplementation in improving sperm parameters after varicocelectomy: a randomized clinical trial. Urol J. 2019;16:495–500.
- Suleiman SA, Ali ME, Zaki ZM, el-Malik EM, Nasr MA. Lipid peroxidation and human sperm motility: protective role of vitamin E. J Androl. 1996;17:530–537.
- Rolf C, Cooper TG, Yeung CH, Nieschlag E. Antioxidant treatment of patients with asthenozoospermia or moderate oligoasthenozoospermia with high-dose vitamin C and vitamin E: a randomized, placebo-controlled, double-blind study. Hum Reprod. 1999;14:1028–1033.
- Vicari E, Calogero AE. Effects of treatment with carnitines in infertile patients with prostato-vesiculo-epididymitis. Hum Reprod. 2001;16:2338–2342.
- Suzuki M, Kurabayashi T, Yamamoto Y, Fujita K, Tanaka K. Effects of antioxidant treatment in oligozoospermic and asthenozoospermic men. J Reprod Med. 2003;48:707–712.
- Balercia G, Mosca F, Mantero F, Boscaro M, Mancini A, Ricciardo-Lamonica G, et al. Coenzyme Q(10) supplementation in infertile men with idiopathic asthenozoospermia: an open, uncontrolled pilot study. Fertil Steril. 2004;81:93–98.
- Piomboni P, Gambera L, Serafini F, Campanella G, Morgante G, De Leo V. Sperm quality improvement after natural antioxidant treatment of asthenoteratospermic men with leukocytospermia. Asian J Androl. 2008;10:201–206.
- Ghanem H, Shaeer O, El-Segini A. Combination clomiphene citrate and antioxidant therapy for idiopathic male infertility: a randomized controlled trial. Fertil Steril. 2010;93:2232–2235.
- Ahmad MK, Mahdi AA, Shukla KK, Islam N, Rajender S, Madhukar D, et al. Withania somnifera improves semen quality by regulating reproductive hormone levels and oxidative stress in seminal plasma of infertile males. Fertil Steril. 2010;94:989–996.
- Nadjarzadeh A, Sadeghi MR, Amirjannati N, Vafa MR, Motevalian SA, Gohari MR, et al. Coenzyme Q10 improves seminal oxidative defense but does not affect on semen parameters in idiopathic oligoasthenoteratozoospermia: a randomized double-blind, placebo controlled trial. J Endocrinol Invest. 2011;34:e224–e228.
- Shukla KK, Mahdi AA, Mishra V, Rajender S, Sankhwar SN, Patel D, et al. Withania somnifera improves semen quality by combating oxidative stress and cell death and improving essential metal concentrations. Reprod Biomed Online. 2011;22:421–427.


 LOKESH. K*
LOKESH. K*
 Veerammal
Veerammal

 10.5281/zenodo.13905320
10.5281/zenodo.13905320