Abstract
The development of pharmaceuticals has Significantly transformed human health, for these drugs to be effective they must be free of impurities and administered correctly. over time various chemical and instrumental methods have been developed to accurately assess drugs. Impurities can arise during the manufacturing, transportation and storage processes, making it crucial to detect and quantify them to ensure Safety Analytical instrumentation and methods are vital for this purpose. This review emphasizes the importance of these techniques in evaluating drug quality, discussing various analytical approaches Such as titration chromatography, spectroscopy, electrophoresis and electrochemistry along with their applications in pharmaceutical analysis.
Keywords
Ibuprofen, Uv-spectrophotometer, analysis.
Introduction
Pharmaceutical analysis is traditionally defined as analytical chemistry dealing with drugs both as bulk drugs Substances & as pharmaceutical products or formulations. pharmaceutical analysis is a branch of practical chemistry that involves a Series of process for identification determination quantification and purification of substance separation of Components of a solution or mixture or determination of structure of chemical compounds. The substance may be in any of the dosage form. Pharmaceutical analysis in a codemia, as well as in the pharmaceutical products industry. other branches of analytical chemistry are also involved, viz. bioanalytical chemistry, daug metabolism Studies. and analytical biotechnology. Analytical chemists are involved many of the studies that constitute his documentation Substance quality and its Specifications are based on Substance analysis and that knowledge is later used for quality control (or) of the substance during full- Scale production produst analysis involves dealing with the various formulations used for toxicological Studies, clinical Studies and marketing.
Objectives:
Pharmaceutical analysis involves various techniques and methodologies to ensure the quality, safety, and efficacy of pharmaceutical products following are some objectives of pharmaceutical analysis :
Quality Control: To assess the purity, potency, and stability of pharmaceutical substances and products.
Identification: To determine the identity of active pharmaceutical ingredients (APIs) and excipients.
Quantification: To accurately measure the concentration of APIs in formulations.
Stability Testing: To evaluate the stability of pharmaceuticals under various conditions and determine shelf life.
Bioavailability Studies: To evaluate the rate and extent of drug absorption in the body
Pharmacokinetics: To study the absorption, distribution, metabolism, and excretion (ADME) of drugs in the body.
Introduction about quality assurance and quality control in pharmaceutical industry
Quality assurance (QA) and quality control (QC) are essential in the pharmaceutical industry to ensure products are safe and effective. Quality Assurance focuses on the systematic processes and procedures that prevent defects in manufacturing and ensure compliance with regulatory requirements. It involves developing guidelines, conducting audits, and training staff to maintain high-quality standards throughout the production process.Quality Control involves the testing and inspection of raw materials, in-process materials, and finished products to verify that they meet predefined specifications. QC activities include laboratory testing, validation processes, and stability studies to ensure product consistency and reliability. Together, QA and QC work to protect public health and improve the quality of pharmaceutical products.
CALIBRATION OF ANALYTICAL INSTRUMENTS:
Analytical instruments are crucial for assessing drugs and pharmaceuticals, ensuring their accuracy and reliability. To maintain their performance, regular calibration and validation are essential to confirm their suitability for the intended purpose.This article focuses on the calibration procedures for various basic analytical instruments used in laboratories and on an industrial scale in scientific institutions. It outlines the steps and precautions required for calibrating instruments such as HPLC, GC, GC-HS, pH Meters, Karl Fischer, Polarimeters, Conductivity Meters, Tablet Friabilators, Hardness Testers, Disintegration Test Apparatus, Dissolution Test Apparatus, Potentiometers, and UV Spectrophotometers.
Preparation of calibration:
- Preparation: Ensure the instrument is clean, functional, and in a controlled environment. Gather required calibration tools, such as certified reference materials, and review the instrument's manual for specific procedures.
- Calibration Process: Test the instrument using a standard reference, compare its readings with the known standard, and make necessary adjustments to minimize any errors. Verify the consistency of results by repeating the test.
- Documentation: Record the calibration details, including the date, standards used, results, and adjustments. Maintain logs for compliance and future reference.
SOP for UV-Spectrophotometer:
1. Ensure the UV Spectrophotometer is clean and properly calibrated,
2. Verify that the correct wavelength range is set.
3. Prepare a blank solution using the same solvent as the samples.
4. Place the blank in a clean cuvette.
5. Prepare samples according to the specified concentration.
6. Fill cuvettes with sample solutions, ensuring no air bubbles.
7. Turnon the UV Spectrophotometer and allow it to warm up for the recommended time.
8. Insert the blank cuvette and zero the instrument at the specified wavelength.
9. Replace the blank with the sample cuvette and record the absorbance.
10. Repeat for all samples.
11. Document absorbance values and analyze data as required.
12. Rinse cuvettes with appropriate solvents and dry them.
13.Turn off the UV Spectrophotometer after use.
ANALYSIS OF MONOGRAPH OF PHARMACEUTICALS
Ibuprofen
- IUPAC Name: 2-(4-Isobutylphenyl) propanoic acid
- Formula: C13H1802
- Molar Mass: 206.28 g/mol
- Density: 0.93 g/cm?3;
- Boiling Point: 157 °C
- Solubility: Soluble in alcohol, slightly soluble in water
- pH: 5.0 to 7.0 (for a 1% solution)
- Category: Nonsteroidal anti- inflammatory drug (NSAID)
- Description: Ibuprofen is a non-opioid analgesic and anti-inflammatory agent used to relieve pain, reduce inflammation, and lower fever. It is widely used as an over-the- counter medication for various conditions, including headaches, toothaches, menstrual cramps, and minor arthritis.
Test 1: Dissolve 0.1 g of ibuprofen in 10 ml of ethanol.Add 1-2 drops of concentrated sulfuric acid; a purple color develops, indicating the presence of ibuprofen.
Test 2: Dissolve 0.1 g of ibuprofen in 10 ml of water. Add 0.05 ml of ferric chloride solution; a violet color develops, confirming the presence of phenolic compounds in ibuprofen.
- Appearance: form White to off-white crystalline powder
Assay of Ibuprofen by using UV Spectrometer:
Preparation of standard solution:
Weigh accurately a quantity of ibuprofen powder equivalent to about 0.1 g of ibuprofen.
Add 20 ml of 0.1 M NaOH and dilute to 100 ml with distilled water. Shake the solution for 15 minutes. Pipette out 10 ml from the stock solution and dilute to 100 ml with distilled water (resulting in a 100 µg/ml solution). From this stock, take aliquots of 0.2 ml, 0.4 ml, 0.6 ml, 0.8 ml,and 1 ml, each diluted to 10 ml with distilled water. This results in solutions with concentrations of 2 µg/ml, 4 µg/ml, 6 µg/ml, 8 µg/ml, and 10 µg/ml, respectively. Measurement of Absorbance: Measure the absorbance of these solutions at a maximum wavelength of approximately 220 nm.
Preparation of Test Solution:
Calculate the average weight of five ibuprofen tablets, crush them to a fine powder.
Weigh accurately an amount of the powder equivalent to 0.1 g of ibuprofen.
Preparation of Stock Solution
Add 20 ml of 0.1 M NaOH and dilute to 100 ml with distilled water. Shake for 15 minutes.
Dilution for Measurement: Pipette out 10 ml from the stock solution and dilute to 100 ml with distilled water (resulting in a 100 µg/ml solution).
Take 1 ml of this resulting solution and dilute to 10 ml with distilled water, obtaining a solution with a concentration of 10 µg/ml.
Measure the absorbance of this test solution at the maximum wavelength of approximately 220 nm.
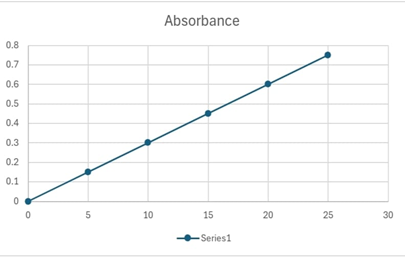
CONCLUSION:
The Analysis Indicates That The Ibuprofen Content In The Tablet Is Consistent With The Labeled Claim (Commonly 400 Mg Or 700 Mg Per Tablet).This Confirms The Quality Of The Tablet And Compliance With Pharmaceutical Standards.
After The Completion Of Report On Analyzing Ibuprofen Via Uv Spectrophotometer, We Understand
Information Of Handling Of Instruments To Get Knowledge Of Calibrations Of Different Analytical Instruments And Glassware. Learned The Importance Of Maintaining Accurate And Detailed Records Of Experiments, Results, And Calibrations For Quality Assurance. Understood The Knowledge About Pharmaceutical Analysis.
Understood The Various Unit Operations Used In Pharmaceutical Industries.
REFERENCES
- Pharmaceutical analysis bock reference Indian Pharmacopoeia
- Pharmaceutical Analysis instrumental methods by Dr. H. N. More
- INSTRUMENTAL METHODS OF CHEMICAL by ANALYSIS GURDEEP R. CHATWAL
- Pharmaceutical analysis practical book written by Dr Devala Rao
- Pharmaceutical Anallysis by Aushutosh Kar
- Mass Spectroscopy by James Barker
- Instrumental Methods of Chemical Analysis by B.K Sharma
- Spectrophotometric identification of Organic Compounds by Silverstein
- Text book of Pharmaceutical Analysis by Kenneth A. Connors
- Instrumental Methods of Analysis by Hober H. Willard, John A. Settl
- DR. A.V. KASTURE, DR.K.R MAHADIKDR.S.G.WADODKAR,DR.H.N.MORE pharmaceutical analysis instrumental method vol 2 published by nirali publication,25th edition 2019 page 1.1


 Dhok kajal *
Dhok kajal *


 10.5281/zenodo.14308629
10.5281/zenodo.14308629