Abstract
At various points throughout the life cycle of a pharmaceutical product, analytical procedures must be established. If these tasks are not appropriately simplified based on scientific knowledge and process understanding, it could result in an extremely expensive and time-consuming approach. The pharmaceutical industry is constantly looking for new guidelines or components to add to or replace the current components of the quality and risk management system The idea of Quality by Design was first introduced by renowned quality expert Joseph M. Juran. (QbD). Analytical method development, or AQbD, can be thought of as an extension of QbD. A methodical approach to development known as "Quality by Design" starts with predetermined objects and places a strong emphasis on process control, product and process understanding, and understanding .contemporary method approach The current review article's primary goal is to outline the various QbD processes while also addressing implementation-related issues. ATP (Analytical Target Profile), CP (Performance Attributes), MODR (Method Operable Design Region), Control Strategy, and Continual Method Improvement are all included in the creation of an analytical method
Keywords
Quality by design, Analytical QbD, Risk Assessment, Control Strategy
Introduction
The goal of pharmaceutical development is to create high-quality products and manufacturing processes that reliably produce the desired results. Scientific understanding is supported in the formulation of the design space, specifications, and production controls by the data and knowledge gathered from pharmaceutical development research and manufacturing experience. Data from research on pharmaceutical development can serve as a foundation for high-quality risk management. Understanding that products cannot be assessed for quality means that quality should be incorporated into the design from the beginning. It is important to view modifications to the formulation and manufacturing processes that occur during the development and lifecycle management phases as chances to learn more and strengthen the foundation of the design space. In a similar vein, adding pertinent information from tests with surprising findings can also be useful.1,2
Design space is proposed by the applicant and is subject to regulatory assessment and approval. Working within the design space is not considered as a change. Movement out of the design space is considered to be a change and would normally initiate a regulatory post approval change process. In all cases, the product should be designed to meet patients’ needs and the intended product performance. Strategies for product development vary from company to company and from product to product. The approach can also vary and should be outlined in the submission. An applicant might choose either an empirical approach or a more systematic approach to product development, or a combination of both.3 A more methodical approach to development, often known as quality by design, can involve, among other things, applying knowledge management (ICH Q10) across the product's lifespan, including prior knowledge, and the findings of studies conducted using design of experiments. It can also involve implementing quality risk management. Such a methodical approach can improve attaining the intended product quality and assist regulators in comprehending a company's business plan. Knowledge acquired during the course of the product lifecycle can be used to refresh understanding of the product and process.4 Prior to the adoption of Quality by Design (QbD) in the pharmaceutical business, in-process testing was conducted via off-line analysis, with product specifications serving as the primary control measure. This led to unforeseen problems during the scaling up process. Furthermore, additional permissions and revalidation came at a higher expense. However, as QbD is being implemented, the situation is shifting. The pharmaceutical business has recently begun to use a methodical approach to development that starts with predetermined goals and places a strong emphasis on understanding goods and processes in order to regulate processes.5
WHAT IS QUALITY BY DESIGN?
Planning, formulating formulas, and manufacturing processes are all incorporated into QbD to guarantee predetermined product requirements. The FDA unveiled a brand-new program called cGMP for the 21st Century: A Risk-Based Approach in 2002. Through this program, the FDA's regulations governing pharmaceutical quality were to be modernized, and a new regulatory framework emphasizing quality system and QbD risk management was to be established. The program has forced the industry to consider methods other than quality by testing (QbT) to guarantee the performance and quality of products. Understanding how process and formulation factors may impact product attributes is a crucial component of quality by design (QbD). This knowledge is necessary for the optimization of these parameters that follows, enabling online monitoring of these parameters during production. A well-understood product and process that reliably achieves its intended performance are the outcome of applying QbD concepts. The information gained throughout development can help establish appropriate process controls and enable the creation of a design space. The term "Analytical QbD" (AQbD) refers to the use of these similar QbD principles to the creation of analytical methodologies.6
Definition [ICH Q 8(R1)]
A systematic approach to development that begins with predefined objectives and emphasizes product and process understanding and process control, based on sound science and quality risk management.7
Application Of QbDs 8
- Analytical Research Development
Reduced variability in analytical attributes to increase method robustness and an advanced level of method comprehension for each crucial element with Method Operable Design Region will offer flexibility for method transfer from AR&D to QC.
- Manufacturing Plant & Quality Control
Detailed idea through model predicting how product will behave with changes in each CMAs and CPPS adjustable within design space along with other QbD tool
- Quality assurance
Through quality risk management during development, batch failures can be eliminated, deviations can be minimized, and expensive investigations can be avoided. This will make the estimation of variability or batch failure easier, faster, and more efficient for root cause analysis.
- Regulatory Affairs
The process of review and approval will become very simple and quick. Additionally, established and validated design space will offer regulatory flexibility for change management following approval.
ELEMENTS OF PHARMACEUTICAL QUALITY BY DESIGN9,10
As ICH guidelines defines the QbD for the pharmaceutical development. ICH Q8 defines the various elements of quality by design. These in combination with the enablers form the fundamental basis for the QbD approach to development. It involves the following key elements during pharmaceutical development
- Define the Quality Target Product Profile
- Identify the Quality Attributes
- Perform a Risk (Assessment) Analysis
- Determine the Critical Quality Attributes and Critical Process Parameters
- Determine the Design Space
- Identify a Control Strategy
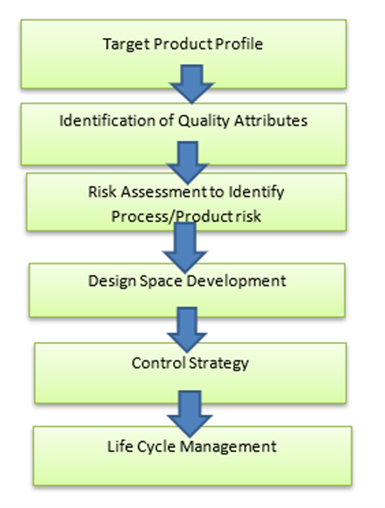
Fig no 1 : Elements of QbD
Quality Target Product Profile (QTPP)
A new guidance from the FDA defines a target product profile. A "prospective and dynamic summary of the quality characteristics of a drug product that ideally will be achieved to ensure that the desired quality, and thus the safety and efficacy of a drug product, is realized" is the definition of the target product profile (TPP). Drug product-quality criteria (e.g., sterility and purity) appropriate for the intended marketed product, as well as dosage form and route of administration, dosage form strength(s), and therapeutic moiety release or delivery and pharmacokinetic characteristics (e.g., dissolution and aerodynamic performance) appropriate to the drug product dosage form being developed. Within the QbD, the idea of TPP in this form and its use are new. An obvious continuation of TPP for product quality is the phrase Target Product Quality Profile (TPQP). The drug product needs to have these qualities in order to consistently provide the therapeutic benefit that the label promises. Formulation scientists are guided by the TPQP in developing formulation strategies and maintaining the efficiency and focus of their work. Identity, assay, dosage form, purity, and stability in the label are all related to TPQP.11
Analytical Target Profile (ATP)
Since QbD is a methodical approach to the design and development of products and processes, defining the aim or goal of the method is the first step in the process. The comprehension of the goods and process is emphasized during this time, and ATP paves the way for the creation of new methods. It outlines the strategy requirements for the intended to be measured technique requirements. The chromatographic method's typical objectives are the separation, measurement, and identification of the drug's active ingredient, impurity, or degradant. One considers impurity to be one of the critical quality attributes (CQA). Understanding earlier synthetic and manufacturing procedures as well as all other potential paths that could result in the encounter of contaminants would be beneficial when handling traces of impurities. The requirements for the procedure are as outlined in the ICH guidelines: accuracy, precision, robustness, and so on. Similar to the conventional approach, the QbD method also requires specific information about the analyte, such as its stability, Pka, PH, UV chromophore, and solubility. ATP can be tuned to produce the optimal technique, which supports these data-driven, rigorous method aims. This offers a framework for developing methods that facilitates additional planning. The ATP fully complies with the ICH guidelines. The sum of all performance requirements needed for the planned analytical application, which guide the method development process, is the analytical target profile. For each of the qualities listed in the management strategy, an ATP associate degree ATP would be developed. The Analytical Target Profile outlines the requirements for the procedure.12
Critical Quality Attributes (CQA)
First, factors that directly affect the goods' quality and safety are sorted out, and their possible influence on method development is investigated. A thorough understanding of the goods and methodology will be used to analyze CQA. A drug product's ability to hold impurities that could directly affect its quality and safety is regarded as a crucial quality attribute for the development of the HPLC method for that specific medicinal ingredient. Schweitzer et al. claim that measurable control over quality parameters, such as product definition, intermediate specification, and process control, can demonstrate safety and efficacy.13
Quality Risk Assessment
Scientific knowledge should be the foundation for any study of the risk to quality, as this ensures patient safety. Outlines methodical procedures for evaluating, managing, discussing, and reviewing quality risks. Covers product development, manufacture, distribution, and lifetime. The following are the methods listed in the ICH Q9 guideline.Failure Mode Effects Analysis (FMEA), Failure Mode, Effects and Criticality Analysis (FMECA), Fault Tree Analysis (FTA), Hazard Analysis and Critical Control Points (HACCP), Hazard Operability Analysis (HAZOP), Preliminary Hazard Analysis (PHA), Risk ranking and filtering, and Supporting Statistical Tools are the other five failure modes and effects analyses.14
Design Space
Design space is defined by ICH Q8 (R2) as the multidimensional combination and interaction of process parameters and input factors (such as material qualities) that have been shown to offer assurance of quality. Moving outside of the Design area is regarded as a change and would typically start a regulatory post-approval change process. Operating inside the Design space is not regarded as a change. The defined range of formulation characteristics and process parameters that have been shown to offer quality assurance is known as the design space. It creates the connection between manufacturing design and development. One unit operation, several unit operations, or the full process can all be built into a design space. The process of getting permission to operate inside a design area without requiring additional regulatory approval involves submitting the design space to the FDA. An established process understanding can be represented using a design space.15
Control Strategy
Management Approach Method performance and product quality are guaranteed by a planned set of controls for CMAs & CMVs\ that are obtained from current extensive method development during lab scale developmental stage. The control strategy is a comprehensive review of the methods used to ensure quality based on knowledge of the existing process and product.To ensure that the procedure stays in the state of control, this phase also involves data collecting and analysis, as well as eventual replication of refined experiments.16
Process Analytical Technology (PAT):
In order to ensure the quality of the end product, PAT is defined as "a system for designing, analysing , and controlling manufacturing through measurements, during processing of critical quality and performance attributes of raw and in-process materials and processes." "Improving understanding and control over the manufacturing process is consistent with our current drug quality system: quality should be built-in or by design; it cannot be tested into products." This is the stated purpose of PAT. The most important and crucial process factors found in the process define the design space. Characterization studies and the permissible bounds for them. The main emphasis of on-, in-, or at-line PAT applications is on these characteristics. Real-time process availability testing (PAT) evaluations have the potential to serve as the foundation for ongoing feedback loops and enhance process resilience. NIR is a helpful instrument for PAT and can be used for RTRT (Real Time Release Testing) since it can track several parameters like dissolution, polymorphism, blend homogeneity, granulation, and particle size.17,18
Advantages of AQbD19,20
- It offers a better degree of medication product quality assurance.
- It provides the pharmaceutical sector with efficiency and cost savings.
- It makes the sponsor more transparent and helps them comprehend the control approach needed to have the drug product approved and eventually go on sale.
- It creates a visible, logical, and predictable process for scaling up, validating, and commercializing
- It encourages innovation to address unmet medical needs.
- It decreases production costs and product rejects while boosting the effectiveness of pharmaceutical manufacturing processes.
- It reduces or gets rid of expensive fines, possible compliance actions, and medication recalls.
- This has prospects for ongoing enhancement.
- It increases the effectiveness of regulatory oversight:
- It simplifies regulatory procedures and production modifications following clearance.
- It concentrated more on CGMP inspections after approval.
- It increases the likelihood of first cycle approval.
FUTURE PERSPECTIVE21,22
The QbD will become habituated to a far greater degree in the future.
Simultaneously, it will also be utilized in the production area because, at the moment, it is frequently employed in the development area, where we typically employ event techniques in the methodology. It happens frequently that after we get to the assembly stage, things stall. This can be because we have to go into a plant that is currently in use for production, or it can be because they will not accept new innovations, especially in the PAT section. It's okay if we can maintain the current production within the constraints of our instruments. But once we've reached the more sophisticated and significant The QbD concept is implemented in tandem by certain regulatory agencies, such as the European Medicines Agency (EMA). In tandem, the EU has released a paper for "Real-Time Release." The EMA gladly accepts applications that deliberately exhibit quality. Applications largely supporting the concept of quality by design (QbD) are receiving some attention from the EMA. The goal of quality intentionally is to ensure that pharmaceutical standards are met through the application of analytical, risk-management, and applied mathematics techniques throughout the design, development, and manufacturing phases of the pharmaceutical industry. Regarding QbD implementation, U.S. authority/EMA cites ICH standards Q8, Q9, Q10, Q11, and Q12 The two projects that ICH is working on right now are "Q13-Continuous Manufacturing" and "Q14-Analytical technique Development.
REFERENCE
- Nishendu P. Nadpara*, Rakshit V. Thumar, Vidhi N. Kalola, Parula B. Patel, QUALITY BY DESIGN (QBD) : A COMPLETE REVIEW, Int. J. Pharm. Sci. Rev. Res., 17(2), 2012; n? 04, 20-28
- Woodcock J, The concept of pharmaceutical quality. American Pharmaceutical Review, 7(6), 2004, 10–15
- Q9: Quality Risk Management. ICH Harmonized TripartiteGuidelines. International Conference on Harmonization of Technical Requirements for Registration of Pharmaceuticalsfor Human Use, 2006.
- Q10:Pharmaceutical Quality System, ICH TripartiteGuidelines. International Conference on Harmonization ofTechnical Requirements for Registration of Pharmaceuticals for Human Use, 2007.
- Lionberger RA, Lee LS, Lee L, Raw A ,Yu LX, Quality bydesign: Concepts for ANDAs, The AAPS Journal, 10, 2008,268–276
- AV Ganorkar, KR Gupta. Analytical Quality by Design: A Mini Review. Biomed J Sci & Tech Res 1(6)- 2017. BJSTR.
- Q8 (R1): Pharmaceutical Development, Revision 1, ICH Harmonized Tripartite Guidelines, International Conference on Harmonization of Technical Requirements for Registration of Pharmaceuticals for Human Use, 2007.
- Asmita Mahapatra,Subramani Nainar Meyyanathan;Analyatical Quality By Design-A Review,JSS college of pharmacy ooty,Ind Res J Pharm & Sci,2020:7(2)
- Delasko, J., Cocchetto, D. M., & Burke, L. B. (2005). Target product profile: beginning drug development with the end in mind. Update.
- Food and Drug Administration CDER. (2007). Draft Guidance for Industry and Review Staff: Target Product Profile- A Strategic Development Tool (March 2007).
- Food and Drug Administration Office of Generic Drugs. (2006). Model Quality Overall Summary
- N. V. V. S. S. Raman, Useni ReddyMallu, and Hanimi Reddy Bapatu, Analytical Quality by Design Approach to Test Method Development and Validation in Drug Substance Manufacturing, Journal of chemistry, 2015
- Q9: Quality Risk Management. ICH Harmonized Tripartite Guidelines. International Conference on Harmonization of Technical Requirements for Registration of Pharmaceuticals for Human Use, 2006.
- Roy S (2012) “Quality by Design-Holistic concept of concept of building quality in pharmaceuticals”. Int. J Pham Biomed Res 3:100-108.
- Ranga S, Jaimini M, Sharma SK, Chauhan BS, Kumar A. A review on design of experiment. International Journal of Research and Reviews in Pharmacy and Applied science. 2013; 3(6): 867-82.
- 16.USP Validation and verification expert panel lifecycle managmen of analyatical procedure Method and development,procedure performance Qualification and procedure performance verification ,http://www.usp.org/sites/default/files/usp_pdf/EN/USPNF/revisions
- Burnham R. How to Select Design of Experiments Software. www.sixsigma.com. Accessed 10 March 2016.
- Yu LX, Amidon G, Khan MA, Hoag SW, Polli J, Raju GK, et al. Understanding pharmaceutical Quality by design. AAPS Journal. 2014; 16:771-83.
- . 19 Sangshetti JN, Zaheer Z, Mahaparale PR and Chitlange SS. Quality by design (QbD) in pharmaceuticals. Unique Publication, Aurangabad. 1st edition; 2015: 20
- 20 Gawade A, Chemate S and Kuchekar A. Pharmaceutical Quality by Design: A New Approach in Product Development. Research and Reviews: Journal of Pharmacy and Pharmaceutical Sciences. 2013; 2:5-11.
- 21.Kadam VR, Patil MP, Pawar VV, Kshirsagar S. A review on: Quality by design (QbD). Asian J Res Pharm Sci 2017;7:197-204.
- Aksu B, Ye?en G. Global regulatory perspectives on quality by design in pharma manufacturing. Pharm Qual Des 2019;2019:19-41.


 Wagh Sneha Sunil *
Wagh Sneha Sunil *
 R. B. Darade
R. B. Darade

 10.5281/zenodo.11235349
10.5281/zenodo.11235349