Wieke M. van Oostveen1 and Elizabeth C. M. de Lange 2,Imaging Techniques in Alzheimer’s Disease: A Review of Applications in Early Diagnosis and Longitudinal Monitoring https://doi.or/10.3390/ijms2204211 Mohammad KHANAHM DI 1, Dariush D.FARHUD 2,3, Maryam MALMIR Genetic of Alzheimer's Disease: A Narrative Review Article http://ijphtums.ac.ir Martha Clare Morris, S.D.1, Christy C. Tangney, Ph.D.2, Yamin Wang, Ph.D.1, FranM. Sacks, M.D.5, David A Bennett, M.D.3,4, and Neelum T. Aggarwal, M.D.3,4 MIND Diet Associated with Reduced Incidence of Alzheimer’s Disease DOI:10.1016/j.jalz.2014.11.00 Emily B. Buttona ,b, Je´roˆme Roberta,b, Tara M. Caffreya,b, Jianjia Fana,b, Wenchen Zhaoa,b, and Cheryl L. Wellington a ,b.HDL from an Alzheimer’s disease perspective Volume 30 Number 3 June 2019 www.co-lipidology.com Tan Sook Ling,1 Shanthini Chandrasegaran,1 Low Zhi Xuan,1 Tong Li Suan,1 Elaine Elaine,1 Durrgashini Visva Nathan,2 Yam Hok Chai,1 Baskaran Gunasekaran,1 and Shamala Salvamani The Potential Benefits of Nanotechnology in Treating Alzheimer’s Disease https://doi.org/10.1155/2021/5550938 Adam S. Fleisher, MD, MAS Introduction Behavioural Neurology 21 (2009) 1 DOI 10.3233/BEN-2009-024 Amir Abbas Tahami Monfared . Michael J. Byrnes Leigh Ann White . Quanwn Zhang Alzimer’s Disease : Epidemiology and Clinical Progression https://doi.org/10.1007/s40120-022-00338-8 Rishi S . Madnani Alzheimer’s disease: a mini-review for the clinician DOI 10.3389/fneur.2023.1178 Alzheimer's disease C. A. Lane, J. Hardy, J. M. Schott https://doi.org/10.1111/ene.13439 Christiane Reitz. Genetic diagnosis and prognosis of Alzheimer’s disease: challenges and opportunities doi:10.1586/14737159.2015.1002469. Armand S. Schachter & Kenneth L. Davis . Alzheimer's disease https://doi.org/10.31887/DCNS.2000.2.2/asschachter Rishi S. Madnani Chen Ma 1,2 , Fenfang Hong 1, and Shulong Yang 3,4,†Amyloidosis in Alzheimer’s Disease: Pathogeny,Etiology, and Related Therapeutic Directionshttps://doi.org/ 10.3390/molecules27041210Alzheimer’s disease: a mini- review for the clinician DOI 10.3389/fneur.2023.1178588 C. A. Lane J. Hardy, J M. Scott Alzheimer's disease https://doi.org/10.1111/ene.13439 Sahil Khan1, Kalyani H. Barve1and Maushmi S. Kumar1 Recent Advancements in Pathogenesis, Diagnostics and Treatment of Alzheimer’s Disease DOI: 10.2174/1570159X1866620052814242 Marcos Vinícius Ferreira Silva1, Cristina de Mello Gomide Loures1, Luan Carlos Vieira Alves1, Leonardo Cruz de Souza 2 , Karina Braga Gomes Borges1 and Maria das Graças Carvalho1 Alzheimer’s disease: risk factors and potentially protective measures http://doi.org/10.1186/s12929-019-0524-y Marta Crous-Bou1, Carolina Minguillón1, Nina Gramunt1,2 and José Luis Molinuevo1,2* Alzheimer’s disease prevention: from risk factors to early intervention DOI 10.1186/s13195-017-0297-z Bruno Duboisa1, Harald Hampela,b,1, Howard H. Feldmanc,1, Philip Scheltensd, Paul Aisene, Sandrine Andrieuf , Hovagim Bakardjiang, Habib Benalih, Lars Bertrami,j, Kaj Blennowk, Karl Broichl, Enrica Cavedob,m, Sebastian Crutchn, Jean-François Dartigueso, Charles Duyckaertsp, Stéphane Epelbauma, Giovanni B. Frisoniq,r , Serge Gauthiers, Remy Genthont, Alida A. Gouwf,u, Marie-Odile Habertv,w, David M. Holtzmanx,y, Miia Kivipeltoz,aa, Simone Listab, José-Luis Molinuevoab ,ac , Sid E. O’Bryantad, Gil D. Rabinoviciae, Christopher Roweaf, Stephen Sallowayag ,ah ,ai, Lon S. Schneideraj, Reisa Sperlingak,al , Marc Teichmanna, Maria C. Carrilloam, Jeffrey Cummingsan, Cliff R. Jack Jrao, Preclinical Alzheimer’s disease: Definition, natural history, and diagnostic criteria doi:10.1016/j.jalz.2016.02.002. Philip Scheltens, Bart De Strooper, Miia Kivipelto, Henne Holstege, Gael Chételat, Charlotte E Teunissen, Jeffrey Cummings, Wiesje M van der Flier Alzheimer’s disease doi:10.1016/S0140-6736(20)32205-4. William B. Granta,? and Steven M. Blakeb Diet’s Role in Modifying Risk of Alzheimer’s Disease: History and Present Understanding DOI 10.3233/JAD-230418 Giuseppe Pasqualetti a, Tony Thayanandan a, Paul Edison a,b,* Influence of genetic and cardiometabolic risk factors in Alzheimer’s disease http://doi.org/10.1016/j.arr.2022.101723 J Cummings 1, P Aisen 2, LG Apostolova 3, A Atri 4, S Salloway 5, M Weiner 6Aducanumab: Appropriate Use Recommendations doi: 10.14283/jpad.2021.41 Hong-Mei Zeng 1,2, Hua-Bo Han 3, Qi-Fang Zhang 4, Hua Bai Application of modern neuroimaging technology in the diagnosis and study of Alzheimer's disease doi: 10.4103/1673-5374.286957 Bruno Dubois1,2†, Christine A. F. von Arnim3*†, Nerida Burnie4,5, Sasha Bozeat6 and Jeffrey Cummings7 Biomarkers in Alzheimer’s disease: role in early and differential diagnosis and recognition of atypical variant https://doi.org/10.1186/s13195-023-01314- 6 Ajit Kumar Thakur,1 Parul Kamboj,1 Kritika Goswami,1 Karan Ahuja2 Pathophysiology and management of alzheimer’s disease: an overview DOI: 10.15406/japlr.2018.07.0023 Weili Xu, Camilla Ferrari and Hui-Xin Wang Epidemiology of Alzheimer's Disease http://dx.doi.org/10.5772/5439 Vasileios Mantzavinos 1,2, Athanasios Alexiou 1,Biomarkers for Alzheimer’s Disease Diagnosis doi: 10.2174/1567205014666170203125942 Zeinab Breijyeh 1, Rafik Karaman 1 ,Comprehensive Review on Alzheimer’s Disease: Causes and Treatment doi: 10.3390/molecules25245789 Elodie Passeri 1,2, Kamil Elkhoury 1, Margaretha Morsink 3, Kerensa Broersen 3, Michel Linder 1, Ali Tamayol 4, Catherine Malaplate 2, Frances T Yen 2,†, Elmira Arab- Tehrany 1,4,,† Alzheimer’s Disease: Treatment Strategies and Their Limitations doi: 10.3390/ijms232213954 Jeffrey L Cummings a,*, Gary Tong b, Clive Ballard c Treatment Combinations for Alzheimer’s Disease: Current and Future Pharmacotherap Options (doi: 10.3233/JAD- 180766) Sahil Khan1, Kalyani H. Barve1 and Maushmi S. Kumar1 ,Recent Advancements in Pathogenesis, Diagnostics and Treatment of Alzheimer’s Disease (DOI: 10.2174/1570159X18666200528142429 Bartosz Twarowski and Mariola Herbet *Alzheimer’s Disease—Patho mechanism, Diagnosis and Treatment: A Review https://doi.org/10.3390/ijms24076518 Judith Neugroschl, MD and Sophia Wang, MD Mount Sinai School of Medicine, New York, NY Alzheimer’s Disease: Diagnosis and Treatment Across the Spectrum of Disease Severity (doi :10.1002/msj.20279.) Jason Weller, Andrew Budson1,2 Current understanding of Alzheimer’s disease diagnosis and treatment (doi: 10.12688/f1000research.14506.1).


 G.V. Srivani*
G.V. Srivani*
 Thakur Vaishnavi
Thakur Vaishnavi
 Kothagadi Veena
Kothagadi Veena
 Poddutoori Manaswini
Poddutoori Manaswini
 Sangem Varsha
Sangem Varsha
 S. K. Kovid
S. K. Kovid
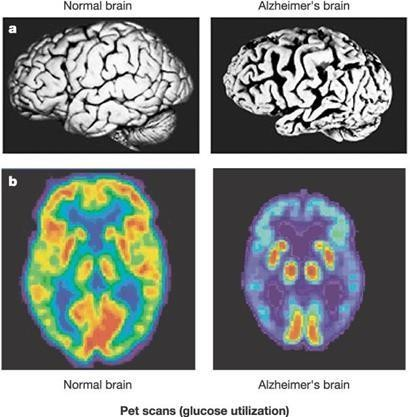
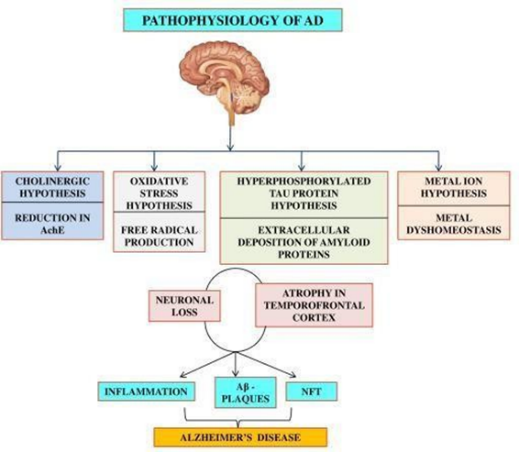
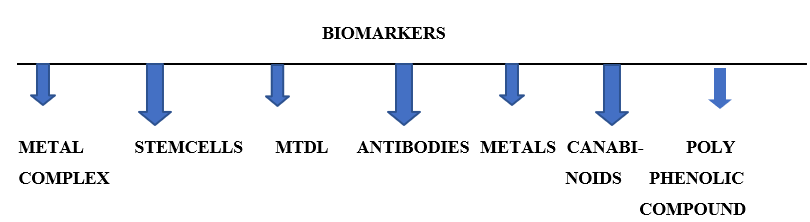
 10.5281/zenodo.14651398
10.5281/zenodo.14651398