Abstract
Antibiotics are the most prescribed medication globally, with India being a leading consumer. However, increased antibiotic consumption has led to an increase in antimicrobial resistance and adverse drug reactions (ADRs). In India; 6% of medical emergency department visits are drug related, with 45% of adverse events being fatal. The Indian Medical Association reports that 4, 00,000 death and 7, 20,000 adverse event occur annually. Despite 895 Adverse drug reaction monitoring Centre’s India contribution to global ADR database is only 2%. The aim of this review is to assess and analyze the most common anti-bacterial drug category associated with adverse drug reaction in Indian population. A total of 26 original articles were obtained based on keywords- adverse drug reaction, antibacterial agent, drug safety alert, drug related problem, Indian population; from electronic database PubMed, Google scholar, and Indian journal available on internet and publishing article in the field of adverse drug reaction. The study was undertaken to determine the pattern, predictability, preventability and effects of ADR due to Anti-microbial agents. In the study, a total of 2586 ADR were reported during 3 year study period; out of them anti-bacterial can contribute 300 ADR (38.65%). The study highlights the importance of Health care professional in monitoring and reporting ADR, highlighting the need for a pharmacovigilance centre to ensure safe and rational use of drug and prevent preventable ADRs. The study emphasizes the importance of early detection and treatment of Adverse Drug Reactions (ADR) in healthcare emphasizing the need for improved patient care and drug safety. It suggest that pharmacovigilance system, sensitization programs and regular prescription audits can help in reducing ADRs due to antibiotics.
Keywords
Adverse drug reaction, Indian Population, antibacterial agents, drug safety alert, Drug related problem
Introduction
Antibiotics are the most prescribed medication worldwide, India is among the leading consumer of antibiotics and its consumption increase from 3.2 billion defined daily dose in the year 2000 to 6.5 billion defined daily dose in 2015. Availability of antibiotics without prescription is another major issue in India therefore increased use of antibiotics is associated with an increase in antimicrobial resistance and an increased incidence of ADR(6). Every pharmaceutical drug that enters the market is expected to have some adverse effects when used by patients outside the clinical trial settings. Adverse drug reactions (ADRs) are a major cause of morbidity and place a substantial unnecessary economic burden on patients as well as on the healthcare system (1). An adverse drug reaction as defined by WHO is “a response to a drug that is noxious and unintended and that occurs at doses normally used in human for prophylaxis, diagnosis, or therapy of disease or for the modification of physiologic function. In India 6% visit to the medical emergency departments are drug related; incidence of adverse event is 4% and death due to adverse event is 14%; in them adverse drug reaction accounts for 45% of all adverse drug event with seriousness of 6.7% and 0.32?ing fatal. Results from a study conducted at one of the Adverse drug reaction monitoring center in south India shows that 0.7?R leads to hospital admission, 3.7?R occur in hospitalized patients with 1.8?ing fatal.(6). >50% of hospitalized patient and >70% of ICU patients receive antibiotics for therapy or prophylaxis of infection; which leads to 35-40% of ADR with unpredictability (1). According to Indian medical association report 2016, in India 4,00,000 death occur due to ADR and 7,20,000 adverse events per annum. On 6th January, 2016 at CDSCO, New Delhi, Indian medical association comes forward and took initiative to work together with PvPi-NCC for promoting patient safety and ADR monitoring in the country. To monitor drug safety, the WHO started programme for international drug monitoring in 1968; involving 10 countries with established national reporting centre. For enhancing pharmacovigilance practices in developing countries; in 2010 WHO collaborate with Indian pharmacopoeia commission with a nationwide ADR monitoring program called PvPi (pharmacovigilance program of India) (4). Pharmacovigilance is defined as the science and activities relating to the detection, assessment, understanding, and prevention of adverse effect or any other drug related problem. India has about nearly 895 AMC centre; these AMC centre collect ADR data from the patients and send it to the National coordinating centre, NCC further sends data to UMC-WHO. In spite of the establishment of 895 AMC; India contribution in the global ADR database is 2% only (4). Antibiotics are most widely misused drug in developing countries in the form of over the counter, self-medication and irrational prescription. There is an urgent need to create awareness among the physicians about detection, reporting, management, and prevention of an ADR. The objective of this review is to assess and analyze the most common antibacterial drug category associated with adverse drug reaction in Indian population.
METHODOLOGY:
A total of 26 original article were obtained based on keywords- Adverse drug reaction, Indian Population, antibacterial agent, drug safety alert, drug related problem; from electronic database PubMed, Google scholar, and Indian journal available on internet and publishing article in the field of adverse drug reaction. Studies publish from 2015 onwards until 31st march, 2023 with only English language article were considered. Finally based on the given below inclusion and exclusion criteria 12 articles were taken into consideration; on these article, analysis was made and the result was outlined and presented in the form of table.

Figure1-: PRISMA FLOWCHART [Studies were included as per the selection criteria]

RESULT:
A retrospective observational study conducted at “Banglore medical college and research institute”, Karnataka, from 2012-2015 under the PvPi. Individual patient data were collected as per the CDSCO form, ADR was assessed and analyzed using ADR assessment tools. A total of 505 ADR were reported; among them antimicrobials can contribute 100 ADR (19.8%); with male predominance (58%) in the age group of 21-40 years. Cutaneous ADR accounted for (58%) with most common clinical manifestation of Maculopapular rash (46%) and then gastrointestinal disorder (35%) coming in the second by AMA. Analysis of ADR according to specific anti-microbial agent (Antibiotic) drug class reveals that cephalosporins accounted for the higher incidence with 35%; then followed by Fluoroquinolones (21%), penicillin (16%), anti-tubercular (14%) and macrolides (11%). The ADR assessment tools reveal that 78% of ADR were of probable causality, moderate severity in 70%, unpredictability in 67% and preventability in 5%. The study concludes that computerized prescribing; monitoring and early recognition is helpful in reducing the incidence and burden of adverse drug reaction.[1] [Table-: 2,3] [Figure-:2,3,4] In a tertiary care teaching hospital Lucknow, Uttar Pradesh; a retrospective observational study was conducted from September 2017 to February 2019 with the aim of understanding the pattern and occurrence of ADR in order to enhance the safety of patient health. A total of 207 ADR were reported among them penicillin contribute maximum number of 6.2?R then followed by cephalosporin 5?R. Males were more prone to ADR than female and the age group 41-50 years accounts 21.25?R. Cutaneous ADR accounts 44.44?R with most common clinical manifestation of skin rashes (66%) and itching (17.3%) comes at second. Based on causality maximum 94.2?R were probable, moderate severity in 53.62% and 97.58?R were non-serious in nature. Rawlings and Thompson criteria of ADR classification reveal 74.87?R belong to type-A category. This study concludes that development of ADR was mainly based on patient age and route of drug administration. There is a need to create awareness among healthcare professional for the establishment of an effective and robust pharmacovigilance system in India. [2] [Table-: 2, 3] [Figure-: 2, 3] A study conducted at Government medical college, Miraj, Maharashtra; 258 ICSR (individual case safety report) were analyzed during the period of July 2015 to June 2016; to determine the pattern, causality, severity of ADR; reported with the use of AMA. The ADR was assessed and analyzed using ADR assessment tools (WHO-UMC & Hartwig Seigel scale).Out of total 258 ADR; antimicrobials(Antibiotic) accounts for 151 ADR, with antiretroviral 21.67% (31ADR), ceftriaxone comes at second 14.68% (21 ADR) then followed by II line anti-T.B. (9.07%), and F.Q. (8.39%). 90 out of 151 ICSR belong to age group 21-40 yrs. CTCAE (common terminology criteria for adverse event) shows skin to be the most affected organ system (41.05%) with Maculopapular rash and fever with chills. WHO-UMC scale reveals possible causative relationship in 50.99?R with moderate severity in 73.42?R. The study concludes that anti-microbial (Antibiotic) are responsible for significant percentage of side effect. To conquer this problem numerous regional AMC have been set up around the nation under PvPi. [3] [Table-: 2,3] [Figure-: 2,3] A retrospective observational study was performed at Chirayu medical college and hospital, Bhopal and data were collected from March 2016 to December 2022. A total of 111 drugs were involved in causing 144 ADR. Antibiotics is the main culprit drug category causing 18% (26) ADR. Cutaneous ADR accounted for 41% (60) ADR with most common clinical manifestation of acute generalized exanthematous pustulosis and skin hyperpigmentation 12?R each. The study concludes that due to irrational use; antimicrobial (Antibiotic) was associated with more ADR in middle low income countries as compared to high income countries. More efforts are needed to motivate and create awareness among healthcare professionals and patients to spontaneously report adverse events which can ultimately strengthen the pharmacovigilance system in India for the safe and effective use of medicines.[4] [Table-: 2,3] In a tertiary care teaching hospital Maharashtra, a retrospective observational study was conducted and previous data from 2017 to 2021 were analyzed to determine the pattern of ADR and impact of sensitization program on reporting rate. A total of 104 ADR were reported; adults in the age group (18-65 yrs.) contribute 78.8?R. Female (58.6%) were more prone to develop ADR than male (42.3%). Antimicrobial (Antibiotic) accounts maximum 22.11?R then followed by anti-tubercular (20.19%) and vaccines (12.4%) ADR. Among the antimicrobials (Antibiotic) Ceftriaxone causes maximum 34.7% (8) ADR. Completeness score of 2021 v/s 2018 was 9.84 and 8.79 respectively, which shows a positive trend in ADR reporting. The study concludes that increasing awareness through sensitization program can play a major role in improving quality and quantity of ADR reporting.[5] [Table-: 2,3]
To analyze and assess antimicrobial (Antibiotic) associated adverse drug reaction; a 4 year retrospective study conducted at PT JNM medical college and associated B.R. Ambedkar hospital, Raipur Chattisgarh, India. A total of 762 ADR were reported, among them anti-microbial were responsible for 54.33?R. Analysis of ADR according to specific antimicrobial drug class reveals Cephalosporin accounted 25.12% then followed by antiviral 21.73% and penicillin 18.84?R respectively. Adults in the age group (19-65) years accounted for 83.33?R with higher incidences in female patient 53.62?R. Skin and subcutaneous tissue disorder accounted 52.56?R with gastro-intestinal disorder coming at second 11.19%. There was a possible causative relationship in 29.71% and seriousness in 6.03?R. This study concludes that periodic analysis of antibiotic safety data will help to formulate guidelines and policies to prevent or reduce the frequency and severity of antibiotic associated adverse drug reaction.[6] [Table-: 2,3] [Figure-: 2,3] A prospective observational 7 month study conducted at PVS hospital Calicut, India. A total of 30 ADR were reported in 3074 inpatients; out of the ADR reported 23.33% were associated with antibiotics. Incidence rate in geriatric patient was higher 50% then followed by adults and pediatric patient. The study found that 80% of the ADR were type-A reactions, 3.33% was severe and 63.33% of ADR were not preventable. The study concludes that establishment of pharmacovigilance centre involving clinical pharmacist can help in reporting and reducing adverse drug reactions. Overuse and misuse of antibiotics leads to increase in prevalence of resistant pathogen and adverse drug reaction among users.[7] [Table-: 2,3] [Figure-: 3,4] In a tertiary care teaching hospital North India, a retrospective observational study was carried out from November 2010 – November 2013 to evaluate and analyze ADR due to anti-microbial (antibiotic). A total of 2586 ADR were reported during 3 year study period. Out of them 392 ADR occur as a result of antibiotics. Ceftriaxone accounts maximum 35.71% (140) ADR, then followed by ciprofloxacin 2.29?R. Most common organ system involved was dermatological (47.77%) adverse drug reaction. It was found that rash (24.28%) was the leading symptoms associated with ceftriaxone, then followed by diarrhea (12.14%), gastritis (12.85%), epigastric pain and hypotension (7.85%) ADR each. Male population was more prone to develop (65.55%) adverse drug reaction as compared to female (35.45%). Based on causality maximum (73.98%) ADR were probable, (6.88%) severe, and (7.14%) ADR were serious in nature. Analysis according to ABC type classification shows maximum (65.06%) ADR as type A reaction. The study concludes that polypharmacy, irrational drug prescription, advancing age are some risk factor which increases the incidences of adverse drug event. Pharmacologist along with physician can correlate and manage ADR promptly in the interest of patient safety.[8] [Table-: 2,3] [Figure-: 2,3] The study at Rajiv Gandhi institute of medical sciences (RIMS) Kadapa district, Andhra Pradesh, India was carried out between July 2016 to June 2017; to determine causality, severity, preventability, and reporting of ADR using the WHO assessment tools and PvPi criteria at RIMS hospital, Andhra Pradesh. Out of 254 suspected ADR reported, antibiotic drug category can contribute 61 ADR (24.01%) with most common drug involving ceftriaxone 14 ADR (20%). Higher incidence of ADR was noted in female patient 129(50.7%). A predominance of gastrointestinal reaction was noted in 67ADR (26.4%). The occurrence of ADR was significantly higher in adult age group (18-65 yrs.) with 181 ADR. There was a possible causative relationship in 48% (124) ADR, seriousness in 65%, and preventability in 54.33%. The study concluded that ADR are more common with polypharmacy and irrational prescribing that directly leads to increase healthcare cost. By developing ADR reporting culture among healthcare professional would results in better patient care. [9] [Table-: 2,3] [Figure-: 2,3,4] A 3year retrospective observational study from March 2016 – February 2019 was conducted; to assess and analyze the incidence of adverse drug reaction due to antibiotics in a tertiary care hospital at “The Department of pharmacology BPS GMC, Sonepat, Haryana.” A total of 776 ADR were reported out of them anti-bacterial can contribute 300 ADR (38.65%). Among the anti-bacterial cephalosporin (51) and penicillin (51) were found to be major culprit to cause adverse event in those patient receiving combination therapy. Majority of the clinical manifestation were related to dermatological reaction (49.33%) (148), then followed by gastrointestinal reaction (33%) (99). Age distribution shows the higher incidences of ADR in adult age group (195) (65%). WHO-UMC scale reveals the possible causative relationship in 67.67 % of adverse event cases. The study concludes that because of self-medication; irrational prescription of antibiotics, serious adverse event and antibiotic resistance will occur. Establishing antibiotic policy and ensuring the best choice of antibiotics should be prescribed by physician can also prevent ADR. [10] [Table-: 2,3] [Figure-: 2] A retrospective observational study was conducted at adverse drug reaction monitoring centre KIMS, Bhuvaneshwar, Odisha from 2015-2020; to analyze and assess the Adverse drug reaction on the basis of pattern and causality. During a 5 year analysis out of 105 children maximum number of 43 ADR were reported in 0-5 year age group. Male population experience higher incidence of ADR (66) as compared to female (39). Antibiotics were the major drug category causing 69 ADR cases, among them ceftriaxone accounts maximum 17 ADR cases then followed by Amoxicillin (14 ADR) comes at second. Antibiotic induced reactions were predominantly found to cause rashes in 48.4?ses. Majority of the patient were found to have been prescribed multidrug therapy therefore there is a possible causative relationship in 34.5?ses and seriousness in 21?ses. The study concludes that beta-lactam antibiotics and cephalosporins were deemed to cause major ADR. Adopting the antibiotic stewardship policy and appropriate antibiotic use by physician or patient can prevent ADR and antimicrobial resistance. [11] [Table-: 2,3] [Figure-: 2] A prospective observational study conducted at tertiary government hospital, Ananthapuram from August 2018 to January 2019 for a period of 6 month to analyze the prescription pattern and also to assess ADR due to antibacterial. Among the 503 prescription which contained 2465 drugs, the average no. of antibiotics per prescription was (mean value 1.8). A total of 26 ADR were reported among them cephalosporin topped the list (38.4%) with maximum ADR reported with ceftriaxone (9 ADR) then followed by Piperacillin + Tazobactum (5 ADR). Majority of patients affected due to ADR were adult age group of 46-60 yrs. (34.6%) and gastrointestinal system (22, 84.6%) was most commonly affected organ system. ADR assessment reveals possible causative relationship in 15 ADR (57.6%). The study concludes that increase in number of drugs per prescription increase the risk of drug interaction, adverse drug reaction and slackened the patient quality of life. Clinical pharmacist along with physician can take part in rational prescribing, regular treatment regimen monitoring to reduce the incidences of ADR due to antimicrobial.[12] [Table-: 2,3] [Figure-: 2]
Tables and Figures:
Table 1.

Table 2-: Most commonly Antibiotics causing ADR

- (*) Antibiotics ADR percentage out of total ADR
- (**) Specific antibiotic drug class ADR percentage out of total antibiotic ADR
- (***) Individual antibiotic drug causing maximum ADR percentage out of specific drug class
- ADR= Adverse drug reaction
- %= Percentage
Table 3-: Most common affected organ system due to ADR
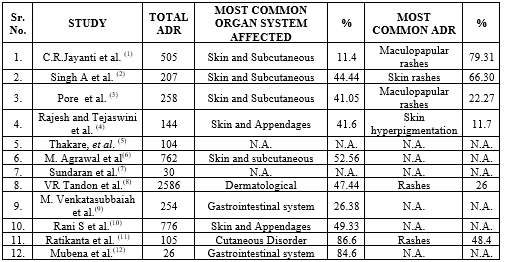
Figure 2-: Causality Assessment graph according to following study using WHO – UMC Scale.

Figure3-: Severity Assessment graph according to following study using Hartwig-Siegel Scale.

Figure 4-: Preventability Assessment graph according to following study using Schumock and Thornton Scale.

CONCLUSION:
Antibiotics which are commonly prescribed; their angiogram can help clinicians in early identification and management of adverse drug reaction; thus to improve the patient health related quality of life. Effective and robust pharmacovigilance systems are crucial for understanding the pattern and occurrence of ADRs for patient safety. Most ADRs were caused by beta-lactam antibacterial. The NCC-PvPI recommends early management of ADRs with dermatologists playing a crucial role in detailed drug history. Creating awareness among healthcare professionals and patients can strengthen India’s pharmacovigilance system. Males were more prone to ADR than female. Sensitization programs can enhance ADR reporting awareness and it also improve reporting quality. Antimicrobials are crucial for treating infections, and vigilance during prescription reduces ADRs, reducing morbidity, mortality, and healthcare system burden. The study identified age categories and drugs prone to ADRs, highlighting the need for future precautions and monitoring, emphasizing the importance of ADR monitoring units. The current study suggests that ADRs due to antimicrobials is a significant health problem. The study highlights the importance of HCPs in monitoring and reporting ADRs, highlighting the need for a pharmacovigilance center to ensure safe drug use and prevent preventable ADRs. Antibiotics which are commonly prescribed their angiogram can help clinicians to identify and manage ADRs early and ensuring patient quality of life. The study found that physicians rationally prescribe antibiotics, adhering to the National Essential Medicine List (86%). Regular prescription audits and awareness about polypharmacy can help to reduce ADRs caused by antibiotics.
REFERENCES:
- Jayanthi C, Reddy N. A profile of adverse drug reactions to antimicrobial agents at a tertiary care hospital.
- Singh A, Jain A, Soni M, Shukla P, Lahon J, Verma AK. Pattern of adverse drug reactions reported at a tertiary care teaching hospital in northern India. International journal of basic and clinical pharmacology. 2020;9(4):625. http://dx.doi.org/10.18203/2319-2003.ijbcp20201189
- Milind Pore S, Ramchandra Burute S, Dinkar Shinde A, Jaiprakash Ramanand S. Pattern of adverse drug reactions reported with use of antimicrobial drugs in a tertiary care hospital. Journal of young pharmacists: JYP. 2018;10(2):213–217. http://dx.doi.org/10.5530/jyp.2018.10.47
- Drug Safety Alerts Issued by the National Coordination Centre for Pharmacovigilance Programme of India: Current Practices and Future Recommendations.
- Thakare V, Patil A, Jain M, Rai V, Langade D. Adverse drug reactions reporting: Five years analysis from a teaching hospital. Journal of family medicine and primary care. 2022;11(11):7316–7321. http://dx.doi.org/10.4103/jfmpc.jfmpc_1043_22
- Agrawal M, Singh P, Joshi U. Antimicrobials associated adverse drug reaction profiling: a four years retrospective study (Pharmacovigilance study). Alexandria Journal of Medicine. 2021;57(1):177–187. http://dx.doi.org/10.1080/20905068.2021.1938425
- Spontaneous adverse drug reaction reporting in a tertiary care hospital in Calicut. Asian journal of pharmaceutical and clinical research
- Richa, Tandon VR, Sharma S, Khajuria V, Mahajan V, Gillani Z. Adverse drug reactions profile of antimicrobials: A 3-year experience, from a tertiary care teaching hospital of India. Indian journal of medical microbiology. 2015;33(3):393–400. http://dx.doi.org/10.4103/0255-0857.158564
- Venkatasubbaiah M, Dwarakanadha Reddy P, Satyanarayana SV. Analysis and reporting of adverse drug reactions at a tertiary care teaching hospital. Alexandria Journal of Medicine. 2018;54(4):597–603. http://dx.doi.org/10.1016/j.ajme.2018.10.005
- Rani S, Sharma B, Tarun, Kumar S, Saini R. Antibiotics-related adverse drug reactions at a tertiary care hospital in North India. International journal of basic and clinical pharmacology. 2019;8(10):2288. http://dx.doi.org/10.18203/2319-2003.ijbcp20194273
- Tripathy R, Das S, Das P, Mohakud NK, Das M. Adverse drug reactions in the Pediatric population: Findings from the adverse drug reaction Monitoring Center of a teaching hospital in Odisha (2015-2020). Cureus. 2021;13(11):e19424. http://dx.doi.org/10.7759/cureus.19424
- Prescription Pattern and Adverse Drug Reaction Monitoring Of Antibacterial Agents in a Tertiary Care Hospital


 Hari Singh Rathore* 1
Hari Singh Rathore* 1
 Mehul Soni 1
Mehul Soni 1
 Bhoomi Soni 1
Bhoomi Soni 1
 Mahendra Singh Rathore 3
Mahendra Singh Rathore 3








 10.5281/zenodo.11484791
10.5281/zenodo.11484791