Abstract
Introduction:
Pharmacogenomics plays a pivotal role in personalized medicine by optimizing treatment efficacy and safety. However, gaps in education and awareness among health care professionals hinder their integration into clinical practice.
Methods:
This observational study assessed the current state of education and awareness of pharmacogenomics among healthcare professionals. A structured survey targeting physicians, pharmacists, and nurses was conducted to assess their knowledge, attitudes, and practices regarding pharmacogenomics.
Results:
The findings revealed significant gaps in the understanding and utilization of pharmacogenomic principles among healthcare professionals. Limited integration into the curricula and insufficient training opportunities have contributed to these gaps.
Conclusion:
Addressing barriers to education and awareness is crucial for advancing the implementation of pharmacogenomics in clinical practice. Strategies, such as integrating pharmacogenomics into healthcare curricula and providing ongoing professional development, are essential for improving patient care and treatment outcomes.
Keywords
Education, awareness, pharmacogenomics, personalized medicine, healthcare professionals, curriculum integration.
Introduction
A. Definition of pharmacogenomics.
Pharmacogenomics, an interdisciplinary discipline at the intersection of pharmacology and genomics, investigates how an individual's genetic makeup influences their response to medications. It seeks to unravel the genetic variations that affect drug metabolism, efficacy, and safety[1]. Understanding these genetic factors will empower healthcare professionals to tailor drug treatment plans, optimize therapeutic outcomes, and minimize the risk of adverse reactions. At the forefront of medical advancements, pharmacogenomics has the potential to significantly improve patient outcomes and refine drug therapy. The rapid evolution of genomic technologies, coupled with increased accessibility to genetic testing, has accelerated the integration of pharmacogenomics into clinical practice. However, for the successful implementation of pharmacogenomics in healthcare, it is imperative to cultivate a well-informed healthcare workforce and raise awareness among the patient population [2]. Consequently, concerted efforts have been made to advance education and awareness of pharmacogenomics. Recent publications have substantially expanded our understanding of pharmacogenomics education and awareness by delving into a diverse range of topics including curriculum development, educational strategies, healthcare provider training, patient engagement, and ethical considerations. Notably, authoritative institutions such as the National Human Genome Research Institute (NHGRI), Clinical Pharmacogenetics Implementation Consortium (CPIC), Pharmacogenomics Knowledgebase (PharmGKB), and Food and Drug Administration (FDA) have played pivotal roles in disseminating knowledge and facilitating pharmacogenomics[3, 4]. Furthermore, findings from a nationwide survey conducted by the Personalized Medicine Coalition (PMC) revealed relatively low adoption of pharmacogenomic testing among physicians, underscoring the need for increased education and awareness among healthcare professionals[5].
By integrating the findings from these studies with credible references, this study seeks to emphasize the crucial significance of education and awareness within pharmacogenomics. This study aimed to offer practical suggestions for improving educational programs in this domain.
B. Importance of education and awareness in pharmacogenomics.
Education and awareness play a crucial role in the field of pharmacogenomics. Pharmacogenomic education and awareness are essential for personalized medicine, for several reasons.
1. Augmenting Healthcare Provider Expertise in Pharmacogenomics.
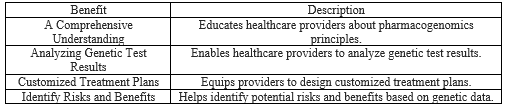
Education and awareness initiatives in pharmacogenomics play a pivotal role in equipping healthcare professionals, including physicians, pharmacists, and clinicians, with an extensive understanding of the principles and applications of pharmacogenomics in personalized medicine. These programs are instrumental in enabling healthcare providers to
a Interpreting Genetic Test Results:
Through participation in these programs, healthcare providers acquire the essential knowledge and skills required to effectively decipher genetic test results[6]. This education empowers them to meticulously analyze and interpret the genetic information provided by these tests. Consequently, they are adept at identifying specific genetic variations that may influence a patient's response to medication.
b Make Informed Medication Decisions:
This heightened understanding permits healthcare providers to make well-informed medication decisions by considering a patient's unique genetic profile and the potential interactions between medications and genetic variations. This judicious approach enables the prescription of tailored and personalized medications, thereby optimizing the treatment outcomes [7]
c Craft Customized Treatment Plans:
Furthermore, education and awareness programs in pharmacogenomics enable healthcare providers to craft personalized treatment plans based on genetic information [7]. By integrating a patient's genetic profile into the decision-making process, health care providers can gain a deeper understanding of the potential risks and benefits of various treatment options. They can adjust medication dosages or select alternative therapies that align more harmoniously with the patient's genetic makeup. This in turn minimizes the risk of adverse reactions and enhances the overall effectiveness of the treatment regimen.
2. Improving Patient Safety through Pharmacogenomic Understanding.
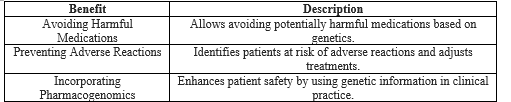
Pharmacogenomic insights can substantially influence the safety and efficacy of drug treatment. This can be achieved by unraveling how an individual's genetic variation influences drug metabolism, response, and the potential occurrence of adverse reactions. Consequently, healthcare providers can engage in more precise and individualized medication decision-making, thereby circumventing potentially harmful or ineffective treatment regimens. Proficiency in pharmacogenomics, facilitated by proper education and awareness, empowers healthcare professionals to
a Proactively identifying at-risk patients:
Education and awareness of pharmacogenomics arm healthcare professionals with the ability to proactively recognize patients who might be susceptible to adverse reactions or poor responses to certain medications based on their genetic profiles [7, 8]. Healthcare providers can fine-tune drug therapies by considering specific genetic variations that affect a patient's drug metabolism or response [9, 10]. This approach minimizes the likelihood of adverse events and optimizes therapeutic outcomes by customizing treatment plans for individual patients.
b Incorporating Pharmacogenomic Data into Clinical Practice:
The capacity to effectively utilize pharmacogenomic information in clinical practice hinges on healthcare professionals' understanding of its significanc[11]. Education and awareness programs play an instrumental role in endowing healthcare providers with the knowledge and skills essential for seamlessly integrating pharmacogenomic data into their decision-making processes. These programs provide healthcare professionals with the requisite tools for interpreting genetic test results, evaluating their implications for medication selection, and applying this information to enhance patient safet[12].
c Supporting Evidence:
Studies by Relling and Evans (2015) and Johnson et al. (2017) provide further evidence and insights into the substantial impact of education and awareness in pharmacogenomics on augmenting patient safety [13, 14]. By leveraging pharmacogenomic insights, healthcare providers can make informed decisions, reduce the risk of adverse events, and enhance patient outcome. This knowledge paves the way for a personalized medical approach that considers an individual's genetic makeup, thereby yielding safer and more effective treatments.
3. Ethical and Societal Considerations.
As pharmacogenomics is steadily being integrated into clinical practice, it has become imperative to educate healthcare providers on the ethical and societal implications of this transformative field, thereby ensuring the responsible and equitable implementation of personalized medicine. However, several key considerations in this domain require further investigation.
a Informed Consent:
One pivotal ethical consideration pertains to obtaining informed consent. Educating healthcare providers about pharmacogenomics equips them with means to effectively communicate with patients and secure informed consent for genetic testing. Patients should possess a clear understanding of the benefits, risks, and potential consequences of pharmacogenomic testing to enable them to make informed decisions regarding their participation in such endeavors[15].
b Privacy Concerns:
Privacy issues form another significant facet of pharmacogenomics. Healthcare providers must be aware of the concerns surrounding collection, storage, and utilization of genetic information. Healthcare providers can ensure that patient information is managed appropriately and confidentially by acquainting themselves with the ethical and legal frameworks governing data protection and patient privacy, healthcare providers can ensure that patient information is managed appropriately and confidentially[16].
c Equitable Access:
Equitable access to pharmacogenomic testing is paramount. Awareness programs can aid healthcare providers in comprehending the potential influence of socioeconomic factors on access to genetic testing[17]. By encouraging discussions and fostering awareness of these issues, healthcare providers can contribute to the realization of equal opportunities for all patients to benefit from pharmacogenomic testing regardless of their socioeconomic background.
d Responsible Navigation:
Education of healthcare providers regarding the ethical and societal implications of pharmacogenomics enables them to navigate these intricate matters in a responsible and ethical manner. By fostering open discussions and promoting awareness, healthcare providers can actively participate in the development of guidelines and policies that address ethical concerns and advocate equitable access to pharmacogenomic testing [18].
4. Fostering Collaborative Synergy.
Pharmacogenomic education plays an indispensable role in fostering effective communication and collaboration among diverse stakeholders, including researchers, educators, policymakers, and industry professionals [19]. Collaboration has emerged as a link between the multiple facets of the pharmacogenomic landscape.
a Standardized Protocols:
Collaborative endeavors are essential for the standardization of genetic testing protocols to ensure uniformity and reliability across healthcare settings. Educating stakeholders about the pivotal importance of standardized protocols stimulates collaboration in the formulation and implementation of guidelines that yield results characterized by accuracy and reproducibility [20].
b Integration into Electronic Health Records (EHRs):
The integration of pharmacogenomic data into electronic health records (EHRs) holds the promise of enabling healthcare providers to access and harness patient-specific pharmacogenomic data for well-informed medication decisions [21-23]. This integration necessitates a symphony of collaboration among healthcare providers, EHR vendors, and technology experts, who work to design interoperable systems proficient in capturing, storing, and retrieving pharmacogenomic data.
c Guideline and Policy Establishment:
Another critical arena where collaboration is imperative is the establishment of guidelines and policies [21-23]Collaboration among researchers, policymakers, and industry professionals ensures that these guidelines and policies resonate with the best interests of patients. This, in turn, promotes the responsible and equitable use of pharmacogenomic information [23, 24].
5. Enhancing Therapeutic Outcomes in Personalized Medicine.
Seamless integration of pharmacogenomic education and awareness is paramount for improving therapeutic outcomes in personalized medicine. This integration empowers health care providers to make well-informed decisions rooted in individual genetic variations, ultimately amplifying treatment efficacy and ensuring patient safety. The following key elements underscore the critical role of integration:
a Tailored treatment selection:
Understanding the influence of genetic variations on drug response equips healthcare providers with means to personalize treatment selection [25, 26]. By harnessing pharmacogenomic information, providers can expertly circumvent the prescription of ineffective or potentially harmful medications, thereby significantly increasing the likelihood of attaining optimal therapeutic outcomes[27].
b Precision Dosing:
Precision dosing is a vital aspect of pharmacogenomic education. Providers gain profound insights into drug metabolism and clearance, enabling them to calibrate dose adjustments that align precisely with patients' genetic profiles [28]. This personalized approach ensures optimal medication dosage and intensifies treatment efficacy, while minimizing the risk of toxicity[29].
c Adverse event mitigation
Pharmacogenomic education facilitates the identification of genetic markers associated with drug reactions. This enables providers to customize medication choices and effectively mitigate adverse events effectively [30]. The alignment of treatment plans with pharmacogenomic insights serves to substantially reduce the incidence of adverse reactions, ultimately enhancing therapeutic outcomes and patient safety[30].
d Treatment Response Monitoring:
The application of pharmacogenomic testing to treatment response monitoring is of paramount importance[31]. By vigilantly tracking the genetic markers associated with treatment response, providers can make well-timed adjustments to drug regimens, maximize therapeutic outcomes, and ensure long-term success of the treatment.
e Patient Engagement and Shared Decision-Making:
As elucidated by Khan et al. (2018), patient engagement and shared decision-making represent pivotal aspects of pharmacogenomic education [32]. Educating patients about how their genetic profile influences their medication response empowers them to make active treatment decisions[33]. This collaborative dynamic between provider and patient fosters engagement, amplifies treatment adherence, and ultimately magnifies therapeutic outcomes [34].
6. Preventing Treatment Failure.
Understanding the influence of genetic factors on treatment outcomes is imperative for effective healthcare. This section delves into pharmacogenomic strategies aimed at averting treatment failures by equipping healthcare professionals with essential knowledge and embracing personalized medicine tailored to individual genetic makeups.
a. Genetic insights:
Acquiring profound genetic insights into drug responses is critical for preventing treatment failure. The comprehension of genetic underpinnings not only enhances our capacity to predict and address potential treatment inefficiencies, but also serves as a linchpin for developing tailored therapeutic approaches to optimize patient outcomes[35].
b. Equipping Healthcare Professionals: Providing healthcare practitioners with pharmacogenomic knowledge enables them to discern patients who might not respond favorably to standard therapies because of genetic variations[36]. This paves the way for exploring alternative treatment options or making the necessary dosage adjustments.
c. Multiple Avenues of Prevention:
c.1. Tailored Medication Selection:
The Education and awareness of pharmacogenomics allow healthcare professionals to account for an individual's genetic variances when choosing medications and dosage levels, thereby enhancing patient outcomes. Averting Adverse Reactions: By identifying individuals at heightened risk of experiencing adverse drug reactions through pharmacogenomic testing and educating healthcare professionals about these genetic peculiarities, it becomes feasible to steer clear of detrimental medications while selecting more suitable alternatives[37].
c.2. Enhanced Treatment Responses:
The inclusion of pharmacogenomic data in treatment decisions resulted in notable improvements in response rates and overall treatment outcomes, as observed in patients with diverse medical conditions, suggesting that utilizing pharmacogenomic information when making treatment decisions led to significant enhancements in how patients responded to treatments and improved their overall outcomes across various medical conditions[38].
d. Supporting Personalized Medicine: Education and awareness in pharmacogenomics endorse the adoption of a personalized medical approach, in which treatments are finely tuned to an individual's unique genetic traits, optimize treatment plans, and diminish the likelihood of treatment failures[39].
e. Empowering Patients:
Educating patients on pharmacogenomics empowers them to actively engage in their treatment decisions, elevating medication adherence and consequently curbing treatment failure. These findings underscore the potential advantages of pharmacogenomic education and awareness in preventing treatment failure[40].
7. Enhancing Cost-Effectiveness.
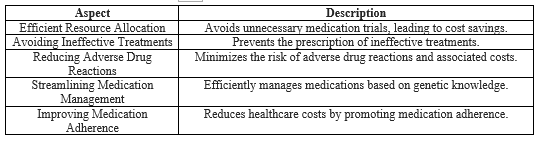
The integration of pharmacogenomic education into healthcare presents a promising avenue for optimizing resource utilization and streamlining healthcare costs. This section outlines how education and awareness in pharmacogenomics contribute significantly to enhancing cost-effectiveness through judicious resource allocation and minimizing futile medical interventions.
a Informed Resource Allocation:
The infusion of pharmacogenomic education and awareness significantly bolsters the cost-effectiveness of healthcare interventions. Educating healthcare professionals to discern patients who stand to benefit from pharmacogenomic testing, education, and awareness allows for optimized resource allocation and maximizes cost-effectiveness[41].
b Steering Clear of Unnecessary Trials:
This approach aids in steering clear superfluous medication trials and mitigating the likelihood of adverse drug reactions, ultimately leading to substantial cost savings[42].
c Essential Role of Pharmacogenomics:
Pharmacogenomics plays a pivotal role in cost-effectiveness by circumventing the prescription of ineffective treatments [43]. By identifying individuals who are unlikely to respond based on their genetic profiles, healthcare professionals can save time and resources otherwise spent on futile medication trials [44].
d Preventing Harm and Reducing Costs:
Pharmacogenomic testing has the added benefit of identifying patients at heightened risk of adverse drug reactions. This empowers healthcare professionals to avoid potentially harmful medications and prevents associated healthcare costs, such as emergency visits and hospitalization[45].
e Personalized Treatment Optimization:
The inclusion of education and awareness in pharmacogenomics is a cost-effective measure that reduces the trial-and-error approach to medication selection and dosing. By considering a patient's genetic profile, healthcare professionals can make well-informed decisions, thereby curtailing the expenses associated with ineffective treatments and additional medical visits [46].
f Patient-Centric Understanding:
Educating patients about pharmacogenomics nurtures their understanding of how their genetic makeup influences their response to medication. This, in turn, fosters adherence to personalized treatment plans, diminishing the need for supplementary interventions and the accompanying healthcare costs[47].
g Advancing Outcomes and Trimming Costs:
Through optimization of medication usage, avoidance of ineffective treatments, prevention of adverse drug reactions, minimization of trial-and-error approaches, and promotion of personalized medicine and medication adherence, educational and awareness initiatives substantiate improved outcomes and decreased healthcare costs[48]. In conclusion, education and awareness programs in pharmacogenomics empower healthcare providers to offer personalized care and improve quality and safety. Of treatment. Integrating this knowledge improves patient safety, reduces adverse reactions, and signifies a shift towards individualized healthcare. These initiatives foster collaboration, disseminate knowledge, innovate solutions, advance personalized medicine, and enhance therapeutic outcomes. Pharmacogenomic education prevents treatment failure by leveraging genetic insights and empowering both healthcare professionals and patients. It stands as a proactive force, improving patient care while significantly contributing to cost-effectiveness through informed resource allocation and patient-focused care. Overall, these educational strategies optimize healthcare resources, leading to a patient-centric cost-effective model (Table I).
Table I: Significance of Education and Awareness in Pharmacogenomics.




II. Pharmacogenomic Education.
A. Integration into healthcare curricula.
Integrating pharmacogenomic education into healthcare curricula is vital for effectively preparing healthcare professionals to utilize genetic information in patient care. By incorporating pharmacogenomics into training, future healthcare providers can enhance treatment outcomes, improve patient safety, and advance the precision medicine field[49]. Several overviews have considered the integration of pharmacogenomic education into healthcare curricula for professional benefit.
1. Medical Schools:
The American College of Medical Genetics and Genomics (ACMG, 2015) has provided guidelines for integrating genetics and genomics education into medical school curricula, including pharmacogenomics. It offers insights into curriculum content, teaching methods, and assessment strategies[50]. Integrating pharmacogenomic education into medical schools offers several professional benefits to future healthcare providers. Several strategies can be employed to integrate pharmacogenomics education into medical school curricula. These include developing dedicated pharmacogenomics courses, incorporating pharmacogenomics content into existing courses such as pharmacology or therapeutics, providing practical training on genetic testing and interpretation, and offering opportunities for experiential learning through case studies or clinical rotations[51]. Collaboration with genetic laboratories, research institutions, and industry partners can enrich the educational experience by providing access to resources, expertise, and real-world applications of pharmacogenomics. The key points are as follows:
a Enhanced Clinical Decision-Making:
Pharmacogenomics education teaches medical students how genetic variations influence drug response and metabolism. This understanding helps make informed decisions regarding medication selection, dosage adjustments, and potential drug-drug interactions[52, 53]. This will enable healthcare providers to practice precision medicine and tailor treatment plans according to the genetic profile of an individual patient.
b Enhancing Treatment Outcomes:
Pharmacogenomics education equips healthcare professionals with the knowledge and skills required to interpret and apply genetic information in clinical practice. Understanding how genetic variations affect drug responses enables healthcare providers to make informed treatment decisions and prescribe medications tailored to individual patients[54]. This personalized approach can improve treatment outcomes by maximizing drug efficacy and minimizing adverse reactions.
c Improved Patient Safety:
Adverse drug reactions are a significant concern in healthcare. Pharmacogenomics education empowers healthcare professionals to identify patients at increased risk of adverse reactions due to genetic factors. By considering genetic information, healthcare providers can proactively adjust medication regimens and dosages to mitigate these risks, improve patient safety, and reduce healthcare costs associated with adverse events[55]. This proactive approach reduces the likelihood of adverse reactions and improves patient outcomes.
d Advancing Precision Medicine:
Pharmacogenomics is a cornerstone of precision medicine, which seeks to deliver individualized treatments based on the unique characteristics of each patient. Integrating pharmacogenomic education into medical school curricula will enable healthcare professionals to embrace this paradigm shift in patient care. They can learn how to incorporate genetic testing into their practice, interpret genetic test results, and tailor treatment accordingly. By integrating pharmacogenomics into medical education, healthcare professionals can actively contribute to the advancement of precision medicine and its potential to revolutionize healthcare delivery[56]. It fosters a patient-centered approach and improves treatment efficacy.
e Efficient medication management:
Pharmacogenomic knowledge allows healthcare providers to streamline medication management. By understanding genetic variations in drug metabolism and responses, medical students can anticipate potential drug interactions and make informed decisions regarding medication selection, dosage adjustments, and treatment duration[57]. This has led to the development of more efficient and effective medication-management strategies.
f Keeping pace with scientific advances,
the field of pharmacogenomics is rapidly evolving, and discoveries and technologies are continually emerging. Integrating pharmacogenomic education into medical school curricula ensures that future healthcare professionals are equipped with the latest knowledge and skills in this field. By staying updated on scientific advancements, healthcare providers can provide state-of-the-art care, engage in ongoing research, and contribute to further advancements in pharmacogenomics[58]. This collaboration between academia and research institutions has driven advancements in the field and facilitated evidence-based practice.
g Professional Competitiveness:
With the growing importance of pharmacogenomics in healthcare, medical professionals with knowledge and skills in this field have a competitive edge. Integrating pharmacogenomics education in medical schools ensures that graduates are well-prepared to meet the evolving needs of personalized medicine and effectively contribute to the patient care field[59].
h Multidisciplinary Collaboration:
Pharmacogenomics involves collaboration among various healthcare professionals, including physicians, pharmacists, genetic counselors, and laboratory scientists. Integrating pharmacogenomic education into healthcare curricula promotes interdisciplinary collaboration and fosters a team-based approach to patient care. Healthcare professionals knowledgeable about pharmacogenomics can effectively communicate and collaborate with other experts in the field, leading to comprehensive and coordinated patient management[60, 61]. In conclusion, integrating pharmacogenomic education into medical school curricula is crucial for healthcare professionals to leverage genetic information in patient care. It enhances treatment outcomes, improves patient safety, supports the advancement of precision medicine, facilitates multidisciplinary collaboration, and ensures that healthcare providers are equipped with the latest knowledge and skills. By embracing pharmacogenomic education, medical schools can play a pivotal role in shaping the future of personalized medicine and optimizing patient care.
2. Pharmacy Schools:
The American Association of Colleges of Pharmacy (AACP) offers resources and guidelines for integrating pharmacogenomics into pharmacy school curricula. It includes educational modules, case studies, and best practices for teaching pharmacogenomics. Integrating pharmacogenomic education into pharmacy schools offers several professional benefits to future pharmacists. The key points are as follows:
a Improved Medication Selection and Management:
Pharmacogenomics education equips pharmacy students with the knowledge and skills to incorporate genetic information into their medication selection and management. This enables pharmacists to personalize treatment plans based on individual patients' genetic profiles and optimize therapeutic outcomes Pharmacists can use this knowledge to identify medications that are more likely to be effective and avoid those that may cause adverse reactions[62].
b Enhanced Medication Safety:
Pharmacogenomics education in pharmacy schools enhances medication safety by helping pharmacists identify patients at a higher risk of adverse drug reactions due to genetic variations. Pharmacists can use this information to adjust medication dosages, select alternative medications, or provide appropriate monitoring and counseling to minimize potential harm[63]. This proactive approach improves patient safety and reduces the incidence of adverse drug events.
c Facilitated medication counseling:
Pharmacogenomic knowledge allows pharmacists to provide targeted medication counseling to patients. This can explain how genetic variations can influence medication responses and their potential side effects. Pharmacists can also address patient concerns, promote medication adherence, and empower patients to make informed decisions regarding treatment[64, 65]. Pharmacogenomics education enhances pharmacists' ability to engage in effective patient counseling and support.
d Advancing Precision Medicine:
Integrating pharmacogenomics education into pharmacy schools’ advances in precision medicine. Pharmacists are crucial in implementing personalized medical approaches by integrating genetic information into medication management[66]. By embracing pharmacogenomics, future pharmacists can actively participate in interdisciplinary teams and collaborate with other healthcare providers to deliver individualized patient care.
e Professional Development and Competitiveness:
Knowledge and skills in pharmacogenomics provide graduates with a competitive advantage in the job market. With the increasing importance of personalized medicine, pharmacists proficient in pharmacogenomics are well positioned to meet the evolving needs of the healthcare system. Integrating pharmacogenomics education in pharmacy schools ensures that graduates are prepared for the expanding role of pharmacists in precision medicine[67].
3. Nursing Schools:
The International Council of Nurses (ICN, 2020) provides guidelines for incorporating genetics and genomics, including pharmacogenomics, into nursing curricula. This highlights the importance of genetic competency for nurses, and offers resources to educators. Integrating pharmacogenomic education into nursing schools offers several professional benefits to future nurses. The key points are as follows:
a. Enhanced Medication Safety:
Pharmacogenomics education equips nursing students with knowledge of genetic variations that affect drug responses. This knowledge enables nurses to identify patients at an increased risk of adverse drug reactions and helps them make informed decisions regarding medication selection, dosing, and monitoring[68]. By incorporating pharmacogenomics into their practice, nurses can contribute to improving medication safety and reducing adverse events.
b. Personalized Patient Care:
Pharmacogenomics education in nursing schools enables nurses to provide personalized patient care based on individual genetic profiles. By understanding how genetic variations influence medication responses, nurses can advocate individualized treatment plans that lead to better patient outcomes. This personalized approach enhances patient satisfaction and engagement in health care[69].
c. Improved Medication Adherence:
Nurses educated in pharmacogenomics are vital in promoting medication adherence. By explaining to patients how their genetic variations affect medication response, nurses can help patients understand the importance of adherence to prescribed medications[70]. Nurses can address these concerns and provide education to support patients in adhering to their medication regimens and optimizing treatment effectiveness.
d. Effective Interdisciplinary Collaboration:
Integrating pharmacogenomics education in nursing schools prepares future nurses to collaborate effectively with other healthcare professionals in interdisciplinary teams. Nurses can actively contribute to discussions and decision making by leveraging their understanding of pharmacogenomics to provide valuable insights[71]. This collaboration ensures comprehensive patient care and promotes a team-based approach for personalized medicine.
e. Professional Growth and Competitiveness:
Knowledge and skills in pharmacogenomics increase nurses’ competitiveness in the healthcare job market. As the field of pharmacogenomics continues to advance, there is high demand for healthcare providers with expertise in this area. Nurses with pharmacogenomics education can contribute to research, quality improvement initiatives, and implementation of precision medicine practices, thus advancing their professional growth[72].
4. Other healthcare professional programmers:
Integrating pharmacogenomic education into other professional healthcare programs, such as genetic counseling, physician assistance programs, and allied health professionals, can offer several benefits. The key points are as follows:
a Enhanced Patient Care:
Pharmacogenomics education equips healthcare professionals in other fields with knowledge about genetic variations and their impact on drug response. This knowledge allows them to provide personalized and evidence-based patient care by considering their genetic profiles when making treatment decision[73]. By integrating pharmacogenomics into their practice, healthcare professionals can improve patient outcomes and optimize therapeutic interventions.
b Improved Medication Selection and Dosage:
Understanding pharmacogenomics helps healthcare professionals choose the most appropriate medications and dosages for individual patients. Healthcare professionals can minimize the risk of adverse drug reactions and ensure treatment efficac[74]. Pharmacogenomic education enables healthcare professionals to make informed decisions regarding drug selection and dosage adjustment, thereby improving patient safety and treatment outcomes.
c Effective Interdisciplinary Collaboration:
Integrating pharmacogenomics education into other professional healthcare programs facilitates collaboration within the interdisciplinary healthcare teams. Healthcare professionals with pharmacogenomic knowledge can contribute to discussions, provide valuable insights, and collaborate with other team members to deliver personalized patient care[75]. This collaboration fosters a team-based approach to precision medicine and ensures comprehensive and coordinated healthcare delivery[76].
d Research and Innovation:
Pharmacogenomics education provides healthcare professionals with the necessary knowledge and skills to actively participate in research and innovation in the field. Professionals in other healthcare fields can contribute to ongoing research, quality improvement initiatives, and development of best practices for pharmacogenomic implementation [77]. By staying updated with the latest advancements in pharmacogenomics, these professionals can contribute to evidence-based practices and drive innovation in personalized medicine.
e Professional Growth and Career Advancement:
Knowledge and expertise in pharmacogenomics enhance professional growth and career advancement opportunities for healthcare professionals in other fields. As pharmacogenomics has become increasingly integrated into clinical practice, healthcare professionals with specialized training in this area are in high demand [78]. By acquiring pharmacogenomics education, professionals can position themselves as leaders in precision medicine and expand their career prospects.
e.1. The National Human Genome Research Institute (NHGRI) offers resources for genomic medicine education, including pharmacogenomics, for various professional healthcare programs. It provides educational materials, curricular guidelines, and case studies to support the integration of genomic medicine into various disciplines.
e.2. The American Society of Clinical Oncology (ASCO) developed a personalized medicine curriculum that included pharmacogenomics. It aims to equip oncology professionals with the knowledge and skills required to provide personalized cancer treatments. These resources provide valuable guidance and educational materials for integrating pharmacogenomics into health care curricula. They support educators and institutions in implementing effective teaching strategies and ensuring that future healthcare professionals are equipped with the necessary knowledge and skills in pharmacogenomics. By integrating pharmacogenomics education, healthcare curricula can prepare students to apply personalized medical approaches and deliver optimal patient care. Integrating pharmacogenomic education into healthcare programs sharpens the professionals' understanding of the genetic impact of drug responses, refines clinical decisions, fosters precision medicine, and ensures patient safety. This integration also cultivates interdisciplinary collaboration, driving personalized patient care in an evolving healthcare landscape (Table II).
Table II: Incorporating Pharmacogenomics Education into Healthcare Curricula

B. Continuing education and professional development.
Continuing education and professional development play a crucial role in keeping healthcare professionals updated with the latest advances in pharmacogenomics. Healthcare professionals can enhance their knowledge and skills in applying pharmacogenomics in clinical practice through workshops, seminars, online courses, webinars, and certification programs. Here is an overview of continuing education opportunities in pharmacogenomics, along with relevant resources:
1. Professional Organizations:
a. Workshops, conferences, and seminars.
Many professional organizations and scientific conferences host workshops and seminars on pharmacogenomics. These events led experts, researchers, and industry leaders to share knowledge and insights. Some notable organizations and conferences include the following.
a.1. The National Human Genome Research Institute (NHGRI) provides workshops and seminars on pharmacogenomics for healthcare professionals[79]. These events covered topics such as genetic testing, interpretation of results, and clinical implementation.
a.2. The International Conference on Genomics and Pharmacogenomics provides a platform for in-depth discussions through keynotes, presentations, and exhibitions. The conference underlined the significance of pharmacogenomics in tailoring drugs for diseases and highlighted trends in biomarkers and personalized medicine.
a.3. The American Society for Clinical Pharmacology and Therapeutics (ASCPT): ASCPT organizes an annual meeting that features workshops and seminars on various aspects of clinical pharmacology, including pharmacogenomics[80].
a.4. American Association of Colleges of Pharmacy (AACP): AACP conducts workshops and seminars on pharmacogenomics education and its integration into pharmacy curricula[81].
a.5. Precision Medicine Conference (PMC): This conference focuses on "Pharmacogenomics: Convergence of Pharmacology & Genomics," exploring personalized healthcare based on genetic characteristics. The conference covers diverse topics such as biomarkers, COVID-19 treatments, AI integration, oncology, and more, uniting experts worldwide to advance the field of PM [82].
a.6. The Precision Medicine World Conference (PMWC): PMWC is a global event that allows experts to discuss the latest innovations in precision medicine, featuring multidisciplinary collaboration and cutting-edge presentations on genomic sequencing, AI applications, and ethical considerations. It provides networking opportunities, showcases innovative technologies, addresses policy and regulatory challenges, and includes patient perspectives, contributing to improved personalized healthcare outcomes[83].
a.7. National Institutes of Health (NIH): NIH offers various training programs, workshops, and seminars on genomics and pharmacogenomics through the National Human Genome Research Institute (NHGRI) and National Institute of General Medical Sciences (NIGMS)[84].
a.8. Clinical Pharmacogenomics at St. Jude provides Implementation Resources for Professionals encompassing PG4KDS Publications, PG4KDS Presentations, Select Pharmacogenomics Posters, Pharmacogenomics Videos, Pharmacogenomic Competencies, and other resources[85].
In summary, professional organizations and conferences such as NHGRI, ASCPT, AACP, Precision Medicine Conference, PMWC, and NIH actively contribute to advancing pharmacogenomics through workshops, seminars, and events, fostering collaboration among experts. These platforms have propelled discussions on genetic testing, drug customization, biomarkers, and personalized medicine, significantly impacting healthcare. Table III provides comprehensive details of these influential organizations and conferences in the field of pharmacogenomics.
b. Online Courses, tutorials, and Webinars: Online courses, tutorials, and webinars offer convenient and accessible continuing education opportunities in pharmacogenomics. These platforms provide self-paced learning modules, live webinars, and recorded lectures delivered by experts in the field. Some unique online resources include the following.
1. Webinars and online tutorials
a. Pharmacogenomics Knowledge Base (PharmGKB):
It offers webinars and online tutorials covering various topics in pharmacogenomics, including drug-gene interactions, genetic testing, and implementation strategies.
b. Professional Organizations (e.g., ASHP, AANP):
Host webinars on pharmacogenomics, covering topics ranging from basic concepts to practical implementation strategies. These webinars provide an opportunity for healthcare professionals to remain updated on the latest developments in the field.
2. Online Courses:
a. ISCC-PEG Pharmacogenomics Learning Series:
The PGx Project Group in Pharmacogenomics has created peer-reviewed web-based educational materials tailored for healthcare professionals to promote the integration of pharmacogenomics into clinical practice. These resources offer flexibility, allowing individuals to access them at no cost or opt for continuing education credits depending on their specific requirements. The content undergoes an annual review, and the University of Pittsburgh hosts this online series[86].
b. RxGenomix (in collaboration with CE Impact):
Offers an 8-hour CE course that equips pharmacists with the fundamental knowledge and skills required to implement pharmacogenomics into their practice and provide optimal treatment. RxGenomix, in collaboration with CEimpact, has developed an 8-hour continuing education (CE) course to equip pharmacists with the essential knowledge and skills to incorporate pharmacogenomics into their practice and provide optimal patient care. This course, supported by RxGenomix, covers the fundamentals of pharmacogenomics and its applications in pharmaceutical practice[87]. Additionally, for those who prefer shorter introductory courses, one-hour introductory courses are available for both pharmacists and physicians. Interested individuals can access further details and register for any of the three courses through the link provided.
c. The "Implementing Pharmacogenomics into Clinical Practice Certificate Program (2nd Edition)" is a comprehensive educational offering approved by the Florida Board of Pharmacy, providing 28.0 general recertification hours. This program is tailored to pharmacists with an interest in pharmacogenomics and personalized medicine. Participants could earn 28.0 the general hours of continuing education for pharmacists. It falls under the category of a practice or certificate program and does not receive external financial support. This program was designed to offer a self-paced and fully online learning experience. The participants had up to 16 weeks from the date of registration or until the UAN expiration date, whichever came first, to complete all coursework. It is important to note that this course will no longer be eligible for continuing education credit after its expiration[88].
d. Integrating Pharmacogenomics into Clinical Practice:
Certificate Program Online CME Course: Mayo Clinic offers an online certificate program for Integrating Pharmacogenomics into Clinical Practice, catering to healthcare professionals. Participants can access 16+ hours of content, including videos and case studies, at their own pace, earning CME credit and a certificate upon completion. It is open to pharmacists, physicians, nurse practitioners, physician assistants, nurses, students, and other healthcare team members[89].
3. Genetic Education Modules
The Clinical Pharmacogenetics Implementation Consortium (CPIC) provides online educational modules, known as PharmGenEdTM. These modules cover various aspects of pharmacogenomics, including drug-gene interactions, therapeutic guidelines, and clinical case studies[90]. It is important to note that the availability of workshops, seminars, and online courses can vary over time. It is recommended that websites of professional organizations, educational institutions, and online platforms be regularly checked for up-to-date information on continuing education opportunities in pharmacogenomics. Continuing education in pharmacogenomics through workshops and seminars is crucial for healthcare professionals to remain informed about advancements in this field. By participating in workshops, seminars, online courses, webinars, and certification programs, healthcare professionals can stay up-to-date with the latest advancements, guidelines, and best pharmacogenomic practices, thereby providing more personalized and effective patient care (Table III).
Table III: Workshops, Conferences, Seminars, Online Courses, Tutorials, and Webinars Providing Pharmacogenomic Education by Professional Organizations and Events
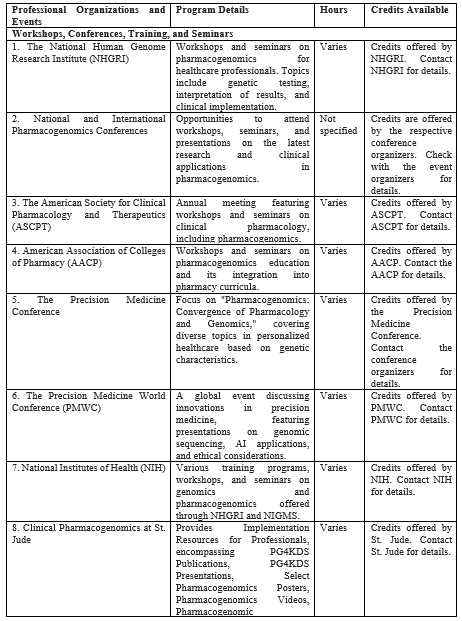
This table compiles professional organizations, events, and online resources focused on pharmacogenomics education. It includes details of program specifics, hours, and available credits for continuing education including workshops, conferences, seminars, online courses, webinars, and genetic education modules.
4. Pharmacogenomics Education Programs: Several institutions and organizations offer specialized educational programs in pharmacogenomics that include certificate, master ’s, and PhD programs that assess professionals' knowledge and competency in this field. Some notable programs include the following.
a. Professional organizations
Some organizations in the USA offer certificate programs in pharmacogenomics or personalized medicine.
a.1. (American Society of Health-System Pharmacists) offers a pharmacogenomics certificate as part of its professional certificate line. The certificate program aimed to educate pharmacists on integrating pharmacogenomics into their practice to enhance medication use. Pharmacogenomics focuses on how an individual's genetic makeup influences their medication response, allowing personalized and more effective therapy. The certificate program offers up to 20 hours of ACPE-accredited continuing education credit[91].
a.2. The American Board of Personalized Medicine administers
a certified pharmacogenomic specialist (CPGS) certification program. This program evaluates an individual's understanding and proficiency in pharmacogenomics. Certification is obtained through an examination that assesses knowledge and competency in different areas of pharmacogenomics, including the clinical application and interpretation of genetic testing results. The certificate program offers up to 24 hours of ACPE-accredited continuing education credit[92].
a.3. The Association of Molecular Pathology (AMP)
Certificate Program on Pharmacogenomics provides comprehensive training on the fundamentals of pharmacogenomics and expert consensus recommendations for clinical pharmacogenomic genotyping assays. The program includes approximately 4.5 hours of lecture content, pre-tests, post-lecture tests, downloadable resources, and an AMP Certificate of Completion. It offers CME and CMLE credits and is designed for molecular pathologists, laboratory professionals, oncologists, genetic counselors, and other healthcare professionals with prior exposure to molecular diagnostic testing. The topics covered include an introduction to pharmacogenetics and recommendations for genotyping alleles related to CYP2C19, CYP2C9, warfarin-sensitivity genes, and CYP2D6. Successful program completion required passing the post-test with a score of 75% or higher. The program is available until December 31, 2024, and CME/CMLE credits must be claimed by December 31, 2023[93].
a.4. The Test2LearnTM pharmacogenomics certificate program developed by NACDS and the University of Pittsburgh School of Pharmacy offers interactive training for both pharmacists and learners. It utilizes real genetic data to enhance the interpretation and application of genetic test results, thereby enabling appropriate recommendations for patients and collaboration with prescribers. The program provides education on pharmacogenomic principles, focusing on the practical implications in various disease states. It includes a personal genomic testing kit, offers 20 credit hours for continuing education, and grants access to the Test2LearnTM platform. Completion of the program allows individuals to educate others on pharmacogenomics and earn 20 credit hours for ACPE/CME[94]. It equips the participants with the necessary knowledge and skills to enhance patient care.
a.5. Genomind: The Pharmacogenomic Certificate Program,
offered in collaboration with the Albany College of Pharmacy and Health Sciences, provides healthcare providers, pharmacists, nurses, and students with the opportunity to earn up to 15 CME credits. The program covers various topics in pharmacogenomics, expands beyond psychiatry, and equips participants with the skills to confidently apply pharmacogenomic information in clinical practice[95].
In conclusion, these pharmacogenomics education programs, ranging from certificates to comprehensive training, equip healthcare professionals with vital knowledge for tailoring therapies based on an individual's genetic makeup. Notable programs such as those offered by ASHP, ABPM, AMP, Test2LearnTM, and Genomind not only provide substantial continuing education credits, but also empower professionals to effectively integrate pharmacogenomics into clinical practice, fostering personalized patient care across diverse disease domains (Table VI).
b. Universities and institutions:
The field of pharmacogenomics, which focuses on tailoring medical treatment to an individual's genetic makeup, has gained prominence in the healthcare sector. Several universities and institutions in the United States offer comprehensive programs and degrees related to pharmacogenomics and personalized medicine. These programs provide students and healthcare professionals with the knowledge and skills necessary to understand and apply pharmacogenomics principles in clinical practice. Table VI shows that some universities in the USA States offer programs in pharmacogenomics or personalized medicine.
C. Educational resources and tools
Educational resources and tools are essential for healthcare professionals seeking to expand their pharmacogenomics knowledge and skills. Resources, such as textbooks, reference materials, online databases, platforms, interactive case studies, and simulations, provide valuable information and practical applications in this rapidly evolving field. Here is an overview of the educational resources and tools in pharmacogenomics along with relevant examples.
1. Textbooks and Reference Materials
Pharmacogenomics textbooks and reference materials are valuable resources for healthcare professionals seeking in-depth knowledge and understanding of the field. These resources comprehensively cover key concepts, research findings, and practical applications of pharmacogenomics. Examples of textbooks and reference materials that are widely recognized in the field are as follows:
a. Pharmacogenomics:
A Primer for Clinicians, 1st Edition by Jerika T. Lam Mary A. Gutierrez, and Samit Shah This book represents an essential asset in the swiftly developing area of pharmacogenomics, containing case studies, valuable clinical insights, and recommendations for treatment[96]. This guide is an invaluable evidence-based resource for pharmacogenomics that shows the transition from genetics to genomics within the pharmaceutical domain. It comprehensively encompasses the basics of pharmacogenomics, genotyping tests and their substantiated evidence, practical application in clinical settings, and ethical, legal, and social aspects. Additionally, it features insightful case studies, practical clinical insights, and recommendations for treatments and options based on CPIC, PharmGKB, and FDA guidelines.
b. "Pharmacogenomics:
Methods and Protocols," edited by Federico Innocenti, Ron H. N. Schaik: This reference book focuses on laboratory methods and techniques used in pharmacogenomic research. It includes detailed protocols for genotyping, gene expression analysis, and other molecular biology techniques relevant to pharmacogenomics[97].
c. Principles of Pharmacogenetics and Pharmacogenomics
edited by Russ B. Altman, David Flockhart, and David B. Goldstein 2012. This comprehensive book explores personalized medicine, highlighting the driving forces behind this shift and the significance of pharmacogenetics and pharmacogenomics. It emphasizes patient advocacy, the pharmaceutical industry's move towards targeted therapies, and the use of biomarkers for more effective and safer medications. This book discusses the integration of biomarkers into medical care, the need for effective communication, and the potential of genetic testing to improve drug selection and dosing. The goal is to educate healthcare providers to deliver precise and tailored treatment, making it valuable to students and professionals in medicine and pharmacies [98].
d. Principles of Pharmacogenetics and Pharmacogenomics"
by Innocenti and Ratain. This textbook provides a comprehensive introduction to the principles of pharmacogenetics and pharmacogenomics, including discussions on genetic variation, drug response variability, and personalized medicine[99]. They have both scientific and clinical applications.
e. "Pharmacogenomics: An Introduction and Clinical Perspective"
by Joseph S. Bertino: This book introduces pharmacogenomics from a clinical perspective. This study explored the impact of genetic variation on drug responses and provided insights into the application of pharmacogenomics in clinical settings. Distinguished by its diverse global authorship comprising PharmDs, MDs, PhDs, and social scientists, "Pharmacogenomics" serves as an indispensable and easily accessible overview of this dynamic field. It comprehensively covers essential subjects ranging from individual molecules to systemic diseases, while examining the latest evolving technologies that continually reshape this area of study. The book is divided into two parts. The first section addresses the fundamental aspects of pharmacogenomics, encompassing ethical considerations, regulatory insights, scientific foundations, drug metabolism, and a concise exploration of molecular genetics and testing. The subsequent section emphasizes the pragmatic utilization of pharmacogenomics across various medical disciplines, such as cardiovascular medicine, immunology, neurology, and other specialized areas [100].
f. "Pharmacogenomics:
The Search for Individualized Therapies," by MA Li Wong edited by Julio Licinio and Ma-Li Wong 1st edition: This comprehensive textbook covers various aspects of pharmacogenomics, including genetic testing, biomarkers, and the integration of pharmacogenomics into clinical decision-making. The ethical, legal, and social consequences of pharmacogenomics have also been investigated. This publication stands out as a pioneering comprehensive work in the groundbreaking domain of pharmacogenomics, poised to transform pharmaceutical research. Along with esteemed editors, contributors’ rosters were eminent figures in the field. It provides an extensive examination of all facets of pharmacogenomics, offering a complete portrayal of its application in various diseases. Anyone engaged in pharmaceutical research and drug development should stay abreast of this innovative and revolutionary approach[101].
2. Online Databases and Platforms.
Some notable online databases and platforms in the field of pharmacogenomics include the following.
a. Pharmacogenomics Knowledgebase (PharmGKB)
PharmGKB is a widely used resource that integrates information on genetic variations, drug-gene interactions, and drug dosing guidelines. It provides curated data, including pharmacogenetic associations, drug labels, and clinical guidelines, to support personalized medical approaches[102].
b. Clinical Pharmacogenetics Implementation Consortium (CPIC):
The CPIC is an initiative that develops guidelines for translating pharmacogenomic information into clinical practice. These guidelines provide recommendations on drug dosing and selection based on individual genetic variations, helping healthcare professionals to make informed treatment decisions[103].
c. Drug-Gene Interaction Database (DGIdb):
The DGIdb is a comprehensive resource that consolidates information on drug-gene interactions from various sources. It includes data on drug targets, biomarkers, and pharmacogenomic associations, facilitating the exploration of genomic influences on drug response [104].
d. The Pharmacogenomics Research Network (PGRN):
PGRN is a collaborative research network that focuses on studying the impact of genetic variations on drug response. Their websites provide access to research findings, publications, and tools for pharmacogenomic analysis[105, 106].
e. Open Targets:
Open Targets integrate genetic, genomic, and drug discovery data to identify potential drug targets and biomarkers. It combines genetic association data with biological and pharmacological knowledge, thereby aiding the discovery of new therapeutic targets[107].
f. PGX for Pharmacists:
This podcast highlights the crucial role of pharmacists in utilizing pharmacogenomics (PGx) and precision medicine to improve patient healthcare outcomes. Exploring the power and impact of PGX as an innovative tool for pharmacists will enable the development of personalized and effective treatment strategies. Gain insights into the latest advancements and practices in PGx implementation for optimal patient care field[108].
3. Interactive Case Studies and Simulations
Interactive case studies and simulations are valuable tools in pharmacogenomics that allow healthcare professionals, researchers, and students to explore real-world scenarios and gain practical experience in applying pharmacogenomic principles. These interactive resources provide a dynamic learning environment and help users develop critical thinking skills and decision-making abilities related to personalized medicine and drug therapies. Examples of interactive case studies and simulations for pharmacogenomics include the following:
a. Pharmacogenomics Education Program (PharmGenEd™)
PharmGenEd offers interactive case studies designed to simulate real-world scenarios. These case studies enable healthcare professionals to apply their pharmacogenomic knowledge to patient care, thereby enhancing critical thinking and decision-making skills[109].
b. The Pharmacogenomics Virtual Summit 1:
Held in February and March 2021, this summit focuses on introducing pharmacogenomics and its benefits to patient care. The event featured panel discussions with experts, covering topics such as health equity and economics and providing credit for Continuing Medical Education (CME) and Continuing Pharmacy Education (CPE)[110].
c. Stanford Pharmacogenomics Program's
Interactive Case Studies Stanford University offers interactive case studies across various clinical specialties. These studies allow users to explore patient histories, review genetic information, and evaluate the impact of genetic variations on drug response [111, 112].
d. MyRx Virtual Pharmacogenetic Testing Consultations:
Launched at the University of Florida, this service provides patients with insight into drug response and medication optimization through virtual pharmacogenetic testing consultations. Clinical pharmacists with pharmacogenomic expertise aim to enhance drug therapy based on unique genetic variations, thereby making testing more accessible to a wider population[113].
e. Pharmacist Letter:
An evidence-based drug therapy resource authored by pharmacists. It keeps professionals updated on the latest clinical findings, offers drug charts and comparison tables, and provides access to a vast pharmacy continuing education (CE) library field[114].
f. SimPHARM:
SimPHARM™ is an immersive web-based clinical training tool offering real-life patient scenarios for students, facilitating dynamic decision-making and interactive case-building, and promoting reflective learning in a flipped classroom style. It supports collaborative team training, diverse treatment options, and scalable simulations, enhancing inter-professional education and smarter therapeutic decisions for improved clinical outcomes[115].
g. National Institutes of Health (NIH) Case Study Repositories:
Various institutions and organizations, including the NIH, provide online repositories for precision medicine case studies. These resources present clinical scenarios where pharmacogenomics is crucial, enabling healthcare professionals to apply their knowledge to practical contexts[116].
In summary, healthcare professionals benefit from diverse resources such as articles, online databases, and case studies, fostering deeper insights and keeping them updated in pharmacogenomics. Online platforms offer accessibility to genetic information and enhance tailored drug treatments, whereas interactive case studies facilitate hands-on learning, critical thinking, and practical pharmacogenomic implementations. These resources collectively support professionals in expanding their knowledge and skill sets for pharmacogenomic integration into clinical practice (Table V).
III. Awareness of Pharmacogenomics among Healthcare Professionals
Awareness of pharmacogenomics among healthcare professionals is crucial for effectively integrating genetic information into patient care. It involves understanding the principles, benefits, and limitations of pharmacogenomics, and is updated with the latest research and guidelines. However, various factors can affect healthcare professionals' awareness of pharmacogenomics. Here is an overview of the awareness of pharmacogenomics among healthcare professionals along with relevant resources.
A. Survey Studies on Healthcare Professionals' Knowledge:
• A systematic review and meta-analysis examined the knowledge and attitudes of medical and pharmacy students regarding pharmacogenomics. These findings provide insights into students' current understanding and attitudes towards this field, highlighting the need for targeted educational interventions to enhance their knowledge and awareness of pharmacogenomics[117].
• Pharmacogenomic Tests into Daily Clinical Practice:
A General Survey for Polish Healthcare Workers: This study examines the readiness of healthcare workers in Poland to integrate pharmacogenomic tests into daily clinical practice. The survey highlighted healthcare workers' current knowledge of and attitudes towards pharmacogenomics and identified potential barriers to its implementation. These findings provide insights into strategies for enhancing the integration of pharmacogenomic tests in clinical settings in Poland[118].
• Assessment of healthcare professionals' knowledge, attitudes, and perceived challenges of clinical pharmacogenetic testing:
This study assessed the knowledge, attitudes, and perceived challenges of healthcare professionals regarding clinical pharmacogenetic testing in Egypt. These findings shed light on healthcare professionals' current understanding and perspectives in this context, providing valuable insights for developing educational initiatives and strategies to overcome the challenges of implementing pharmacogenetic testing[119].
• Assessment of healthcare professionals' Knowledge and perception of pharmacogenomics before and after a pharmacogenomics training program
(Lamberts et al., 2018) is a study that assessed healthcare professionals' knowledge and perception of pharmacogenomics before and after a training program. This highlights the need for educational initiatives to improve awareness and knowledge of pharmacogenomics[120].
• " Knowledge and attitudes concerning pharmacogenomics among healthcare professionals":
This survey examines the knowledge, attitudes, and implementation of pharmacogenetics and pharmacogenomics among healthcare professionals. This paper examines the knowledge and attitudes of healthcare professionals towards pharmacogenomics, identifies gaps in awareness, and emphasizes the importance of education. This study provides insights into healthcare professionals' current understanding of and attitudes towards pharmacogenomics, emphasizing the importance of targeted educational interventions to improve their knowledge and promote positive attitudes in this field. These findings will contribute to ongoing efforts to integrate pharmacogenomics into clinical practice[121].
• "Knowledge of Pharmacogenetics among Healthcare Professionals and Faculty Members of Health Training Institutions in Ghana":
This paper explores the knowledge of pharmacogenetics among healthcare professionals and faculty members of health training institutions in Ghana. This study highlights gaps in the understanding and awareness of pharmacogenetics among these professionals, indicating the need for targeted educational interventions to enhance their knowledge of this field. These findings can inform the development of educational programs and strategies to improve the integration of pharmacogenetics into healthcare practices in Ghana[122].
B. Barriers to Awareness and Implementation:
• Lack of Training and Education: " summary of this paper in three lines:"
(Giri et al., 2018) discusses the lack of training and educational resources in pharmacogenomics as a significant barrier to awareness and implementation. This emphasizes the need for comprehensive education to bridge this gap[123].
• Limited Access to Pharmacogenomic Testing:
"Barriers to clinical implementation of pharmacogenomic testing" (Virelli et al., 2021) is a review that highlights the limited availability and access to pharmacogenomic testing as a barrier to the widespread awareness and implementation of pharmacogenomics. It discusses the challenges and potential solutions for increasing accessibility[124].
• Ethical and Legal Considerations:
"Ethical, legal, and social considerations in pharmacogenomics" (Gershon et al., 2014) explore the ethical and legal considerations surrounding pharmacogenomics, which can influence healthcare professionals' awareness and implementation. It addresses issues such as privacy, informed consent, and equity[125].
C. Strategies to Improve Awareness
• Professional Guidelines and Recommendations:
The Clinical Pharmacogenetics Implementation Consortium (CPIC) provides evidence-based guidelines for implementing pharmacogenomics in clinical practice. These guidelines serve as a valuable resource for healthcare professionals to improve awareness and guide decision making [126, 127].
• Collaborations and Partnerships:
Establishing partnerships between academic institutions, professional organizations, and industry is pivotal for advancing pharmacogenetic education. Successful collaboration models underscore the significance of these alliances[128].
• Integration into Clinical Practice Guidelines:
Advocating for the integration of pharmacogenomics within pharmacotherapy guidelines emphasizes the importance of incorporating these genomic principles into established clinical practices, thereby fostering greater awareness and implementation[110].
• These resources provide insights into the awareness of pharmacogenomics among healthcare professionals and offer strategies to enhance their knowledge and integration. By addressing barriers, such as lack of training and education, limited access to pharmacogenomic testing, and ethical and legal considerations, healthcare professionals can improve their awareness and effectively incorporate pharmacogenomics into patient care (Table VI).
IV. Patient Education and Empowerment
Patient education and empowerment are crucial aspects of pharmacogenomic implementation, enabling patients to understand the benefits and limitations of pharmacogenomic testing and actively participate in treatment decisions[129, 130]. Here is a detailed overview of patient education and empowerment in pharmacogenomics along with relevant resources.
A. Importance of Patient Awareness:
Patient awareness is vital in pharmacogenomics because it allows patients to comprehend how their genetic makeup influences their responses to medications. When informed about pharmacogenomics, patients can actively engage in shared decision making with healthcare providers to ensure personalized and effective treatment[131]. Awareness empowers patients to advocate for themselves and to make well-informed decisions about their healthcare.
B. Patient-Centered Communication:
Effective patient-centered communication is crucial for patient education and empowerment in pharmacogenomics. Healthcare providers should engage in open and transparent discussions with patients and explain the concept of pharmacogenomics, the relevance of genetic testing, and how it can affect treatment decisions. They should address patients' questions, concerns, and values, fostering a collaborative relationship based on mutual trust and shared decision-making[132, 133].
C. Educational Materials for Patients.
1. Brochures and Pamphlets
• Understanding Pharmacogenomics: The Importance of Your Genetic Makeup (National Human Genome Research Institute):
This resource provides an overview of pharmacogenomics, explaining the role of genetic variations in drug responses. This helps patients to understand the significance of pharmacogenomics in their healthcare decisions[134].
• "The Mayo Clinic offers a patient brochure explaining pharmacogenomics and its relevance to personalized medicine. It guides patients in understanding how genetic testing can inform medication decisions, empowering them to actively participate in their treatment[135].
2. Online Resources and Videos
• MedlinePlus (National Library of Medicine):
MedlinePlus provides reliable information regarding pharmacogenomics through articles, videos, and links to resources. Patients can access educational materials to gain a better understanding of pharmacogenomics and its implications for their healthcare [136].
• "Pharmacogenomics Explained" (Mayo Clinic Center for Individualized Medicine):
Mayo Clinic offers online resources and videos that explain pharmacogenomics, genetic testing, and personalized medicine. These resources aim to empower patients with knowledge to actively engage in treatment decisions[137].
3. Support Groups and Patient Advocacy Organizations
• Genetic Alliance:
Genetic Alliance is a nonprofit organization that advocates for individuals and families affected by genetic conditions. They provided educational resources, support networks, and access to research and clinical trials. Patients can engage with support groups and patient advocacy organizations to gain knowledge, share experiences, and advocate personalized care[138]. These educational resources, such as brochures, online materials, videos, and support groups, help patients understand the relevance of pharmacogenomics, genetic testing, and personalized medicine. They empower patients to actively participate in their healthcare decisions, engage in meaningful discussions with healthcare providers, and advocate personalized and effective treatment. In line with the discussion of patient education and empowerment in pharmacogenomics, Table VI summarizes the crucial aspects, emphasizing the importance of patient awareness, patient-centered communication, and the diverse educational resources available to aid patients in understanding and actively engaging in their healthcare decisions.
V. Public Health Initiatives and Policy Considerations
Pharmacogenomics has immense potential to improve public health outcomes by enabling personalized medicine approaches. Several public health initiatives and policy considerations have been established to harness the benefits of pharmacogenomics. Here, we provide an overview of these aspects, along with the relevant resources.
A. Government Initiatives and Funding: Government initiatives play a crucial role in promoting pharmacogenomics research, education, and implementation. Funding agencies and organizations provide grants and support to advance pharmacogenomic knowledge and its integration into healthcare systems[139].
• National Institutes of Health (NIH):
NIH supports research and educational initiatives in pharmacogenomics through various funding mechanisms. Their Pharmacogenomics Research Network (PGRN) facilitates collaboration among researchers, fostering advancements in the field[140, 141].
• The National Human Genome Research Institute (NHGRI):
The NHGRI is a part of the NIH and promotes research on genomics and its applications, including pharmacogenomics. They offer funding opportunities and resources to accelerate the translation of genomic discoveries into an improved healthcare field[142, 143].
B. Regulatory Aspects and Guidelines Regulatory
agencies have developed guidelines and policies to ensure the safety and effectiveness of pharmacogenomic information in clinical practice.
U.S. Food and Drug Administration (FDA):
The FDA provides regulatory oversight and guidance for pharmacogenomic tests and medications. They evaluated the safety and efficacy of pharmacogenomic tests and incorporated genomic information into drug labeling to inform healthcare professionals and patients[144].
• Clinical Pharmacogenetics Implementation Consortium (CPIC):
CPIC develops guidelines for implementing pharmacogenomics in clinical practice[145]. Their evidence-based guidelines aid healthcare professionals in interpreting genetic test results and in making informed medication decisions[146].
C. Ethical, Legal, and Social Implications (ELSI): Pharmacogenomics raises ethical, legal, and social considerations that need to be addressed for responsible implementation.
• "Ethical, Legal, and Social Issues in Pharmacogenomics" (National et al. Institute):
This resource explores ELSI associated with pharmacogenomics, including privacy, informed consent, and equity. This highlights the importance of considering the ethical, legal, and social implications of policy development and implementation[147].
D. Health Disparities and Access to Pharmacogenomic Education:
Addressing health disparities and ensuring equitable access to pharmacogenomic education are crucial for public health initiatives[148].
• "Reducing Health Disparities:
An Action Plan for Advancing Racial and Ethnic Equity in Genomic Medicine" (National et al. Institute): This action plan focuses on reducing health disparities in genomic medicine, including in pharmacogenomics. It provides strategies to improve access, education, and implementation to ensure equitable healthcare delivery[149]. These public health initiatives and policy considerations, supported by government funding, regulatory guidelines, and ELSI frameworks, aim to facilitate the integration of pharmacogenomics into the healthcare system[150]. By addressing health disparities and ensuring equitable access to education, they strive to maximize the benefits of pharmacogenomics for public health and improve patient outcomes. Pharmacogenomics, an ever-evolving discipline, holds tremendous promise in improving patient care and treatment outcomes. However, the successful integration of pharmacogenomics into healthcare systems requires attention to several future directions and challenges. (Table VII).
Table IV: Pharmacogenomics and Personalized Medicine Training Programs

This table provides an extensive overview of certificate and training programs in pharmacogenomics (PGx) and personalized medicine offered by professional organizations and universities in the USA. It covers various aspects, such as program details, target audience, credit availability, and delivery modes, serving as a comprehensive resource for individuals seeking education and expertise in this rapidly evolving field.
Note that This list may not encompass all universities that provide programs in pharmacogenomics or personalized medicine. It is advisable to visit university websites to obtain specific information on the program details, admission criteria, curricula, and faculty expertise.
VI. FUTURE DIRECTIONS AND CHALLENGES
Pharmacogenomics, an ever-evolving discipline, holds tremendous promise in improving patient care and treatment outcomes. However, the successful integration of pharmacogenomics into healthcare systems requires attention to several future directions and challenges. This section delineates these essential considerations, along with pertinent resources that offer valuable insights into these domains(Figure 1).
A. Advances in Pharmacogenomics Research and Technologies
Ongoing research and technological advances have significantly influenced the field of pharmacogenomics. The noteworthy resources in this sphere are as follows.
• "Pharmacogenomics in the Era of Personalized Medicine’ (Nature et al.):
Explore recent breakthroughs in pharmacogenomic research, emphasizing the potential of next-generation sequencing and bioinformatics tools to guide personalized treatment decisions [151].
• "Emerging Trends and Advances in Pharmacogenomics" (Pharmacogenomics Journal):
Provide an overview of emerging trends and technologies, including the utilization of genomic data, electronic health records, and artificial intelligence, and examine their implications for personalized medicine [152].
B. Integration of Pharmacogenomics into Precision Medicine
Pharmacogenomics plays a pivotal role in the broader scope of precision medicine. The future directions of this study are as follows.
• "Precision Medicine in the Age of Pharmacogenomics:
Achievements and Remaining Challenges" (British Journal of Clinical Pharmacology): Discusses integrating pharmacogenomics into precision medicine, highlighting achievements and challenges associated with implementing personalized treatments based on genetic variations[153].
• "Promise and Challenges of Precision Medicine:
A Conceptual Overview" (Journal of Pharmacogenomics and Pharmacoproteomics): Provides a conceptual overview of precision medicine, elucidates the role of pharmacogenomics, and delves into challenges and future directions regarding its incorporation into precision medicine approaches[154].
C. Addressing Barriers and Challenges in Education and Awareness
Realizing the full benefits of pharmacogenomics necessitates addressing the impediments to education and awareness. Noteworthy resources addressing these issues include the following.
• "Barriers to the Implementation of Pharmacogenomics in Clinical Practice:
A Systematic Literature Review" (Journal of Personalized Medicine): Identifies and examines barriers to implementing pharmacogenomics, including education, awareness, and clinical integration challenges, emphasizing the need for strategies to overcome these hurdles[155].
• "Pharmacogenomics Education and Clinical Decision Support Implementation: The Views of Pharmacists in the United States" (pharmacy):
Explore pharmacists' perspectives on pharmacogenomics education and clinical decision support implementation, identify barriers, and propose improvement strategies[156]. By addressing these challenges and propelling advancements in pharmacogenomics research, integrating it into precision medicine, and bolstering education and awareness initiatives, healthcare professionals and stakeholders can pave the way for personalized and optimized patient care. Remaining abreast of the latest research and actively overcoming barriers will facilitate successful pharmacogenomic implementation, maximizing its benefits for patients.

Figure 1. A clear visual representation of the Advancing Pharmacogenomics for Personalized Medicine: Overcoming Challenges and Future Directions
CONCLUSION
In conclusion, education and awareness of pharmacogenomics are crucial for improving patient outcomes and advancing personalized medicine. This review highlights the importance of integrating pharmacogenomics into healthcare curricula, providing continuing education to healthcare professionals, and ensuring the availability of educational resources and tools. It also discusses the current state of awareness among healthcare professionals, identifies barriers to implementation, and proposes strategies to enhance awareness. Additionally, patient education and empowerment are essential to promote the understanding and utilization of pharmacogenomics. This review recognizes the significance of public health initiatives, government support, and policy considerations in facilitating the integration of pharmacogenomics into healthcare systems. Looking towards the future, advancements in pharmacogenomics research and technologies hold promise for improving patient care and treatment outcomes. The integration of pharmacogenomics into precision medicine presents an exciting opportunity for personalized and tailored therapies. However, it is important to address challenges and barriers such as limited access to testing, ethical considerations, and health disparities in access to education. To advance these areas, it is recommended to strengthen education and training, foster collaboration and partnerships, promote patient-centered communication, ensure government support and policies, and address health disparities. By focusing on these recommendations, healthcare systems can embrace the potential of pharmacogenomics to deliver personalized and effective treatments, leading to improved patient outcomes.
GRANTS AND/OR FUNDING INFORMATION:
Not applicable. This review paper did not receive any external funding or grant for publication.
ACKNOWLEDGEMENT:
Not applicable.
CONFLICT OF INTEREST STATEMENT: The authors declare no conflict of interest regarding the publication of this paper.
ETHICAL APPROVAL AND INFORMED CONSENT:
Ethical approval and consent to participate were not included in this review. This study relied solely on information gathered from previously published scientific articles, online programs offered by universities, conferences, seminars, and publicly available information from companies or organizations that provide training or certificates in pharmacogenomics. No primary research involving human subjects has been conducted in this study.
PLAIN LANGUAGE SUMMARY:
This study is dedicated to advancing knowledge and promoting awareness of pharmacogenomics, and exploring the relationship between genes and an individual's response to medications. This emphasizes the importance of tailoring treatments based on their genetic composition to enhance their safety and efficacy in healthcare. This paper outlines the significance of education in pharmacogenomics, ethical considerations, collaboration among experts, and potential for personalized treatment rooted in genetics. Furthermore, it discusses the integration of pharmacogenomics into healthcare education and patient initiatives, and foresees future challenges in the field.
CONSENT FOR PUBLICATION:
Not applicable. No personal or identifiable data from any participant or source were included in this review. This manuscript references and synthesizes information from the previously published scientific literature, online programs, conferences, seminars, and publicly available sources.
AUTHOR DETAILS:
The manuscript, titled "Advancing Education and Awareness of Pharmacogenomics: Insights from Recent Publications: A Review," was solely conducted by Asma S. Mohamed Elmabruk Alsarrah. No other contributors were involved in the design, implementation, analysis, or writing of this manuscript.
AVAILABILITY OF DATA AND MATERIAL:
Not applicable. This review paper synthesizes information obtained from the existing scientific literature and publicly accessible sources, and no specific datasets or original materials are used.
DATA AVAILABILITY STATEMENT:
This review paper on Advancing Education and Awareness of Pharmacogenomics: Insight from Recent Publications does not contain the original data. The study exclusively utilizes information and findings obtained from referenced and cited scientific literature and online programs offered by universities and conferences and relies solely on available information from companies or organizations providing training or certificates in pharmacogenomics. All sources and references cited in this paper are duly acknowledged and provided in the reference list for transparency and further examination.
CONCLUSION:
In conclusion, education and awareness of pharmacogenomics are crucial for improving patient outcomes and advancing personalized medicine. This review highlights the importance of integrating pharmacogenomics into healthcare curricula, providing continuing education to healthcare professionals, and ensuring the availability of educational resources and tools. It also discusses the current state of awareness among healthcare professionals, identifies barriers to implementation, and proposes strategies to enhance awareness. Additionally, patient education and empowerment are essential to promote the understanding and utilization of pharmacogenomics. This review recognizes the significance of public health initiatives, government support, and policy considerations in facilitating the integration of pharmacogenomics into healthcare systems. Looking towards the future, advancements in pharmacogenomics research and technologies hold promise for improving patient care and treatment outcomes. The integration of pharmacogenomics into precision medicine presents an exciting opportunity for personalized and tailored therapies. However, it is important to address challenges and barriers such as limited access to testing, ethical considerations, and health disparities in access to education. To advance these areas, it is recommended to strengthen education and training, foster collaboration and partnerships, promote patient-centered communication, ensure government support and policies, and address health disparities. By focusing on these recommendations, healthcare systems can embrace the potential of pharmacogenomics to deliver personalized and effective treatments, leading to improved patient outcomes.
REFERENCES:
1. Wake, D.T., et al., Pharmacogenomics: Prescribing Precisely. Med Clin North Am, 2019. 103(6): p. 977-990.
2. Oates, J.T. and D. Lopez, Pharmacogenetics: An Important Part of Drug Development with A Focus on Its Application. Int J Biomed Investig, 2018. 1(2).
3. Rohrer Vitek, C.R., et al., Healthcare provider education to support integration of pharmacogenomics in practice: the eMERGE Network experience. Pharmacogenomics, 2017. 18(10): p. 1013-1025.
4. Barbarino, J.M., et al., PharmGKB: A worldwide resource for pharmacogenomic information. Wiley Interdiscip Rev Syst Biol Med, 2018. 10(4): p. e1417.
5. Luzum, J.A. and M.J. Luzum, Physicians' attitudes toward pharmacogenetic testing before and after pharmacogenetic education. Per Med, 2016. 13(2): p. 119-127.
6. Oni-Orisan, A., et al., An Introductory Tutorial on Cardiovascular Pharmacogenetics for Healthcare Providers. Clin Pharmacol Ther, 2023.
7. Zhang, Z., et al., Genetic literacy series: clinical application of pharmacogenetics for adverse reactions to antiepileptic drugs. Epileptic Disord, 2019. 21(4): p. 330-336.
8. Li, D., et al., Linking Pharmacogenomic Information on Drug Safety and Efficacy with Ethnic Minority Populations. Pharmaceutics, 2020. 12(11).
9. Dutta, P., et al., Genetic variants in African?American and Hispanic patients with breast cancer. Oncol Lett, 2023. 25(2): p. 51.
10. Rakshith, H., et al., Sex differences in drug effects and/or toxicity in oncology. Current Research in Pharmacology and Drug Discovery, 2023: p. 100152.
11. Rai, V., et al., THE ROLE OF PHARMACOGENETICS IN PERSONALISED MEDICINE: CURRENT PERSPECTIVES AND FUTURE DIRECTIONS. International Research Journal of Modernization in Engineering, Technology and Science, 2023. 05.
12. Krebs, K. and L. Milani, Translating pharmacogenomics into clinical decisions: do not let the perfect be the enemy of the good. Human Genomics, 2019. 13(1): p. 39.
13. Relling, M.V. and W.E. Evans, Pharmacogenomics in the clinic. Nature, 2015. 526(7573): p. 343-50.
14. Luzum, J.A., et al., The Pharmacogenomics Research Network Translational Pharmacogenetics Program: Outcomes and Metrics of Pharmacogenetic Implementations Across Diverse Healthcare Systems. Clin Pharmacol Ther, 2017. 102(3): p. 502-510.
15. Salari, P. and B. Larijani, Ethical Issues Surrounding Personalized Medicine: A Literature Review. Acta Med Iran, 2017. 55(3): p. 209-217.
16. Brothers, K.B. and M.A. Rothstein, Ethical, legal and social implications of incorporating personalized medicine into healthcare. Per Med, 2015. 12(1): p. 43-51.
17. Magavern, E.F., et al., Health equality, race and pharmacogenomics. Br J Clin Pharmacol, 2022. 88(1): p. 27-33.
18. Rahma, A.T., et al., Knowledge, Attitudes, and Perceived Barriers toward Genetic Testing and Pharmacogenomics among Healthcare Workers in the United Arab Emirates: A Cross-Sectional Study. J Pers Med, 2020. 10(4).
19. Research and Education Poster Abstracts Presented at the 122nd Virtual Annual Meeting of the American Association of Colleges of Pharmacy, July 19-22, 2021. Am J Pharm Educ, 2021. 85(7): p. 8737.
20. Caudle, K.E., et al., Standardization can accelerate the adoption of pharmacogenomics: current status and the path forward. Pharmacogenomics, 2018. 19(10): p. 847-860.
21. Hicks, J.K., et al., Integrating pharmacogenomics into electronic health records with clinical decision support. Am J Health Syst Pharm, 2016. 73(23): p. 1967-1976.
22. Gammal, R.S., et al., Documenting Pharmacogenomic Test Results in Electronic Health Records: Practical Considerations for Primary Care Teams. J Pers Med, 2021. 11(12).
23. Amano, A., et al., Perspectives on the Intersection of Electronic Health Records and Health Care Team Communication, Function, and Well-being. JAMA Network Open, 2023. 6(5): p. e2313178-e2313178.
24. Chhibber, A., et al., Genomic architecture of pharmacological efficacy and adverse events. Pharmacogenomics, 2014. 15(16): p. 2025-48.
25. Jarvis, J.P., et al., Real-World Impact of a Pharmacogenomics-Enriched Comprehensive Medication Management Program. J Pers Med, 2022. 12(3).
26. Pritchard, D.E., et al., Strategies for integrating personalized medicine into healthcare practice. Personalized Medicine, 2017. 14(2): p. 141-152.
27. Alrajeh, K.Y. and Y.M. Roman, Pharmacogenetic Perspective for Optimal Gout Management. Future Pharmacology, 2022. 2(2): p. 135-152.
28. Weitzel, K.W., et al., Educational strategies to enable expansion of pharmacogenomics-based care. Am J Health Syst Pharm, 2016. 73(23): p. 1986-1998.
29. Subhan, M.A., et al., Recent Advances with Precision Medicine Treatment for Breast Cancer including Triple-Negative Sub-Type. Cancers, 2023. 15(8): p. 2204.
30. Uppaluri, K., et al., Translational Pharmacogenomics Augmenting Pharmacotherapy. J Clin Genet Hered, 2023. 1(1): p. 1-18.
31. Hippman, C. and C. Nislow, Pharmacogenomic Testing: Clinical Evidence and Implementation Challenges. J Pers Med, 2019. 9(3).
32. Guyatt, G.H., et al., Patient engagement and shared decision-making. J Gen Intern Med, 2014. 29(4): p. 562.
33. Moraes, J.C., et al., Nurse empowerment through Pharmacogenetics. Rev Lat Am Enfermagem, 2020. 28: p. e3265.
34. Kwame, A. and P.M. Petrucka, A literature-based study of patient-centered care and communication in nurse-patient interactions: barriers, facilitators, and the way forward. BMC Nursing, 2021. 20(1): p. 158.
35. disorders, N.N.s.r.s.g.m.u.s.u. National Institute on Drug Abuse. 2023, March 22 [cited 2023 December 23]; Available from: https://nida.nih.gov/news-events/news-releases/2023/03/new-nih-study-reveals-shared-genetic-markers-underlying-substance-use-disorders.
36. Nakatani, K. and T. Nobori, [Pharmacogenomics]. Rinsho Byori, 2013. 61(11): p. 1018-25.
37. Simona, A., et al., Polygenic risk scores in pharmacogenomics: opportunities and challenges-a mini review. Front Genet, 2023. 14: p. 1217049.
38. Bradley, P., et al., Improved efficacy with targeted pharmacogenetic-guided treatment of patients with depression and anxiety: A randomized clinical trial demonstrating clinical utility. J Psychiatr Res, 2018. 96: p. 100-107.
39. Goetz, L.H. and N.J. Schork, Personalized medicine: motivation, challenges, and progress. Fertil Steril, 2018. 109(6): p. 952-963.
40. Norris, M., et al., Lessons from clinical implementation of a preemptive pharmacogenetic panel as part of a testing pilot program with an employer-sponsored medical plan. Front Genet, 2023. 14: p. 1249003.
41. Morris, S.A., et al., Cost Effectiveness of Pharmacogenetic Testing for Drugs with Clinical Pharmacogenetics Implementation Consortium (CPIC) Guidelines: A Systematic Review. Clin Pharmacol Ther, 2022. 112(6): p. 1318-1328.
42. Sultana, J., P. Cutroneo, and G. Trifirò, Clinical and economic burden of adverse drug reactions. J Pharmacol Pharmacother, 2013. 4(Suppl 1): p. S73-7.
43. Flowers, C.R. and D. Veenstra, The role of cost-effectiveness analysis in the era of pharmacogenomics. Pharmacoeconomics, 2004. 22(8): p. 481-93.
44. van der Wouden, C.H., et al., Cost-Effectiveness of Pharmacogenomics-Guided Prescribing to Prevent Gene-Drug-Related Deaths: A Decision-Analytic Model. Frontiers in Pharmacology, 2022. 13.
45. Apted, T., & Huff, Pharmacogenomics for Improved Outcomes and Decreased Costs in Health Care. The American Journal of Managed Care (AJMC), 2023.
46. David, V., et al., An Analysis of Pharmacogenomic-Guided Pathways and Their Effect on Medication Changes and Hospital Admissions: A Systematic Review and Meta-Analysis. Front Genet, 2021. 12: p. 698148.
47. Asiedu, G.B., et al., An assessment of patient perspectives on pharmacogenomics educational materials. Pharmacogenomics, 2020. 21(5): p. 347-358.
48. Aremu, T.O., et al., Medication Adherence and Compliance: Recipe for Improving Patient Outcomes. Pharmacy, 2022. 10(5): p. 106.
49. Kuželi?ki, N.K., et al., Pharmacogenomics education in medical and pharmacy schools: conclusions of a global survey. Pharmacogenomics, 2019. 20(9): p. 643-657.
50. Richards, S., et al., Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet Med, 2015. 17(5): p. 405-24.
51. Li, J., et al., Clinical pharmacy undergraduate education in China: a comparative analysis based on ten universities’ training programs. BMC Medical Education, 2023. 23(1): p. 83.
52. McDermott, J.H., et al., The Implementation of Pharmacogenetics in the United Kingdom. Handb Exp Pharmacol, 2023.
53. Aboelbaha, S., et al., Mental Health Prescribers’ Perceptions on the Use of Pharmacogenetic Testing in the Management of Depression in the Middle East and North Africa Region. Pharmacogenomics and Personalized Medicine, 2023. 16: p. 503-518.
54. Martin-Sanchez, F., et al., Personalized Precision Medicine for Health Care Professionals: Development of a Competency Framework. JMIR Med Educ, 2023. 9: p. e43656.
55. Micaglio, E., et al., Role of Pharmacogenetics in Adverse Drug Reactions: An Update towards Personalized Medicine. Front Pharmacol, 2021. 12: p. 651720.
56. Snyder, M.W., et al., Cell-free DNA Comprises an In Vivo Nucleosome Footprint that Informs Its Tissues-Of-Origin. Cell, 2016. 164(1-2): p. 57-68.
57. Primorac, D., et al., Pharmacogenomics at the center of precision medicine: challenges and perspective in an era of Big Data. Pharmacogenomics, 2020. 21(2): p. 141-156.
58. Kalman, L.V., et al., Pharmacogenetic Allele Nomenclature: International Workgroup Recommendations for Test Result Reporting. Clin Pharmacol Ther. 99(2):172-185., 2015. 99(2).
59. Venugopal, M.L., et al., Mapping Australian pharmacy school curricula for content related to pharmacogenomics. Explor Res Clin Soc Pharm, 2022. 8: p. 100192.
60. Formea, C.M., W.T. Nicholson, and C.R. Vitek, An inter-professional approach to personalized medicine education: one institution's experience. Personalized medicine, 2015. 12(2): p. 129-138.
61. Gammal, R.S. and E. Fieg, Pharmacist and genetic counselor collaboration in pharmacogenomics. Am J Health Syst Pharm, 2022. 79(18): p. 1516-1520.
62. Marcinak, R., M. Paris, and S.R.M. Kinney, Pharmacogenomics Education Improves Pharmacy Student Perceptions of Their Abilities and Roles in Its Use. Am J Pharm Educ, 2018. 82(9): p. 6424.
63. Elewa, H. and A. Awaisu, Pharmacogenomics In Pharmacy Practice: Current Perspectives. Integr Pharm Res Pract, 2019. 8: p. 97-104.
64. Owusu?Obeng, A., et al., Emerging roles for pharmacists in clinical implementation of pharmacogenomics. Pharmacotherapy: The Journal of Human Pharmacology and Drug Therapy, 2014. 34(10): p. 1102-1112.
65. Wang, Y.-T., et al., Opportunities for pharmacists to integrate pharmacogenomics into clinical practice. The pharmacogenomics journal, 2020. 20(2): p. 169-178.
66. Hicks, J.K., et al., Precision Pharmacotherapy: Integrating Pharmacogenomics into Clinical Pharmacy Practice. J Am Coll Clin Pharm, 2019. 2(3): p. 303-313.
67. Kennedy, M.J., Personalized medicines - are pharmacists ready for the challenge? Integr Pharm Res Pract, 2018. 7: p. 113-123.
68. Kagan, I., et al., A Mixed-Methods Study of Nurse Managers' Managerial and Clinical Challenges in Mental Health Centers During the COVID-19 Pandemic. J Nurs Scholarsh, 2021. 53(6): p. 663-670.
69. Knisely, M.R., J.S. Carpenter, and D. Von Ah, Pharmacogenomics in the nursing literature: an integrative review. Nurs Outlook, 2014. 62(4): p. 285-96.
70. Lee, B.O., [Precision Health and Nursing Care in the Digital Age]. Hu Li Za Zhi, 2022. 69(2): p. 4-6.
71. Lee, J.J. and C.L. Clarke, Nursing students' attitudes towards information and communication technology: an exploratory and confirmatory factor analytic approach. J Adv Nurs, 2015. 71(5): p. 1181-93.
72. Goddard, K.A.B., et al., Establishing the Medical Actionability of Genomic Variants. Annual Review of Genomics and Human Genetics, 2022. 23(1): p. 173-192.
73. Ingelman-Sundberg, M., D.W. Nebert, and V.M. Lauschke, Emerging trends in pharmacogenomics: from common variant associations toward comprehensive genomic profiling. Human Genomics, 2023. 17(1): p. 105.
74. Klein, M.E., M.M. Parvez, and J.-G. Shin, Clinical Implementation of Pharmacogenomics for Personalized Precision Medicine: Barriers and Solutions. Journal of Pharmaceutical Sciences, 2017. 106(9): p. 2368-2379.
75. Lee, Y.M., et al., Advancing Pharmacogenomics-Based Care Through Interprofessional Education. Am J Pharm Educ, 2023. 87(5): p. 100007.
76. Just, K.S., et al., Educating the Next Generation of Pharmacogenomics Experts: Global Educational Needs and Concepts. Clin Pharmacol Ther, 2019. 106(2): p. 313-316.
77. Just, K.S., et al., Medical education in pharmacogenomics—results from a survey on pharmacogenetic knowledge in healthcare professionals within the European pharmacogenomics clinical implementation project Ubiquitous Pharmacogenomics (U-PGx). European Journal of Clinical Pharmacology, 2017. 73(10): p. 1247-1252.
78. Kabbani, D., et al., Pharmacogenomics in practice: a review and implementation guide. Frontiers in Pharmacology, 2023. 14.
79. Institute, N.H.G.R. Home page. n.d.; Available from: https://www.genome.gov/.
80. (ASCPT), A.S.f.C.P.a.T. Home page. n.d.; Available from: https://www.ascpt.org/.
81. (AACP), A.A.o.C.o.P. Home Page. n.d.; Available from: https://www.aacp.org/.
82. website, P.M.C. The International Precision Medicine Conference. n.d.; Available from: https://precision-medicine.magnusconferences.com/program/scientific-sessions/pharmacogenomics-convergence-of-pharmacology-genomics.
83. Precision Medicine World Conference (PMWC). n.d.; Available from: https://www.pmwcintl.com.
84. (NIGMS), N.I.o.G.M.S. Home Page. n.d.; Available from: https://www.nigms.nih.gov/.
85. Jude, C.P.a.S. Implementation Resources for Professionals. n.d.; Available from: https://www.stjude.org/treatment/clinical-trials/pg4kds-pharmaceutical-science/implementation-resources-for-professionals.html.
86. Institute, N.H.G.R. ISCC-PEG Pharmacogenomics Learning Series. n.d. [cited 2023 10/23]; Available from: https://www.genome.gov/ISCC-PEG/pharmacogenomics-learning-series.
87. rxgenomix, Pharmacogenomics: A Training for Pharmacists, Education, Editor. n.d., rxgenomix. p. https://rxgenomix.com/education/.
88. Florida, U.o. Pharmacogenomics & Precision Medicine Online Graduate Program. n.d.; Available from: https://onlinepim.pharmacy.ufl.edu/pm-search/?gad_source=1&gclid=Cj0KCQjwhfipBhCqARIsAH9msbn6ChkuggnfD_hVFHZ7zxAx6q6csygIxTIYpQW-49vzWHx5ezvEpD0aAghFEALw_wcB.
89. Clinic, M., Integrating Pharmacogenomics into Clinical Practice: Certificate Program Online CME Course, O.C. Courses, Editor. 2022, Mayo Clinic: Mayo Clinic. p. https://ce.mayo.edu/online-education/content/integrating-pharmacogenomics-clinical-practice-certificate-program-online-cme-course#group-tabs-node-course-default1.
90. Clinical Pharmacogenetics Implementation Consortium (CPIC). n.d. 2023]; The Clinical Pharmacogenetics Implementation Consortium (CPIC) is an international consortium that provides guidelines and resources for the use of pharmacogenetic tests in patient care. CPIC offers freely available, peer-reviewed, evidence-based clinical practice guidelines, fostering the integration of pharmacogenomics into clinical practice for healthcare professionals. It is endorsed by organizations such as ASHP and ASCPT and is indexed in PubMed as clinical guidelines.]. Available from: https://cpicpgx.org/.
91. Pharmacists, A.S.o.H.-S., Pharmacogenomics Certificate, P. Certificate, Editor. 2021, American Society of Health-System Pharmacists. p. https://elearning.ashp.org/products/8883/pharmacogenomics-certificate.
92. Pharmacy, A.A.A.o.c. APPLIED PHARMACOGENOMICS CERTIFICATE PROGRAM 2022. 2022; https://www.accp.com/store/product.aspx?pc=AC_PGX22].
93. Pathology, A. f. M. AMP Certificate Program Pharmacogenomics: Genes, Drugs, and Genotyping: AMP Pharmacogenomics Guidelines, EDUCATION, Editor. 2022, Association for Molecular Pathology. p. https://educate.amp.org/local/catalog/view/product.php?productid=373.
94. test2learn, The Test2Learn™ Pharmacogenomics Certificate Program, Programs, Editor, test2learn. p. https://www.test2learn.org/pgxcertificate/.
95. better, G.T.s.b.t. Continuing Medical Education: Pharmacogenomic Certificate Program. 2022; Available from: https://genomind.com/providers/continuing-medical-education-pharmacogenomic-certificate-program/.
96. Shah, S., J.T. Lam, and M.A. Gutierrez, Pharmacogenomics: A Primer for Clinicians. 2021: McGraw-Hill Education.
97. Innocenti, F. and R.H.N. van Schaik, Pharmacogenomics [electronic resource] : Methods and Protocols / edited by Federico Innocenti, Ron H.N. van Schaik. 2nd 2013. ed. Methods in Molecular Biology, 1015. 2013, Totowa, NJ: Humana Press.
98. Principles of Pharmacogenetics and Pharmacogenomics. 2012, Cambridge: Cambridge University Press.
99. Altman, R.B., D. Flockhart, and D.B. Goldstein, Principles of Pharmacogenetics and Pharmacogenomics. 2012: Cambridge University Press.
100. Bertino, J.S., Pharmacogenomics: An Introduction and Clinical Perspective.
101. Licinio, J. and M.L. Wong, Pharmacogenomics: The Search for Individualized Therapies. 2002: Wiley.
102. PharmGKB, Pharmacogenomics Knowledgebasea, PharmGKB, Editor., PharmGKB. p. https://www.pharmgkb.org/.
103. (CPIC®), C.P.I.C., Clinical Pharmacogenetics Implementation Consortium (CPIC). n.d, Clinical Pharmacogenetics Implementation Consortium (CPIC®). p. https://cpicpgx.org/.
104. Freshour, S.L., et al., Integration of the Drug–Gene Interaction Database (DGIdb 4.0) with open crowdsource efforts. Nucleic Acids Research, 2020. 49(D1): p. D1144-D1151.
105. (DGIdb), D.-G.I.D. Drug-Gene Interaction Database (DGIdb). p. https://www.dgidb.org/.
106. (PGRN), T.P.R.N., The Pharmacogenomics Research Network (PGRN) n.d., The Pharmacogenomics Research Network (PGRN) p. https://www.pgrn.org/.
107. opentargets, The Open Targets Platform. n.d., opentargets. p. https://www.opentargets.org/.
108. NETWOEK, P.P., in PGX FOR PHARMACISTS.
109. (PharmGenEd™), T.P.E.P. General Genomics. 2016; https://genomicseducation.net/resource/pharmacogenomics-education-program-pharmgened].
110. Rigter, T., et al., Implementation of Pharmacogenetics in Primary Care: A Multi-Stakeholder Perspective. Frontiers in Genetics. 2020. 11.
111. Venkatachalapathy, P., et al., Pharmacogenomics and Personalized Medicine in Type 2 Diabetes Mellitus: Potential Implications for Clinical Practice. Pharmgenomics Pers Med, 2021. 14: p. 1441-1455.
112. (SCGPM), S.C.f.G.a.P.M., Stanford Pharmacogenomics Program's Interactive Case Studies.
113. Thomas, D., et al., Pharmacy Student Perceptions of a Virtual Pharmacogenomics Activity. Healthcare (Basel), 2022. 10(2).
114. Pharmacist’s Letter. Available from: https://trchealthcare.com/store/individual/pharmacists-letter/.
115. Pharmacy, A.A.o.C.o. SimPHARM. 2020 [cited 2023 Dec 23]; Available from: https://www.aacp.org/sites/default/files/2020-05/SimPHARM w IPE Data Sheet Feb. 2020.pdf.
116. (NIH), N.I.o.H., Using Genes to Guide Prescriptions.
117. Li, C., et al., Knowledge and attitudes of medical and pharmacy students about pharmacogenomics: a systematic review and meta-analysis. Pharmacogenomics J, 2023.
118. Tafazoli, A., et al., Toward Integration of Pharmacogenomic Tests into Daily Clinical Practice: A General Survey for Polish Healthcare Workers. Med Sci Monit, 2023. 29: p. e940119.
119. Nagy, M., et al., Assessment of healthcare professionals' knowledge, attitudes, and perceived challenges of clinical pharmacogenetic testing in Egypt. Per Med, 2020. 17(4): p. 251-260.
120. Makrygianni, D. et al., Pharmacy students’ attitudes and intentions of pursuing postgraduate studies and training in pharmacogenomics and personalized medicine. Human Genomics, 2023. 17(1): p. 27.
121. Arafah, A., et al., Knowledge, Attitude and Perception of Pharmacy Students towards Pharmacogenomics and Genetics: An Observational Study from King Saud University. Genes (Basel), 2022. 13(2).
122. Kudzi, W., B.S. Addy, and B. Dzudzor, Knowledge of Pharmacogenetics among Healthcare Professionals and Faculty Members of Health Training Institutions in Ghana. Ghana Med J, 2015. 49(1): p. 50-6.
123. Giri, J., et al., Education and Knowledge in Pharmacogenomics: Still a Challenge? Clin Pharmacol Ther, 2018. 103(5): p. 752-755.
124. Virelli, C.R., A.G. Mohiuddin, and J.L. Kennedy, Barriers to clinical adoption of pharmacogenomic testing in psychiatry: a critical analysis. Transl Psychiatry, 2021. 11(1): p. 509.
125. Gershon, E.S., N. Alliey-Rodriguez, and K. Grennan, Ethical and public policy challenges for pharmacogenomics. Dialogues Clin Neurosci, 2014. 16(4): p. 567-74.
126. Lee, C.R., et al., Clinical Pharmacogenetics Implementation Consortium Guideline for CYP2C19 Genotype and Clopidogrel Therapy: 2022 Update. Clinical Pharmacology & Therapeutics, 2022. 112(5): p. 959-967.
127. Johnson, J.A., et al., Clinical Pharmacogenetics Implementation Consortium (CPIC) Guideline for Pharmacogenetics-Guided Warfarin Dosing: 2017 Update. Clin Pharmacol Ther, 2017. 102(3): p. 397-404.
128. Ahmed, F., et al., Strengthening the Bridge Between Academic and the Industry Through the Academia-Industry Collaboration Plan Design Model. Frontiers in psychology, 2022. 13: p. 875940-875940.
129. Subasri, M., et al., Pharmacogenomic-based personalized medicine: Multistakeholder perspectives on implementational drivers and barriers in the Canadian healthcare system. Clin Transl Sci, 2021. 14(6): p. 2231-2241.
130. Anderson, A.N., A.R. Chan, and Y.M. Roman, Pharmacogenomics and clinical cultural competency: pathway to overcome the limitations of race. Pharmacogenomics, 2022. 23(6): p. 363-370.
131. Weitzel, K.W., et al., A stepwise approach to implementing pharmacogenetic testing in the primary care setting. Pharmacogenomics, 2019. 20(15): p. 1103-1112.
132. Nguyen, K. and K. Wiisanen, Chapter Nine - Patient-facing clinical decision support for pharmacogenomic precision medicine, in Clinical Decision Support for Pharmacogenomic Precision Medicine, B. Devine, R.D. Boyce, and K. Wiisanen, Editors. 2022, Academic Press. p. 203-225.
133. Bright, D., M. Worley, and B.L. Porter, Patient perceptions of pharmacogenomic testing in the community pharmacy setting. Research in Social and Administrative Pharmacy, 2021. 17(4): p. 744-749.
134. Institute, N.H.G.R., Understanding Pharmacogenomics: The Importance of Your Genetic Makeup, N.I.o.H. (NIH), Editor. nd, National Institutes of Health (NIH). p. https://www.genome.gov/genetics-glossary/Pharmacogenomics.
135. Clinic, M., Pharmacogenomics Patient Brochure n.d. p. https://www.mayo.edu/research/search/search-results?q=Pharmacogenomics.
136. MedlinePlus, Pharmacogenomics., MedlinePlus, Editor. n.d. p. https://vsearch.nlm.nih.gov/vivisimo/cgi-bin/query-meta?v:project=medlineplus&v:sources=medlineplus-bundle&query=pharmacogenomics&_gl=1*5slkbs*_ga*NjE1MTEzMDk5LjE2ODc2MzQyMDA.*_ga_7147EPK006*MTY4NzYzNDE5OS4xLjEuMTY4NzYzNDcwOC4wLjAuMA..*_ga_P1FPTH9PL4*MTY4NzYzNDE5OS4xLjEuMTY4NzYzNDY4NS4wLjAuMA..&_ga=2.213528788.1519314817.1687634200-615113099.1687634200.
137. Clinic, M., PHARMACOGENOMICS FOR PATIENTS, C.A.P.F.I. MEDICINE, Editor. n.d. p. https://www.mayo.edu/research/centers-programs/center-individualized-medicine/patient-care/pharmacogenomics/patients.
138. Alliance, G., About Genetic Alliance. n.d., Genetic Alliance. p. https://www.geneticalliance.org/about-genetic-alliance/.
139. Dubashi, B. and B. Ramadass, Funding Organizations for Science and Health Care, in Grant writing for medical and healthcare professionals, S.C. Parija and V. Kate, Editors. 2023, Springer Nature Singapore: Singapore. p. 33-52.
140. Yee, S.W., et al., Expanding Precompetitive Multisector Collaborations to Advance Drug Development and Pharmacogenomics. Clinical Pharmacology & Therapeutics, 2020. 107(1): p. 96-101.
141. Claw, K.G., et al., A framework for enhancing ethical genomic research with Indigenous communities. Nature Communications, 2018. 9(1): p. 2957.
142. Manolio, T.A., Implementing genomics and pharmacogenomics in the clinic: The National Human Genome Research Institute's genomic medicine portfolio. Atherosclerosis, 2016. 253: p. 225-236.
143. Schatz, M.C., et al., Inverting the model of genomics data sharing with the NHGRI Genomic Data Science Analysis, Visualization, and Informatics Lab-space. Cell Genomics, 2022. 2(1).
144. Hess, G.P., et al., Pharmacogenomic and pharmacogenetic-guided therapy as a tool in precision medicine: current state and factors impacting acceptance by stakeholders. Genetics Research, 2015. 97: p. e13.
145. Rahawi, S., et al., Knowledge and attitudes on pharmacogenetics among pediatricians. J Hum Genet, 2020. 65(5): p. 437-444.
146. Relling, M.V., et al., The Clinical Pharmacogenetics Implementation Consortium: 10 Years Later. Clinical Pharmacology & Therapeutics, 2020. 107(1): p. 171-175.
147. Parker, L.S., Ethical, Legal, and Social Issues in Pharmacogenomics, in Pharmacogenomics: A Primer for Clinicians, J.T. Lam, M.A. Gutierrez, and S. Shah, Editors. 2021, McGraw Hill: New York, NY.
148. Roman, Y.M., Editorial: The role of pharmacogenomics in addressing health disparities: the path, the promise, and the barriers. Frontiers in Genetics, 2023. 14.
149. Shaaban, S. and Y. Ji, Pharmacogenomics, and health disparities are we helping? Front Genet, 2023. 14: p. 1099541.
150. Educational and Ethical Considerations for Genetic Test Implementation Within Health Care Systems. Network and Systems Medicine, 2020. 3(1): p. 58-66.
151. White, C., et al., Pharmacogenomics in the era of personalised medicine. Med J Aust, 2022. 217(10): p. 510-513.
152. van der Lee, M., et al., Technologies for Pharmacogenomics: A Review. Genes (Basel), 2020. 11(12).
153. Chenoweth, M.J., et al., Global Pharmacogenomics Within Precision Medicine: Challenges and Opportunities. Clin Pharmacol Ther, 2020. 107(1): p. 57-61.
154. Chambliss, A. B. and D.W. Chan, Precision Medicine: From Pharmacogenomics to Pharmacoproteomics. Clinical Proteomics, 2016. 13(1): p. 25.
155. Youssef, E., et al., A Theory-Informed Systematic Review of Barriers and Enablers to Implementing Multi-Drug Pharmacogenomic Testing. J Pers Med, 2022. 12(11).
156. Tomcsanyi, K.M., et al., Veterans Health Administration: Implementation of pharmacogenomic clinical decision support with statin medications and the SLCO1B1 gene as an exemplar. Am J Health Syst Pharm, 2023.


 Asma Elmabruk*
Asma Elmabruk*











 10.5281/zenodo.11665725
10.5281/zenodo.11665725