As an alternative to traditional needle injections, a number of non-invasive administrations have lately surfaced. The most appealing of them is the transdermal drug delivery system (TDDS), which has a low rate of rejection, exceptional simplicity of administration, and exceptional patient convenience and persistence. In addition to the pharmaceutical industry, TDDS may find use in the skin care sector, which includes cosmetics. This approach can avoid nonspecific drug distribution to tissues that are not the drug's target and local drug concentration buildup because it primarily involves local administration. Transdermal delivery is hampered by a number of physicochemical characteristics of the skin, which have led to a great deal of research into ways to get over these barriers. In this review, we list the various kinds of TDDS approaches that are now available and critically examine their individual benefits and drawbacks, characterization techniques, and potential. The high efficiency that TDDS possesses has been demonstrated by advancements in research on these alternative approaches, and it is anticipated that these technologies will find use in a multitude of fields.
Transdermal drug delivery, Skin, Characterization, Cosmetics
Transdermal drug delivery is the non-invasive process of delivering drugs to the circulatory system from the surface of the skin the largest and most accessible organ in the human body through its layers. Comparing TDDS to oral and traditional injectable techniques, there are numerous benefits (Table 1). It lessens the strain that eating orally frequently puts on the liver and digestive system. It improves patient compliance and reduces negative pharmacological adverse effects brought on by transient overdose. Convenience is another benefit, which stands out in particular for patches that only need to be applied once a week. Adherence to medication therapy by patients can be improved with such a basic dose schedule. Transdermal patch development and design can be characterized as state of the art. The creation of TDDS is a multidisciplinary endeavor that includes basic viability Following a series of investigations that begin with the choice of drug molecule and continue through the demonstration of adequate drug flux in an ex vivo and in vivo model, a drug delivery system that satisfies every demanding requirement unique to the drug molecule (physicochemical and stability factors), the patient (comfort and cosmetic appeal), the manufacturer (scale up and manufacturability), and above all the economy [1]. Oral medication administration is the most widely used method. Both major benefits and downsides of this route of administration are present, including first-pass metabolism, drug breakdown in the gastrointestinal system as a result of enzymes, and pH. A unique drug delivery mechanism was created in order to get around these problems1. Transdermal drug delivery systems (TDDS), sometimes referred to as patches, are dosage forms intended to distribute a drug in a way that is therapeutically effective over the patients skin (Figure 1) [2].
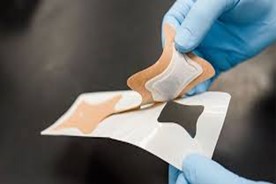
Figure 1: Transdermal Patch
Transdermal delivery prevents pulsed entry into systemic circulation and permits continuous input of medications with brief biological half-lives in addition to enabling controlled, continuous drug administration. With a transdermal patch, the pace at which the liquid medication within the reservoir can seep through the skin and into the bloodstream is controlled by a unique membrane. Transdermal drug delivery has several significant benefits, including suppression of hepatic first-pass metabolism, improvement of therapeutic efficacy, and preservation of the medication's constant plasma level [3].
The skins physiology:
The skin on an adult average has a surface area of around 2 m2 and receives approximately 1/3 of the blood that circulates throughout the body. The epidermis, the topmost layer of skin, is made up of highly cornified (dead) cells embedded in a continuous matrix of lipid membranous sheets. It comprises four morphologically distinct regions: the basal layer, spiny layer, stratum granulosum, and highest stratum corneum (Figure 2). Ceramides, cholesterol, and free fatty acids make up the distinct composition of these extracellular membranes. Every square centimeter of the human skin is known to have between 200 and 250 sweat ducts and 10 to 70 hair follicles. It is among the human body's easiest organs to reach [34].

Figure 2: Structure of skin
Skin pathways for delivery systems of transdermal drugs:
There are a number of ways that medications can penetrate and enter the skin when they are applied topically. Medications can enter the body through the appendages (transappendageal) or the stratum corneum (transepidermal) (Figure 3). There are two distinct routes that may be identified for penetration into the stratum corneum: the transcellular route, which alternates between passing through the lipid lamellae and corneocytes, and the intercellular route, which follows the convoluted journey along the lipid lamellae. It is widely acknowledged that the intercellular pathway is the primary means of penetration into the stratum corneum. The thickly cross-linked cornified membrane that covers the keratinocytes is the primary source of this. It is not possible to fully rule out transcellular transport for tiny hydrophilic molecules like water. The follicular duct or the duct of the eccrine sweat glands are included in the appendage route, also known as the shunt route. Whereas the follicular duct's substance is lipophilic, the eccrine sweat glands' contents are primarily hydrophilic. The primary cause of this is the sebum released into the follicular duct aperture. It is widely acknowledged that intact stratum corneum is the primary route via which passive skin permeation occurs due to its high surface area [35-38].

Figure 3: Pathways for permeation of drug across the skin barrier
Different Transdermal Patch Types:

Figure 4: Types of Transdermal Patches
Transdermal medical patches generally fall into four categories, as seen in Figure 4: drug-in adhesive, reservoir, matrix, and micro-reservoir systems. Reservoir or matrix systems are the most common categories for patches that are sold commercially [39].
1. The Matrix Method:
Drugs are evenly distributed within polymer matrices that are hydrophilic or lipophilic. Affixed to drug-containing discs with regulated thickness and surface area is the resultant drug-containing polymer.
2. System of Reservoirs:
The medication is released through the microporous rate-controlling membrane in this arrangement, which holds the drug reservoir between the backing layer and the membrane. Within the reservoir chamber, the medicine may be disseminated in a solid polymer matrix or exist in the forms of a gel, suspension, or solution.
3. Micro-Reservoir System (Multilaminate):
This system combines a matrix dispersion system with a reservoir. In order to construct thousands of non-leaching tiny drug reservoirs, the drug is prepared here by first suspending drug solids in an aqueous solution of a water-soluble liquid polymer and then uniformly dispersing the solution in a lipophilic polymer.
4. Adhesive Drug System:
The most basic type of membrane permeability control system is this one. This system's adhesive layer, which holds the many layers together, is drug-containing. The backing and liner are layered with the medication combination.
The fundamental components of TDDS:
Polymer Matrix:
The polymer regulates the medication's release from the apparatus. To be employed in a transdermal system, a polymer needs to meet the following requirements. The following polymers could be helpful for transdermal devices [40].
Drug:
The medicine must be carefully chosen in order to build a transdermal drug delivery system that works. Some of the desired characteristics of a medication for transdermal distribution are listed below [40,41].
Physical-Chemical Properties:
The medication's molecular weight should be less than or equal to 1000 Daltons. The medication needs to exhibit a preference for both hydrophilic and lipophilic phases. Severe partitioning properties are incompatible with effective transdermal medication administration. The medication's melting point need to be low [41].
Biological Properties:
The medication ought to be strong, requiring a daily dosage of a few milligrams or less. The medication must to have a brief half-life (t1/2). The medication must not cause allergic reactions or skin irritation. Transdermal administration is a good option for medications that break down in the gastrointestinal tract or are rendered inactive by the hepatic first-pass effect. Because transdermal administration has a nearly zero-order release profile, tolerance to the medication cannot developed. Drugs that must be delivered continuously or that have negative effects on tissues other than the intended target can also be designed for transdermal delivery [41].
Permeation Enhancers:
Also known as promoters of Permeation, these substances are able to transfer the sorption of medications from drug delivery devices onto the skin without possessing any inherent medicinal qualities. Drug flow across the skin can be expressed as follows [42].
J = D Xdc/dx
Where, C is the concentration of the diffusing species, x is the spatial coordinate, and D is the diffusion coefficient, which depends on the size, shape, and flexibility of the diffusing molecule as well as the membrane resistance. Even though there are a lot of different boundary conditions and membrane heterogeneities in the solution for J, the preceding equation contains the fundamental ideas of flux enhancement. The diffusion coefficient is correlated with the size, shape, and energy needed to create a hole for diffusion, and the concentration gradient has a thermodynamic origin. Therefore, the following factors must be taken into account to improve flux across membranes:
- Thermodynamics (lattice energies, distribution coefficients).
- Size and form of molecules.
- lowering the energy needed to create a membrane-wide molecular hole.
Solvents:
These substances may improve penetration by enlarging the skin's polar channels and lipids becoming more fluid. Examples includes, laurocapram (Azone), pyrrolidones-2-pyrrolidone, alkyl homologs of methyl sulfoxide, dimethyl acetamide, and dimethyl formamide, water alcohols (methanol and ethanol), propylene glycol, glycerol, silicone fluids, and isopropyl palmitate.
Surfactants:
It has been suggested that these substances improve the transport of hydrophilic medicines down polar pathways. A surfactant's capacity to modify penetration depends on its head group and hydrocarbon chain length. Since these substances irritate the skin, it is necessary to strike a balance between irritation and penetration enhancement. Anionic surfactants have a significant ability to permeate and interact with skin. These surfactants have the ability to cause significant changes after they are absorbed by the skin. According to reports, cationic surfactants cause more irritation than anionic surfactants, and their ability to improve skin penetration has not received much research. The nonionic surfactant class is the most extensively researched and has long been acknowledged as having the lowest potential for causing irritation among the three main classes of surfactants. Some surfactants that are frequently used are:
Anionic Surfactants:
Dioctyl sulphosuccinate, Sodium lauryl sulphate, Decodecylmethyl sulphoxide etc.
Nonionic Surfactants:
Pluronic F127, Pluronic F68, etc. Bile.
Salts:
Sodium taurocholate, Sodium deoxycholate, Sodium tauroglycocholate. Miscellaneous Chemicals: These include urea, a hydrating and keratolytic agent; N, N-dimethyl-mtoluamide; Calcium thioglycolate; Anticholinergic agents. Some potential permeation enhancers have recently been described but the available data on their effectiveness are sparse. These include eucalyptol, di-o-methyl-betacyclodextrin and soyabean casein.
Additional Ingredients:
Adhesives:
Up until now, pressure-sensitive adhesive has been used to adhere transdermal devices to the skin. The device's front or the rear of the device with the peripheral extension can be where the pressure-sensitive adhesive is placed. The following requirements should be met by both adhesive systems. Should not disrupt the natural skin flora or irritate or sensitize the skin. Should firmly adhere to the skin during the dosage interval and not allow activities like exercising or bathing to alter its place. It cought to be simple to delete Shouldn't cling to the skin in an unwashable way.
Should make both microscopic and macroscopic excellent (intimate) contact with the skin.
Backing Membrane:
Because they are flexible, backing membranes allow printing, offer a strong attachment to the drug reservoir, and keep the drug from escaping the dosage form through the top. For example, metallic plastic laminate, plastic backing with absorbent pad and occlusive base plate (aluminum foil), sticky foam pad (flexible polyurethane) with occlusive base plate (aluminum foil disc), etc., are examples of impermeable materials that shield products from the skin while they are being used.
Release Liner:
Release liner stops contamination and medication loss during storage by preventing migration of the drug into the adhesive layer. As a result, it is thought of as a component of the main packing material as opposed to the drug's dose form. The release liner consists of two layers: an occlusive (polyethylene, polyvinyl chloride) or non-occlusive (paper fabric) base layer, and a silicon or Teflon release coating layer. Metalized laminate and polyester foil are other materials used in the production of TDDS release liners [40-42].
Transdermal patch preparement:
Mercury Substrate Method:
In this technique, the necessary quantity of medication is dissolved in a fixed volume of polymer solution together with plasticizer. The aforementioned solution should be shaken for a while to create a uniform dispersion. It should then be set aside until all air bubbles have been eliminated before being poured into a glass ring that is set over the mercury surface in a glass petri dish. An inverted funnel placed above the petri dish regulates the solvent's rate of evaporation. It is necessary to preserve the dried films in a desiccator [43-47].
Circular Teflon Mould Method:
This method uses solutions in an organic solvent that include different concentrations of polymers. Half as much of the same organic solvent is used to dissolve the calculated amount of medication. adding plasticizer to the solution of drug polymer. After stirring the entire mixture, pour it into a circular Teflon mold. And the teflon mold was used to place an inverted glass funnel to control the pace of solvent vaporization. For a whole day, the solvent is left to evaporate. A desiccator is required for the storage of the dried films [48].
Glass Substrate Method:
After letting the polymeric solutions swell for a while, the necessary amount of plasticizer and medication solution are added, and everything is mixed for ten minutes. In order to remove any trapped air, it is also left aside for a while before being poured into a dry, clean anumbra petriplate. To regulate the solvent evaporation rate, place a glass funnel upside-down over the petriplate. The dried films are removed and kept in a desiccator after being left overnight [49,50].
Using the IPM Membranes Method:
This technique involves dispersing the medication in a solution of water and propylene glycol that contains carbomer 940 polymers, then stirring the mixture with a magnetic stirrer for 12 hours. Triethanolamine is to be added to the dispersion in order to neutralize it and make it viscous. If the drug's solubility in aqueous solution is extremely low, solution gel can be created using buffer pH 7.4. The IPM membrane will incorporate the gel that has formed [51].
Using EVAC Membranes Method:
Polyethylene (PE) and ethylene vinyl acetate copolymer (EVAC) membranes can be utilized as rate control membranes to prepare the target transdermal therapeutic system. Gel is made with propylene glycol if the medication is not soluble in water. Propylene glycol is used to dissolve the drug; Carbopol resin is then added to the mixture and neutralized using a 5% w/w sodium hydroxide solution. The medication (in gel form) is applied to a backing layer sheet that covers the designated area. To create a leak-proof device, a rate-regulating membrane will be placed over the gel and the borders will be sealed with heat [52,53].
Aluminum-Backed Adhesive Film Method:
When a loading dose of more than 10 mg is used in a transdermal drug delivery system, unstable matrices may be produced. The adhesive film approach with aluminum backing is appropriate. Since the majority of medications and adhesives are soluble in chloroform, it is the solvent of choice for preparing the same. Adhesive substance is added to the drug solution and dissolved once the drug is dissolved in chloroform. Aluminum foil is used to line a specially constructed aluminum former, and cork blocks that fit firmly are used to blank off the ends [54,55].
Asymmetric TPX Membrane Method:
The backing membrane for a prototype patch can be made from heat-sealable polyester film (type 1009, 3m) that has a concave diameter of 1 cm. The drug sample is injected into the concave membrane, sealed with an adhesive, and covered with an asymmetric TPX {poly (4-methyl-1-pentene)} membrane [56].
Transdermal patch evaluation test:
Drug Excipient Interaction Studies:
To create a stable product, the medicine and excipients must be compatible, and any potential chemical and physical interactions must be found. By contrasting their physiochemical characteristics, such as assay, melting endotherms, distinctive wave numbers, and absorption maxima, among others, interaction investigations are frequently conducted utilizing thermal analysis, FT-IR studies, UV, and chromatographic techniques [56,57].
Drug Content:
A predetermined portion of the patch has to dissolve in a predetermined volume of an appropriate solvent. After that, the mixture must be filtered through a filter medium before the drug content is determined using the appropriate technology (UV or HPLC). Every value is the average across three samples [58,59].
Weight Uniformity:
Prior to testing, the produced patches must be dried for four hours at 60°C. A predetermined patch area needs to be divided into several sections, then weighed using a digital balance. From the individual weights, the average weight and standard deviation values must be determined [59].
Thickness of the Patch:
To guarantee the thickness of the prepared patch, the thickness of the drug-loaded patch is measured at various sites using a digital micrometer. The average thickness and standard deviation for the same are then calculated [59].
Flatness Test:
The flatness test involves cutting three longitudinal strips from each film at different points, such as the center, the left side, and the right side. Every strip's length was measured, and the percentage of constriction (0% constriction = 100% flatness) was used to calculate the length variation resulting from non-uniformity in flatness [60].
Percentage Moisture Uptake:
To maintain 84% RH, the weighted films must be stored in desiccators with saturated potassium chloride solution at room temperature for 24 hours. The films must be reweighed after 24 hours in order to calculate the percentage of moisture absorption using the formula below [61,62].
Percentage moisture uptake = [Final Weight-Initial Weight/ Initial Weight] × 100
Moisture Loss:
Weigh each of the manufactured films separately and store them at 40°C in a desiccator filled with calcium chloride. The films must be reweighed after 24 hours in order to calculate the percentage of moisture loss using the formula below [63].
% Moisture Loss = [Initial wt – Final wt/ Final wt] × 100
Water Vapor Transmission Rate (WVTR) Studies:
Transmission cells were equal-diameter glass vials. These transmission cells underwent extensive washing before being dried for a while at 100oC in an oven. The cells were filled with approximately 1g of anhydrous calcium chloride, and the corresponding polymer film was fixed over the brim. The cell was precisely weighed and preserved at a relative humidity of 84% in a closed desiccator filled with a saturated potassium chloride solution. After being stored, the cells were removed and weighed. The following formula was used to determine the amount of water vapor conveyed [63,64].
Water Vapor Transmission Rate = Final Weight –Initial Weight/ Time X Area
It is expressed as the number of grams of moisture gained/hr/cm.sq.
Swellability:
After weighing the 3.14 cm2 patches, they were placed in a petri dish with 10 ml of double-distilled water and left to ingest. The patch's weight was increased at predetermined intervals until a steady weight was noted. The formula used to calculate the degree of swelling (S) is as follows [65]:
S (%) = Wt – Wo / Wo × 100
Where, S represents the percentage of swelling. The weight of the patch at time t is represented by Wt, and the weight at time zero by Wo.
Folding Endurance:
To fold an area-specific strip evenly and again until it breaks, cut it at the same spot. The value of the folding endurance was determined by counting how many times the film could be folded in the same direction without breaking [66].
Polariscope Examination:
This test is meant to use a Polariscope to look at the drug crystals that are extracted from the patch. To determine if the drug is present in the patch in an amorphous or crystalline form, a particular surface area of the piece must be retained on the object slide and examined for drug crystals.
Percentage Elongation Break Test:
To find the percentage elongation break, measure the length shortly before the break point. The percentage elongation may be found using the formula that is mentioned below [67].
Proportion of elongation = [L1-L2 / L2] × 100
where, L1 is each strip's ultimate length and L2 is each strip's starting length.
Tensile Strength:
A universal strength testing machine was used to assess the film's tensile strength. The machine had a sensitivity of 1 g. It was made up of two grips for load cells. While the upper one is adjustable, the lower one is fixed. A 4-by-1-centimeter test film is placed in between these cell grips, and force is applied gradually until the film breaks. The dial reading in kg is used to determine the film's tensile strength. This is how tensile strength is expressed [67].
Tensile strength =Tensile load at break / Cross section area
Probe Tack examination:
In this test, glue is brought into contact with the tip of a clean probe that has a predetermined surface roughness. The test determines when a bond forms between the adhesive and the probe. It breaks mechanically when the probe is removed later. Tack, which is measured in grams, is the force needed to remove the probe from the adhesive at a set rate [67].
Study on Skin Irritation:
Testing for skin sensitivity and irritation can be done on healthy rabbits weighing between 1.2 and 1.5 kg on average. The rabbit's dorsal surface, which measures 50 cm2, needs to be cleansed. The hair should be shaved off of the clean surface, and rectified spirit can be used to clean the surface before representative formulations are applied to the skin. After a full day, the patch is to be taken off, and the skin is to be examined and graded into five categories according to the degree of skin damage [68].
Studies on drug release in vitro:
The drug release from the produced patches can be evaluated using the paddle over disc method (USP equipment V). An adhesive is to be used to fix dry films with a given thickness over a glass plate after they have been cut into a specific shape and weighed. After the apparatus was equilibrated to 32±0.5°C, the glass plate was submerged in 500 milliliters of the phosphate buffer (pH 7.4) or dissolving medium. Next, the paddle was moved at a pace of 50 revolutions per minute and positioned 2.5 centimeters apart from the glass plate. Samples (5 ml aliquots) can be taken out at predetermined intervals for up to 24 hours, and high-performance liquid chromatography (HPLC) or a UV spectrophotometer can be used for analysis. The mean value may be computed, and the experiment needs to be run in triplicate [68].
Studies on in vitro skin penetration:
A diffusion cell can be used to conduct an in vitro permeation research. Male wistar rats weighing 200–250 g has fully developed belly skin. The dermal side of the skin was thoroughly cleaned with distilled water to remove any adhering tissues or blood vessels, and before beginning the experiment, the skin was allowed to equilibrate for one hour in diffusion medium or phosphate buffer pH 7.4. The abdominal region's hair should be carefully removed using an electric clipper.
Diffusion cell with diffusion medium within, set on a magnetic stirrer with a tiny magnetic bead to ensure even diffusant dispersion. The thermostatically regulated heater was utilized to maintain the temperature of the cell at 32 ± 0.5°C. With the epidermis facing upward into the donor compartment, the isolated rat skin piece is to be put between the diffusion cell's compartments. At regular intervals, a specific volume of sample must be taken out of the receptor compartment and replaced with an equivalent volume of fresh medium. Samples must pass through a filtering medium before being subjected to high performance liquid chromatography (HPLC) or spectrophotometric analysis [69]. The permeability coefficients were derived by dividing the flux by the initial drug load (mg cm-2), and flux can be directly estimated as the slope of the curve between the steady-state values of the amount of drug penetrated (mg cm-2) vs. time in hours.
Studies conducted in vivo:
These assessments provide an accurate picture of the effects of the medicine. In vivo investigations allow for the comprehensive exploration of variables that are not possible to examine in vitro.
The following methods can be used to evaluate TDDS in vivo:
Animal models:
The most often utilized animal species for transdermal drug delivery system evaluations include guinea pigs, mice, hairless rats, dogs, and hairless rhesus monkeys.
Human models:
After applying the patch to human volunteers, the last phase of a transdermal device's development entails gathering pharmacokinetic and pharmacodynamic data. Clinical trials have been carried out to evaluate the effectiveness, risk, adverse effects, patient compliance, and other factors.
Stability Studies:
The TDDS samples must be stored for six months at 40±0.5°C and 75±5% relative humidity in accordance with ICH recommendations. The samples were taken out at0,30,60,90, and 180 days, and their drug content was appropriately analyzed [69-72].


 Pratik D. Thakare*
Pratik D. Thakare*
 Pradyumna P. Ige
Pradyumna P. Ige





 10.5281/zenodo.11318946
10.5281/zenodo.11318946