Abstract
The Pharmaceutical industry is undergoing a significant transformation with the integration of Artificial Intelligence (AI). AI is being applied across various stages of drug development, from discovery to clinical trials, to improved efficacy, accuracy, and patient outcome. Machine learning algorithms can analyse vast amount of data to identify potential drug targets, predict efficacy and safety, and streamline clinical trails management.AI-powered tool are also being used to developed personalised medicine approaches, tailoring treatment to individual patients based on their genetic profile, medical histories, and life style factors. Further more, AI can help pharmaceutical companies comply with regulatory requirements and analysed real world data to inform drug development and treatment strategies .As AI technology continuous to evolved, it is expected to revolusatinosed the pharmaceutical industry accelerating drug discovery, improving patient outcome, reducing cost. This Abstract highlights the current applications and future direction of AI in pharmaceuticals, emphasizing its potential to transform the industry.
Keywords
Artificial Intelligence, Drug Discovery, Machine Learning, Clinical Trials.
Introduction
Artificial intelligence (AI) encompasses a range of intelligent functions and behaviours, created through computer models, algorithms, or rule set that enable machines to replicate human cognitive skills, including learning and problem-solving. AI is a branch of computer science dedicated to the examination of complex medical data. Its capacity to analyse large datasets from diverse sources paves the way for new research opportunities in the pharmaceutical and healthcare industries. AI has become an essential resource that harnesses individual expertise to deliver swift solutions to intricate problems. Various sectors are working to improve their operations to fulfil customer expectations and demands, utilizing a variety of strategies. The pharmaceutical industry is crucial in preserving lives, emphasizing innovation and the integration of new technologies to tackle worldwide health issues and respond to recent crises. Recently, traditional approaches to drug design have been supplanted by computer-assisted methods. The integration of artificial intelligence has become prevalent in enhancing the efficiency and speed of drug development. This technology is applied throughout the entire drug design process, minimizing health risks linked to clinical trials and lowering associated costs [3]. In the medical field, artificial intelligence can change many aspects related to the medical field.
The function of artificial intelligence (AI) in the subsequent domains:
• Identification of medical conditions.
• Digital therapy and personalized medicine.
? Radiotherapy
? Retinal structure.
? Cancer
? Additional enduring characteristics.
• Drug discovery:
? Assessment of bioavailability and toxicity potential.
? Clinical Trial Studies:
Development of clinical trial protocols, identification of patients, and their recruitment and enrolment processes. Oversight of monitoring frameworks, ensuring patient adherence, and detection of endpoints.
• Predicting the occurrence of an epidemic or pandemic. [1]

Fig.No.1 Illustrates a potential artificial intelligence (AI) approach to address the challenges faced by the pharmaceutical industry: securig a skilled workforce is essential across all sectors to utilize their knowledge, skills, and capabilities i product development. Additionally, issues related to supply chain interruptions and difficulties in clinical trial processes are highlighted. The increasing frequency of cyberattacks, along with data breaches and security threats, has become a major concern for the industry.
Current Pharmaceutical Challenges:
- Data privacy
- Biases
- Regulatory issues
- Ethical concerns about decision-making need addressing
- Demand Forecasting and Price Fluctuation Assessment.
- Patient Journey Analysis.
- Risk Management.
- Inventory Management.
Due to their numerous advantages, small molecules continue to be a focal point of research within the pharmaceutical industry, aimed at enhancing product quality and consumer satisfaction. The synthesis of synthetic derivatives is cost-effective, and the chemical synthesis process is relatively straightforward. As a result, there is a wide array of reliable and effective small-molecule formulations available in the pharmacy sector. In addition to addressing rare diseases, generic molecules face competition from various innovative small molecules. The introduction of these molecules to the market requires extensive data analysis and clinical trials, which impose additional financial burdens on companies to foster innovation. Despite the challenges posed by small molecules and the uneven distribution of research and discoveries, the bimolecular pharmaceutical sector is experiencing rapid growth. The activities of small molecules are fundamentally based on their reactivity and conformation. [2] Large units called biomolecules are primarily composed of nucleotides or rib-nucleotides for the nucleic acid and amino acids from the protein source. Additionally, the supra molecular arrangement and spatial structure influence their stability and functionality. Adalimumab and insulin represent two highly effective biomolecules. The infusion method is the most practical and preferred means of delivering these compounds, which results in complex pharmacokinetic characteristics. Research involving nucleic acids focuses on two critical aspects: pharmacokinetic modulation and molecular stability. Key goals include enhancing pharmacokinetic exposure and improving molecular forms. To tackle these challenges and address related issues, advancements in technology may prove beneficial. Furthermore, although artificial intelligence offers great potential for improving drug delivery and discovery, it also introduces considerable challenges that necessitate human supervision or expert analysis of the complex outcomes. AI, the approach employed incorporates machine learning and its various subsets, including natural language processing and deep learning. It allows for both supervised and unsupervised learning relies heavily on the selection of the algorithm, which is pivotal. In supervised learning, predictions such as labels or targets are generated based on a range of inputs and characteristics. Conversely, unsupervised classification aims to identify groups that share similar features.
Drug Discovery & Development
The pharmaceutical sector is vital in the drug development process, which encompasses several essential stages: drug discovery, preclinical testing, clinical trials, and regulatory approval. The field of pharmaceutics is integral to each of these stages, beginning with the initial formulation of the drug and continuing through the final phases of clinical testing and securing regulatory approval. The drug discovery process, which focuses on the identification and development of new medications, is intricate and requires significant time investment. Historically, it has depended on labour-intensive techniques, such as high-throughput screening and trial-and-error methods. However, AI methods like natural language processing and machine learning (ML) provide the opportunity to make this process faster and better by providing more accurate and efficient examination of a substantial volume of data [4]. Such novel analytical methods and computational developments over the last decade have fundamentally altered the landscape of drug discovery [5]. Typically, a Drug Discovery Pipeline consists of many phases as shown in Fig.2. Finding new targets from a wide range of proteins that have been linked to illness is the initial stage in target-based discovery. Compound libraries are screened against these targets at high throughput to identify potentially interacting molecules. Compounds will be examined in pre-clinical and clinical studies, optimized for advantageous medicinal characteristics, and, in the best-case scenario, approved by the FDA [5].
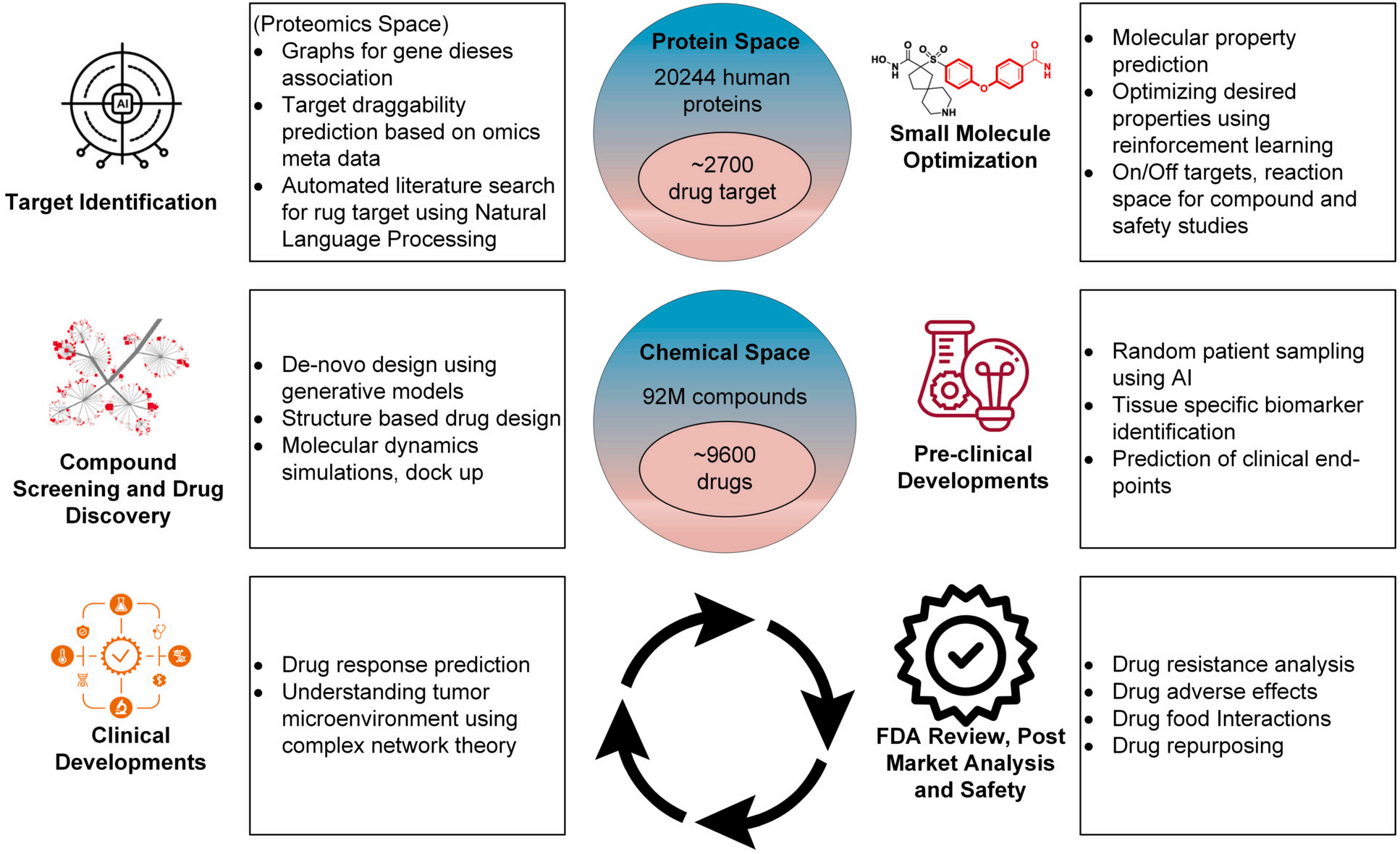
Fig. 2: Drug Discovery Pipeline Phases.
In the drug discovery phase, the field of pharmaceutics is crucial for identifying and selecting the active pharmaceutical ingredient (API) as well as developing the drug formulation. This stage also includes preclinical testing, where the safety and efficacy of the drug are assessed using animal models. The phase of the drug development process is clinical trials. In this phase, the medication is tested on humans to ascertain its safety and effectiveness. Pharmaceutics is involved in the design and execution of the clinical trial themselves as well as the formulation of drug to be used in them. Before a medication can be available for public sale, it must obtain approval from a regulatory agency, such as the US Food and Drug Administration (FDA), after successfully completing clinical trials. Pharmaceutics is responsible for making sure the medication complies with all legal requirements as well as for preparing and submitting the drug application to regulatory bodies. Additionally, pharmaceutics is essential to guaranteeing the quality of medications produced and supplied to the general public.
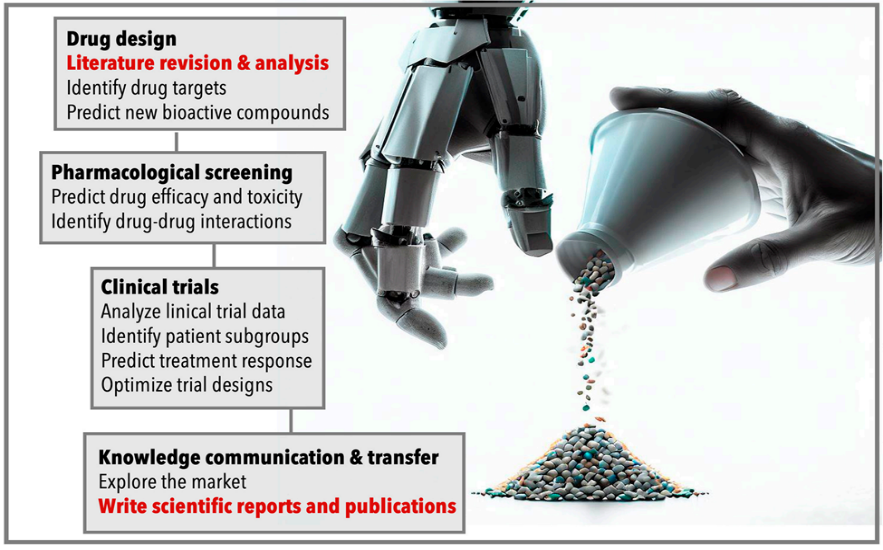
Fig.3: Graphical flowchart of the drug development process of pharmacologically active molecule.
Applications of Drug Discovery
- Identification and prioritization of targets based on the association between genes and diseases.
- Predictions regarding the potential of drug targets.
- Recognition of alternative objectives.
- Designing compounds with preferred characteristics.
- Plans for the synthesis of compounds through chemical reactions.
- Ligand-Driven compound evaluation.
- Tissue-specific biomarkers identification.
- Prediction of biomarkers of clinical end point.
- Automated workflow assistance.
- Clinical trails
AI in Drug Discovery
Machine learning
The fundamental paradigm of machine learning (ML) uses a variety of method-based domains and algorithms to find patterns in data. All automation-based techniques make use of DL and ML, but they differ in that, as shown in Fig.4.Additional classifications for deep learning and machine learning is the branch of ML that works with artificial networks that connect computer components [6]. In the last ten years, significant advancements in computer technology have led to the rise of machine intelligence and artificial learning. Large-scale data collection and processing capabilities have undergone revolutionary advancements as a result. At the same time, it is now unaffordable to introduce new medications to patients and the market [7]. Algorithms for machine learning and optimization have been somewhat used in the past in industrial systems, particularly the production of pharmaceuticals. Additional instances of modeling and then optimizing a manufacturing system using genetic algorithms and machine learning include, Here, pharmaceutical processes like crystallization the subject of our current application have also been subjected to the application of statistical tools like DOE, chemo metric models, and other process analytical technologies [8]. The application of machine learning and deep learning in pharmacogenomics research has demonstrated significant advancements. The use of these methodologies enables the analysis of large datasets, making them valuable tools for drug discovery. By combining genomics with pharmacokinetics, they effectively enhance the process. Deep learning, a subset of machine learning, offers the advantage of representation learning, eliminating the need for manual feature extraction. This advancement significantly improves performance in various machine learning applications, especially in the fields of genomics and drug discovery. The incorporation of artificial intelligence techniques, including machine learning, deep learning, and probabilistic graphical models, surpasses traditional statistical approaches that rely on single or multiple variables. As a result, it brings about a remarkable enhancement in pharmacogenomics. [13]
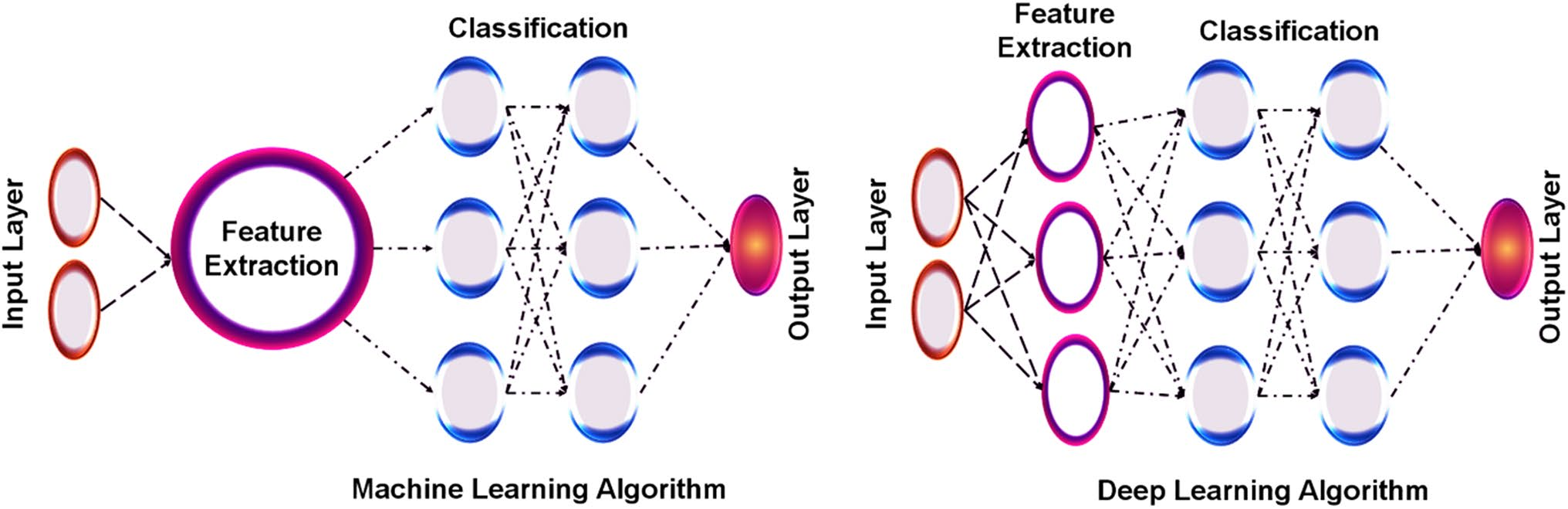
Fig.4: Difference between machine learning and deep learning.
Machine learning applications in structure-based drug design.
Identifying suitable drug candidates and assessing potential targets are foundational steps in the computational drug discovery process. The selection of targets, which involves ranking candidate targets based on their likelihood of success and evaluating the drug potential of lead molecules, is essential for understanding disease pathology. However, due to the complexity of human diseases, the target selection process necessitates advanced methodologies that can integrate diverse data, elucidate the molecular mechanisms underlying disease phenotypes, and help pinpoint patient-specific variations. Innovative techniques such as artificial intelligence and machine learning have been employed to tackle these challenges. For example, deep learning algorithms are used to predict retrosynthetic pathways for small molecules exhibiting specific bioactivity and to design new chemical entities. The SPIDER model for predicting drug equivalence relationships employs self-organizing maps (SOM) as one of the machine learning techniques, leveraging neural network algorithms to convert input vectors into feature maps in an unsupervised fashion. [6]
Machine learning in drug ability prediction
Various machine learning models are employed to evaluate the drug binding pocket. A range of features is utilized to determine the appropriateness of the drug target. Surface Cavity Recognition and Evaluation is a prominent web server that utilizes random forest classifiers. Key elements in the classification process encompass the surface of the protein's active site, the spatial distribution, as well as the shape and geometry of the active site cavities. [6].
Application of Machine Learning:
- Disease Identification/Diagnosis
- Personalized Treatment/Behavioral Modification
- Drug Discovery/Manufacturing
- Clinical Trial Research
- Radiology and Radiotherapy
- Smart Electronic Health Records
- Epidemic Outbreak Prediction
AI with nanotechnology
Artificial Intelligence (AI) is playing an increasingly vital role in the pharmaceutical industry, including areas such as drug formulation and distribution. This shift is largely due to the extended production timelines, elevated costs, and reduced efficiency associated with traditional medical products. Moreover, the development of current formulations relies heavily on research that is often expensive, time-intensive, and prone to errors. A groundbreaking method referred to as "computational pharmaceutics" is integrating big data, artificial intelligence, and multi-scale modeling techniques in the realm of pharmaceutics, suggesting a significant potential shift in drug delivery systems. This innovative approach has arisen from the extraordinary progress in computing capabilities and algorithms over the last ten years. Artificial intelligence (AI) is utilized in various aspects of pharmaceutical product development, including drug distribution, evaluating physical stability, creating in vitro-in vivo correlations, pre-formulation of physical and chemical characteristics, and forecasting activity.
Modern Nano-biotechnology has emerged from four decades of innovative collaboration among biology, medicine, and nanotechnology. Its application in various areas of the medical field is on the rise. Numerous medical challenges, ranging from disease detection to treatment, are benefiting from advancements in Nano-biotechnology. This encompasses drug discovery, personalized medical approaches, cancer therapies, disease diagnostics, pharmaceutical innovations, and the latest medical devices and techniques. Nano-medicine represents a broad field of research and technology that encompasses multiple medical applications, including pain management, disease prevention, treatment and diagnostics, enhancement of human health, trauma damage mitigation, and therapeutic options for various conditions. [10]. Nanotechnology has the potential to revolutionize the field of healthcare diagnostics. It offers improved accuracy, sensitivity, and efficiency in medical tests. One notable application is the use of nanoparticles to detect specific biomarkers, which enhances imaging techniques such as positron emission tomography (PET), computed tomography (CT), and magnetic resonance imaging (MRI). This advancement leads to increased precision, sensitivity, and specificity in these imaging procedures. Additionally, nanotechnology aids in the development of point-of-care diagnostic tests that can quickly and reliably identify cancers, infectious diseases, and various other health conditions. This allows for prompt treatment and implementation of preventive strategies. [10]
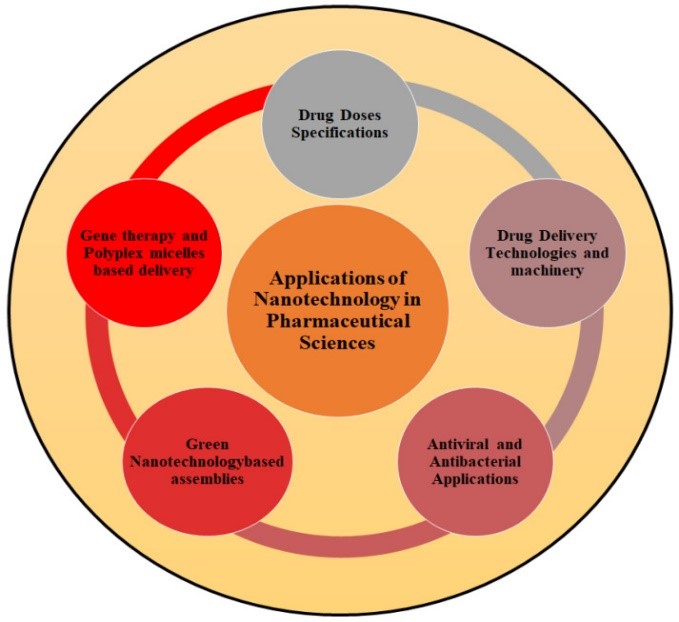
Fig .5. Application of Nanotechnology in Pharmaceuticals
Clinical Trials:
In drug discovery, clinical trials are the most resource-intensive and lengthy phase, requiring considerable financial investment. Despite the extensive time and resources allocated to these trials, the probability of achieving success remains low for those that gain approval from the Food and Drug Administration (FDA). Clinical trials frequently face numerous obstacles that can lead to failure, such as insufficient participant recruitment, drop-outs during the study, adverse reactions to the investigational drug, or inconsistencies in data collection. Failures occurring in the later stages of clinical trials, especially in phases III and IV, can impose significant financial burdens on sponsors. The high costs associated with these trials also influence the pricing of therapies for patients. Consequently, biopharmaceutical companies often factor the research and development expenses of unsuccessful trials into the pricing of approved drugs to ensure profitability. [1]. The execution and oversight of clinical trials encompass several essential components, including trial design, patient recruitment and selection, site selection, monitoring, and data collection and analysis. Patient recruitment and selection, in particular, pose considerable challenges, with 80% of trials exceeding their enrolment timelines and 30% of phase-III trials being halted prematurely due to difficulties in recruitment. Additionally, monitoring in multi-centre global trials is both costly and labour-intensive. Other obstacles include the extended period from the "last subject last visit" to the submission of data to regulatory bodies, necessitating thorough data collection and analysis. However, advancements in AI and digitization are beginning to address these challenges in clinical trials. [1] Artificial intelligence is being utilized to enhance the efficiency of clinical trials while lowering expenses. AI algorithms facilitate patient recruitment, pinpoint appropriate trial demographics, and refine trial protocols. Additionally, AI enables on-going surveillance and evaluation of trial data, which supports adaptive trial designs and accelerates decision-making processes. [2] AI significantly contributes to the improvement of clinical trial design and management. Through the analysis of historical data, AI technologies can optimize trial protocols and anticipate possible medical issues that participants may face. Furthermore, AI aids in boosting participant satisfaction and elevating enrolment rates in clinical research. Artificial intelligence facilitated the assessment of patient feedback gathered from multiple sources, such as patient forums and social media, through sentiment analysis and natural language processing. By leveraging these valuable insights into patient experiences, trial administrators will be more capable of promptly addressing concerns, enhancing their engagement strategies, and boosting participant involvement. The incorporation of AI into clinical trial optimization represents a significant transformation through a data-driven, patient-centred methodology. This advancement enhances the efficiency of processes, reduces expenses, and accelerates the introduction of innovative therapies to the market [14].
1. The Application AI in hiring and selecting patients for clinical trials.
Patient hiring and selection for clinical trials are significantly influenced by AI. The conventional approaches often entail labor-intensive and expensive manual screening and hiring procedures. AI algorithms have the ability to analyze extensive patient data, including electronic health records and genomics, in order to identify appropriate candidates for clinical trials based on specific requirements. Through the automation of the screening process, AI expedites patient hiring , enhances trial enrollment rates, and promotes greater diversity among trial participants[11].
2. Optimizing trial design and enhancing patient stratification through the use of predictive models.
AI-based predictive models play a crucial role in enhancing trial design and refining patient stratification. By examining historical clinical trial data, AI algorithms can uncover variables that affect treatment responses and outcomes. These models are capable of forecasting patient reactions to various interventions, allowing researchers to create more effective and targeted trials. Additionally, AI can determine which patients are more likely to have favorable or adverse responses to particular treatments, thereby supporting personalized medicine strategies in clinical trials[11].
3. AI solutions for the continuous assessment of patient safety and the effectiveness of treatments.
AI-based tools facilitate the continuous oversight of patient safety and treatment effectiveness throughout clinical trials. By evaluating data from multiple sources such as wearable technology, patient feedback, and lab results, AI algorithms can swiftly pinpoint possible adverse events or treatment reactions. This capability allows researchers and healthcare professionals to take timely action, thereby safeguarding patient well-being. Additionally, AI can assess patient data to evaluate treatment effectiveness, aiding researchers in making more informed decisions during clinical trials[11]
Application of AI in Clinical Trials:
- Patient selection and hiring; AI-powered algorithms identify suitable patients,improvimg trial enrollment.
- Data management: AI streamlines data collection, cleaning, and analysis.
- Predictive modeling: AI forecasts patient outcomes, allowing for adaptive trial design.
- Site selection: AI identifies optimal trial sites based on patient demographics and disease prevalence.
- Safety monitoring: AI detects potential safety issurs, enabling prompt action.
- Personalized medicine: AI helps tailor treatments to individual patient characteristics.
- Virtual trails: AI enables remote participation, expanding trial accessibility.
Benefits of AI in Clinical Trials:
- Increased efficiency (up to 30% reduction in trial duration)
- Improved patient outcomes (targeted treatments)
- Enhanced data quality(reduced errors)
- Reduced costa(optimized resource allocation)
- Faster regulatory approvals
- Better trial design (adaptive, Bayesian methods )
- Increased patient engagement (personalized approaches)
Challenges of AI in Clinical Trials:
- Data standardization and integration.
- Ensuring AI explain ability and transparency
- Addressing bias in AI decision making
- Maintaining patient data privacy
- Regulatory frameworks(evolving guidelines)
Pre-clinical research
- Identification of novel targets and prediction of toxicity.
By enhancing and expediting the discovery of novel biological targets, like genes and proteins, artificial intelligence holds promise for addressing unmet medical needs. Access to large pharmacokinetics (PK) and pharmacodynamics (PD) datasets from previous preclinical and clinical research, including data from failed trials, is crucial for developing and improving efficient algorithms that can generate new stable molecules with real therapeutic benefits. However, a major barrier to effectively utilizing AI's potential in drug discovery is the lack of publicly available PK/PD data, which is sometimes caused by competitive or private concerns. Various artificial intelligence methodologies for safety prediction are examined. Notably, there is software applications that can forecast drug toxicity based on target data. Reliable toxicity predictions could potentially supplant conventional in vitro and animal testing as the primary pre-clinical approach. In development pipelines, the models can serve as tools for risk management and prioritization, sending out early warnings for high-risk substances that pose serious safety risks.
Model interpretation presents challenges similar to those found in other areas of artificial intelligence, particularly due to the significant uncertainty that often characterizes the initial stages of research. Improving interpretability and fostering trust in the predictions provided require an understanding of the biological mechanisms at work as well as model attributes.
[12].
Importance of pre-clinical research
- Assessment of dosage, toxic levels, pharmacological effects, and related factors.
• Compliance with regulatory agency requirements.
• It is essential to evaluate the safety of the drug in animal models prior to human trials.
• Analysis of the drug's kinetic profile to inform the choice of administration route.
Several steps in pre-clinical research
- Identify a drug target
- Develop a bioassay
- Screen the drug in the assay
- Establish effective and toxic doses
- File for approval as investigational new drug
AI In Pharmaceutical Formulation
1. AI in the Development of Controlled-release tablets
The development of controlled-release formulations requires the use of pharmacokinetic simulations and artificial neural networks (ANN). Here, the ANN model for 22 tablet formulations of a particular model medicine incorporates three tablet characteristics—hardness, particle size, and moisture content—as well as seven formulation factors. The cumulative percentage of drug release measured in vitro at 10 distinct sampling intervals is the source of the results produced by this model. Using input and output data that have been processed with chemical software improves the ANN model's functionality. Using two particular in vivo release profiles and two desirable in vitro dissolution profiles, this advanced artificial neural network (ANN) model is used to forecast the best tablet formulations. One important component is the disintegration process. [15]
2. AI in Development of Immediate-release tablets
To increase the tablet's strength, Turkoglu created a formulation for direct compression tablets that contained hydrochlorothiazide. Caravan and Peck developed a caffeine-containing tablet formulation in a different trial, emphasizing the various components of each formulation, such as the type and concentration of diluents and binders, along with processing parameters like the granulator type and the method of binder addition. They assessed the characteristics of the resulting granules and tablets, focusing on disintegration time, hardness, and friability. Both studies showed that neural networks performed better than traditional statistical methods. Consequently, a number of genetic algorithms and neural networks were used to better investigate Caravan and Peck's findings. This research confirmed that the limits placed on each component and processing variable, as well as the relative relevance of the output qualities, had an impact on the optimal formulation. Furthermore, the same data was examined through the lens of neuron-fuzzy computing, which led to the creation of effective guidelines that emphasize the most critical factors for each asset. [15]
3. AI in Product Development
The development of pharmaceutical products involves a multifaceted optimization challenge that requires careful adjustment of both formulation and process variables. The nature of these models makes them particularly effective in addressing the optimization issues encountered in pharmaceutical product development. Artificial Neural Networks (ANN) have demonstrated superior fitting and predictive capabilities in the formulation of solid dosage forms, particularly in studies examining how various factors, including formulation and compression parameters, influence the physicochemical properties of tablets. This advancement has resulted in a valuable resource for enhancing micro-emulsion-based drug delivery systems, significantly reducing the need for extensive experimental trials. Furthermore, it has enabled the forecasting of phase behavior in quaternary systems that include oil, water, and two surfactants. ANN is utilized to simulate aerosol behavior, aiming to use this method to assess and develop pulmonary drug delivery systems. Fuzzy logic stands out as an effective method for addressing problems, particularly in situations involving control and decision-making. The integration of neural networks with fuzzy logic presents a promising approach that enhances the adaptability and effectiveness of the method, resulting in more dependable outcomes. [15]
4. AI in Hard Gelatine capsule shell formation development
Artificial neural networks (ANN) and expert systems (ES) are essential tools for decision-making in the development of hard gelatin capsules. ANNs are designed to replicate certain functions of human cognition, including generalization, learning, prediction, and abstraction based on expertise. Through the use of ANNs, researchers can transform gathered data and statistics into actionable insights, enabling formulators to establish targeted guidelines for future formulations or anticipate the properties of hypothetical formulations. In 2005, Wendy I. Wilson created a capsule shell formulation for BCS class II medications by improving the Expert Network for analytical needs. The capsule expert system, serving as a centralized resource for developing powders in hard gelatin capsules, has been utilized globally, even though it mainly offers recommended formulations. Multiple model drugs, including carbamazepine, ketoprofen, naproxen, diazepam, chlorpropamide, and ibuprofen, were formulated and evaluated for their dissolution efficacy. Preliminary assessments revealed substantial mistakes and restricted forecasting precision in the system. [15]
Application of AI in Pharmaceuticals
1. in drug discovery:
- Drug screening
- Drug design
- Drug repurposing
- Poly-pharmacology
2. Pharmaceutical product development:
- Nano-robots drug delivery
- Controlled insulin release
- Combination drug delivery
- Nano-medicine
3. Drug synergism and antagonism prediction
4. Medical diagnosis and clinical diagnosis
5. Research and Development (R&D)
6. Quality control and quality assurance (QA and QC)
7. Pharmaceutical manufacturing
8. Clinical application:
- Radiology
- Oncology
- Cardiology
- Gastroenterology
- Ophthalmology
- Surgery
9. Drug prescription and precise medication
Advantages and Disadvantages of AI in pharmaceuticals
CONCLUSION
The pharmaceutical sector is consistently advancing its technological capabilities, and the incorporation of artificial intelligence offers a substantial opportunity to decrease the expenses and duration involved in drug development. These AI applications are capable of examining unstructured data and correlating it with existing information to predict accurate results, thereby proving essential for forecasting particular disease diagnoses. [1] Artificial intelligence is transforming drug delivery methods by enabling targeted, personalized, and flexible treatment strategies. Utilizing AI's capabilities in data analysis, pattern identification, and optimization, pharmaceutical scientists and healthcare providers can boost drug efficacy, minimize adverse effects, and enhance patient results. The integration of computational pharmaceutics, artificial intelligence, and big data is revolutionizing the drug delivery process, resulting in a more effective, economical, and data-informed approach. AI techniques, especially Machine Learning (ML) and Deep Learning (DL), play a crucial role in the pharmaceutical industry. These methods are highly effective at examining the connections between chemical structures and the biological activities of lead compounds obtained from comprehensive pharmaceutical databases. Lately, AI-powered tools have gained significant popularity in computer-aided drug development because of their enhanced data mining skills. The incorporation of AI into the drug development and approval process includes every stage of a medication's lifecycle. Nonetheless, even with the release of reflection papers and strategic action plans related to AI by regulatory authorities, a notable lack of thorough regulatory direction specifically targeting the use of AI in clinical trials persists. [12]
REFERENCES
- Junaid Bajwa,A Usman Munir,B Aditya NoriC and Bryan WilliamsD , Artificial intelligence in healthcare: transforming the practice of medicine.2021; ,Future Healthcare Journal 2021 Vol 8, No 2: e188–94.
- Lalitkumar K. Vora 1, Amol D. Gholap 2, Keshava Jetha 3, 4, Raghu Raj Singh Thakur 1,Hetvi K. Solanki 5 and Vivek P. Chavda 3, Artificial Intelligence in Pharmaceutical Technology and Drug Delivery Design.2023; Pharmaceutics 2023,15, 1916.
- Kit-Kay Mak1,2and Mallikarjuna Rao Pichika2,3, Artificial intelligence in drug development: present status and future prospects.2019; Drug Discovery Today Volume 24, Number 3 March 2019.
- By Alexandre Blanco-González 1,2,3,Alfonso Cabezón 1,2,Alejandro Seco-González 1,2,Daniel Conde-Torres 1,2,Paula Antelo-Riveiro 1,2,Ángel Piñeiro 2,* andRebeca Garcia-Fandino 1, , The Role of AI in Drug Discovery: Challenges, Opportunities, and Strategies. 2023; Pharmaceuticals 2023, 16(6), 891; https://doi.org/10.3390/ph1606089
- Rizwan Qureshi a,e,?, Muhammad Irfan b, Taimoor Muzaffar Gondal c, Sheheryar Khan d, Jia Wu e, Muhammad Usman Hadi f , John Heymach g, Xiuning Le g, Hong Yan h, Tanvir Alam , AI in drug discovery and its clinical relevance .2023; Heliyon 9 (2023) e17575
- Chandrabose Selvaraj1 · Ishwar Chandra1 · Sanjeev Kumar Singh1, Artifcial intelligence and machine learning approaches for drug design: challenges and opportunities for the pharmaceutical industries, 2022; Molecular Diversity (2022) 26:1893–1913.
- Sheela Kolluri1, 3, Jianchang Lin2 , Rachael Liu2 , Yanwei Zhang2 and Wenwen Zhang2,Machine Learning and Artifcial Intelligence in Pharmaceutical Research and Development: a Review,2022; The AAPS Journal (2022) 24: 19.
- Hoi-Ming Chi a,b , Herbert Moskowitz a , Okan K. Ersoy b , Kemal Altinkemer a, ?, Peter F. Gavin c , Bret E. Huff c , Bernard A. Olsen c, Machine learning and genetic algorithms in pharmaceutical development and manufacturing processes.2009; Decision Support Systems 48 (2009) 69–80
- Ayesha Sultana1 , Rahath Maseera2 , Abdul Rahamanulla3 and Alima Misiriya1, Emerging of artifcial intelligence and technology in pharmaceuticals. review.2023; Sultana et al. Future Journal of Pharmaceutical Sciences (2023) 9:65
- Shiza Malik 1, Khalid Muhammad 2,* and YasirWaheed 3,4,*, Emerging Applications of Nanotechnology in Healthcare and Medicine . Review 2023; Molecules 2023, 28, 6624. https://doi.org/10.3390/molecules28186624
- N. Srinivasan & N.Srinivasan , AI Empowering Parmaceuticals:Revalutionizing The Future Of Healthcare, Application of AI In Emerning Reaserach & Education,Volume 2. Review 2023; ISBN : 978-81-965582-7-7
- Scott Askin1,2 · Denis Burkhalter 1,2 · Gilda Calado 1,3 · Samar El Dakrouni 1,4 , Artificial Intelligence Applied to clinical trials: opportunities and challenges. Review 2023; Health and Technology (2023) 13:203–213 https://doi.org/10.1007/s12553-023-00738-2.
- Hamed Taherdoost a,b,* , Alireza Ghofrani c,d , AI's role in revolutionizing personalized medicine by reshaping pharmacogenomics and drug therapy. Review 2024; Intelligent Pharmacy.
- Agyemang Kwasi Sampene1* and Fatuma Nyirenda, Evaluating the efect of artifcial intelligence on pharmaceutical product and drug discovery in China. Review 2024; Sampene and Nyirenda Future Journal of Pharmaceutical Sciences.
- Nagalakshmi Sethuraman,Artificial Intelligence: A New Paradigm for Pharmaceutical Applications in Formulations Development. Review 2020; Indian Journal of Pharmaceutical Education and Research | Vol 54 | Issue 4 | Oct-Dec, 2020 .
- Sahu, Adarsh; Mishra, Jyotika; Kushwaha, Namrata. Artificial Intelligence (AI) in Drugs and Pharmaceuticals . 2022; Combinatorial chemistry & High throughput screening25 (11), 1818-1837, 2022.
- Patil Prasad1, Nrip Nripesh Kumar1,*, Hajare Ashok2, Hajare Digvijay3, Patil Mahadev K.4, Kanthe Rajesh1, Gaikwad Anil T., Artificial intelligence and tools in pharmaceuticals: An overview , 2023; Research Journal of Pharmacy and Technology Year : 2023, Volume : 16, Issue : 4.


 Khairnar Tejaswini *
Khairnar Tejaswini *
 Kapdane Shruti
Kapdane Shruti
 Kawale Sakshi
Kawale Sakshi
 Karad Rutika
Karad Rutika
 Aher Pankaj
Aher Pankaj









 10.5281/zenodo.14836604
10.5281/zenodo.14836604