Abstract
1,3,4-Oxadiazoles are five-membered heterocyclic compounds containing two nitrogen atoms and one oxygen atom. These versatile molecules exhibit a range of biological effects, including antibacterial, antifungal, and antiviral properties. Recent research has demonstrated that many 1,3,4-oxadiazole derivatives surpass the activity of existing antibiotics and other antimicrobial agents. Their potential as novel drugs are promising, making them valuable candidates for future medicine and agriculture applications 1,2,4-Oxadiazoles are five-membered heterocyclic compounds that exhibit a broad spectrum of biological activities, including antibacterial, antifungal, and antiviral properties. Recent research has demonstrated the potential of these derivatives as novel agents against microbial infections. Specifically, some 1,2,4-oxadiazole compounds showed strong antibacterial effects against Xanthomonas oryzae strains, surpassing existing reference drugs. These findings highlight the promise of 1,2,4-oxadiazoles as alternative templates for discovering novel antibacterial agents
Keywords
(AMR) Antimicrobial resistance, Antifungal & Antibacterial activity, Gram positive & Gram-negative bacteria
Introduction
ANTIFUNGAL AND BACTERIAL DATA:
Worldwide, infectious diseases resulting from bacteria and fungus impact millions of individuals. Because organisms are becoming increasingly resistant to the medications now used to treat them, systematic initiatives to find and develop novel antifungals are needed. The antifungal medications used today as previously indicated, delayed, incorrect, or underlying illness diagnoses are frequently the cause of the high morbidity and mortality rate associated with fungal infections. However, other significant issues include low antifungal availability, high toxicity, and species.
specific resistance to antifungals. The many kinds of antifungals available today along with how they work below. [1] At least 3.6 million Americans suffer from the effects of fungus species that are resistant to medications annually. Many fungal illnesses are common in India, and the nation frequently acts as an isolation site for a number of pathogens that are widespread around the world. The presence of fungi-related illnesses passages in the Atharva Veda, an old Hindu text composed between 1500 and 500 BCE, where mycetoma ("pada valmikam," or "anthill foot") is referenced among the Indian populace, indicate that the condition has been in the Indian subcontinent since ancient times. (8) Periodically, reports of several outbreaks of various fungal infections including Candida auris and, more recently, COVID-19-associated mucormycosis have been made from various regions of India. As per the findings of Animesh Ray's (2022) recent study, 4.1% of India's overall population suffers from a serious fungal illness. Additionally, the study highlights how significant the fungal load is in India and how it is frequently underestimated in clinical settings. (7) Since human cells and fungal cells have many characteristics, therapies that work well against fungus may be harmful to human cells as well. In order to effectively decrease fungal survivability without causing further problems for patients, newly developed medications must find a balance. Aspergillus fumigatus's resistance to azoles, Candida glabrata's resistance to echinocandins, and an increase in azole resistance among isolates that are not Candida albicans are the current trends of acquired antifungal resistance. The need for developing novel therapies for fungal infections is growing due to the alarming rise in fungal "superbugs" that are immune to the medications that doctors have been prescribing for a long time.
Fungal infections overview:
For individuals with various comorbidities and increased susceptibility because of things like immunosuppression, the options for treating invasive fungal infections are limited. The increased susceptibility could jeopardize the effectiveness of treatment even in the absence of medication resistance. It is clear that in order to address this problem, new treatment approaches are required, in addition to overcoming the toxicities, side effects, and drug interactions connected to the antifungals that are now market. (8) Because of the emergence of resistance that comes with the extensive use of antifungal medications, treating invasive fungal infections continues to be a difficult issue. Consequently, to treat invasive fungal Infections brought on by resistant organisms, medicinal chemists have concentrated on creating more potent medicines.
The wide range of applications and their unique metabolic profile have made oxadiazoles extremely valuable in medicinal chemistry. Numerous investigations have validated the antifungal properties of oxadiazole derivatives. Owing to nucleophilic substitution processes occurring on the oxadiazole ring, 1,3,4-oxadiazolin-2-thiones are among the oxadiazole derivatives that have drawn the most interest in heterocyclic chemistry as adaptable intermediates willingly.[13] It has been discovered that pyrimidine derivatives contain anticandidal properties. an azole derivative with a pyrimidine group called voriconazole is frequently used as an antifungal medication to treat candidiasis. However, several researchers have also done a great deal of study to find new antifungal drugs with thioether moiety. Notable substances with thioether groups include sulconazole and butoconazole, which are all prescribed as antifungal medications to treat candidiasis. The current work synthesized novel oxadiazole derivatives with the two crucial functional moieties of pyrimidine and thioether, and also elucidated their anticandidal properties. [14] Fungal species can range from beneficial to detrimental. Beyond the practicality, additionally, mild to malevolent diseases are caused by fungi. Numerous living things, including plants, animals, and other fungus, are infected by the fungi. It is imperative that we talk about human pathogenic fungus in particular. Aspergillosis, candidiasis, and other deadly diseases are caused by human pathogenic fungi. People who have compromised immune systems are more vulnerable to infections caused by Aspergillus, Candida, Cryptococcus, Histoplasma, and Pneumocystis. Additionally, fungi can harm skin, nails, hair, and eyes. Mycotoxins, which are secreted by pathogenic fungi and result in food poisoning and deterioration, may also be the cause of fungal activity. These are all very important issues that need to be given top emphasis. Regarding the mode of action, numerous fungicidal mechanisms exist.[23] The suppression of fungal cell wall production is the first identified mechanism of action. The antifungal chemicals prevent the cell wall from being biosynthesised. One can mention penicillin’s and cephalosporins in this group of antifungals. The azole antifungals function by attaching to or targeting ergosterol, which creates pores and makes the membrane leaky. Alternatively, inhibition may take place through blocking the production of ergosterol. Nucleoside antifungal drugs also influence cell division by specifically targeting the microtubule. It has been shown that the heterocyclic medications have strong antifungal effects and are being developed as antifungal medicines. Quinols derivatives find a place and can be cited as an effective class of antifungal compounds among the many heterocycles intended to suppress fungus. Using adaptable pharma cores to derivatize the quinoline molecule might yield a vast array of antifungal medicines. Hereafter, a few recently identified antifungal drugs will be examined in this context.[24]
Fungal Infections (Global Problem):
Account for some of the most difficult human diseases to manage. Fungal-related disease occur in healthy individuals but the most considerably they manifest in severe illness, jeopardizing medical treatment for other serious diseases. Fungal infections led to reduced quality of life for billions of individuals worldwide and the numbers of fungal infections are increasing every year. Candidemia, caused by the Candida family yeast are one of the most sever invasive fungal infections. New emerging pathogenic fungi like, the recently characterised organism C. auris that is able to evade drug therapy targets are making it harder for affected individuals to receive effective medical treatment. In addition, there is an eminent global concern regarding an increase in fungal drug resistance. Azoles, echinocandins and other antifungal drug therapies do not provide the most effective treatment for fungal infections not only for the serious pathogenic organism C. auris but also for the most common fungal pathogen, C. albicans. Therefore, the need for development of new, effective and safe antifungal agents is critical.[25]
Cellular biology of Candida species:
Candida species are genera of a diverse fungal kingdom. Candida is ascomycetous-like fungal species where most family members are anamorphic. C. albicans undergo a diploid life cycle and express a wide-ranging variety of morphological phenotypes due to their ability to phenotypical switch and transitional switch from yeast bud to other forms. The incidence frequency of Candida species isolates differs based on patient characteristics such as, age or ongoing disease, and geographical location. C. albicans and C. parasitosis are predominantly observed in younger individuals and their infection prevalence decreases with age. In comparison, C. glabrata prevalence in candidemia infected patients increased with older age. Underlaying disease is also a factor in for the advancement of candidemia. Candida species are a diverse family of yeast that holds responsibility for IFIs at various ages and geographical position. Infection prevalence per Candida species.[45]
Herbal antifungal oxadiazole:
Fungal and nematode-caused plant diseases are getting worse and have a significant influence on the health and viability of plants. Worse even, when nematodes and fungi are combined, they sometimes create a synergistic relationship that results in a larger crop loss than when each pathogen is present alone or in conjunction with other impacts. More than 8000 species of fungi, such as are currently known to cause plant diseases. These fungi are responsible for 85% of plant diseases and significant economic crop losses.[46]
Plant parasitic nematodes called Meloidogyne incognita, on the other hand, have caused significant harm to cereal crops, forestry, and agricultural crops, respectively. Fungi-caused plant diseases have emerged as a global concern in agriculture. Chemical prevention is the most effective and practical method of controlling nematodes and fungus. Nevertheless, the misuse of currently available pesticides has resulted in the development of drug-resistant bacteria, making the issue even more difficult to manage overall and possibly posing a serious risk to public health.[26]
Furthermore, hardly many pesticide compounds have the dual ability to control nematodes and fungus. To effectively control these plant diseases, it will be crucial to create new, non-resistant pesticides. The market for succinate dehydrogenase inhibitors has grown quickly over the last several years, making them a crucial field of novel chemical development. As transport chain, succinate dehydrogenase is essential to both processes. The SDHI is to inhibit this enzyme. Among them is fluopyram, a brand-new nematicide and fungicide with a unique structure that was effectively created by Bayer combines a fungicide with an amide bridge. including insecticidal, herbicidal, and antifungal properties, among other biological actions. Among these, Monsanto's trioxifene serves as a new kind of seed treatment agent for nematode control. It is a representative nematicide with a core moiety of 1,2,4 oxdiazole. Based on its good control of crop root-knot nematodes in field testing, the industry now believes that 1,2,4-oxadiazole nematicide offers the best chances for market development. Furthermore, it has been observed that compounds of been done on 1,2,4-oxadiazole derivatives against nematodes and fungus.[49] This study also examined the mechanism of action of a number of newly discovered amide fragments that have nematicidal and antifungal properties is clinical isolates of Candida yeast. Finding more potent antifungal medications that can successfully treat these infections is therefore essential. Some herbal products and their active ingredients are included in them. This review's objective is to provide an overview of the present state of research on herbal products and the active ingredients in them that have antifungal efficacy against drug-resistant Candida sp. when used both on their own and in conjunction with antifungal medications. Discovered Because of their large therapeutic windows, carboxylic acid amide fungicides have been extensively used in the design and discovery of agrochemicals.[36]
Scaffold with antifungal activity:
A number of derivatives with better pharmacokinetic profiles and stronger antifungal activities can be designed and synthesized thanks to the scaffold's easy modification. Oxadiazoles are heterocyclic compounds with five members. There exist multiple isomeric forms of oxadiazole that are identifiable in the composition of various medications, such as furamizole, an antibiotic, and zibotentan, an anticancer medicine. The first were created around the close of the 1800s. In the 1950s and 1960s of the 20th century, there was a significant increase in the quantity of research conducted on this chemical. These days, scientists create 1,3,4 oxadiazole derivatives by a variety of techniques, some of which are improved upon earlier approaches. Examples of these techniques include The entire compound's physicochemical and pharmacokinetic properties are impacted by the presence of the 1,3,4-oxadiazole ring. In pharmaceutical chemistry, the 1,3,4 oxadiazole ring has generated interest as a bioisostere for carbonyl-containing compounds, including carboxylic acids, esters, and amides.[15] These characteristics of the 1,3,4-oxadiazole ring have led to a multitude of pharmacological uses for this molecule. The literature claims that for many years, researchers have been creating novel compounds with the 1,3,4 oxadiazole core all around the world. These compounds have demonstrated a variety of biological activities, including Furthermore, a plethora of literature papers attest to the compounds' broad antibacterial action. Researchers have studied antifungal agents. Because list the scientific accomplishments over the past seven years.1,3,4 oxadiazole and combined them into a single unit. True, imidazole rings are present in a lot of current medications. It is impossible to dispute the crucial significance tiny heterocyclic compounds play in medication design. Molecules function as scaffolding with several functions. In this context, the creation of many pharmacological compounds has been greatly aided by the presence of the pyridyl ring, a significant scaffold found in many bioactive molecules.[31] These efforts to develop new drugs have been greatly aided by oxadiazole and its derivatives. The polarity, flexibility, and metabolic stability of the inhibitors can all be altered by adding a, which could disrupt the synthesis of proteins or lipids in pathogens. It also acts as an acceptor of hydrogen bonds creation. As a result, we determined and described the antimicrobial. Many synthesized compounds include the five-membered aromatic ring 1,3,4 Oxadiazole. Because of its unique structural characteristics, they can bind to various enzymes and receptors in biological systems in an effective manner through a variety of weak interactions, triggering a wide range of bioactivities. Scientists now find research on development to be a fascinating subject. Many substances with strong therapeutic potential are being widely employed to treat various illnesses, which has significant development value. the creation of pharmaceuticals with a higher level of activity and lower level of toxicity. Oxadiazoles are heterocyclic compounds with five members that in their structure.[33] Oxadiazole comes in a variety of isomeric forms that are discernible in the composition of several medications, such as the antibacterial furamizole. Derivatives of 1, 3,4oxadiazole show the greatest promise. The first 1,3,4-oxadiazole derivatives were created at the close of the 1800s. The novel structures were produced by a variety of multidirectional processes, such as heat cyclization of 1-acylsemicarbazides and interactions between the proper hydrazides and phosgene. Furthermore, the addition of amino alkyl side chains can significantly increase the parent drug's chemical stability, water solubility, and bioavailability. [35]
Derivatives of documented. It has been demonstrated that these oxadiazoles have analgesic, muscle relaxant, and tranquilizing effects. The literature also reports the presence of central nervous system depressive activity in certain Mannich bases of 5substituted phenyl 1,3,4-oxadiazole 2-thione. These findings led to the synthesis of other Mannich base derivatives of 5-(2,4-dichlorophenyl) that have not yet been published. Potential fungicides: 1,3,4-oxadiazole-2-thiones. Aldehyde, ketone, and amine complexity all had a big impact on how well the percentage yield, temperature, and duration of each synthesized molecule were optimized. the mannich bases of micarbazides derived from aliphatic carbonyl compounds exhibited strong antifungal action.[38] According to statistics, invasive fungal infections claim the lives of around two million people annually; this mortality is three times more than that of malaria, for example. Although synthetic medications have demonstrated great effectiveness in the past, there is a genuine risk of fungal resistance, which arises spontaneously from the use of antifungal agents and has already been identified in numerous unique resistance patterns. Additionally, fungi are a major cause of agricultural losses and the primary initiator of the majority of serious plant diseases. Thus, drug discovery across the various scientific areas is embedded with the identification of new antifungal schedules. Because of the chemical diversity of the various scaffolds they offer, plants provide a significant resource platform for such initiatives and are therefore often used in traditional therapies to fungal diseases.[39]
The antibacterial drug furamizole, the antiretroviral drug antidiabetic agent AZD 3988, and the antihypertensive drugs tiodazosin, and nesadipil. In addition, there is a growing interest in the chemotherapeutic activities of antibacterial, antifungal, antitubercular, and antiviral. agents. Moreover, 1,3,4-Oxadiazole-2(3H)-thiones, derivatives exert their anticancer activities via different mechanisms, such as targeting epidermal growth factor receptors (EGFR), vascular endothelial growth factor receptors (VEGF), focal-adhesion kinase (FAK), histone deacetylases (HDAC), methionine aminopeptidase (MetAP), NF-?B (nuclear factor ?B), poly(ADP-ribose) polymerase (PARP-1), thymidine phosphorylase (TP), telomerase, thymidylate synthase (TS), zinc-finger protein 143 (ZNF143), and tubulin polymerase, 1,3,4-oxadiazoles were proved to exhibit potent anti-inflammatory , antioxidant , antidiabetic and monoamine oxidase (MAO) inhibitory activities. Besides, 1,3,4-oxadiazole derivatives are highly attractive compounds in the development of organic light-emitting diodes (OLEDs). Furthermore, 3,4-dimethoxyphenyl moiety represents an essential motif in various chemotherapeutic agents.[41] Invasive fungal infections due to Candida species are being increasingly shown as a main reason of morbidity and mortality in the healthcare environment. These infections typically occur due to the effect of five most general pathogens C. glabrata, C. albicans, C. krusei, C. parapsilosis, and C. tropicalis. Each of these organisms has unique virulence potency, antifungal susceptibility and epidemiology; however, when considered as a whole, important organism-related infections are often referred to as invasive candidiasis. The high prevalence of antifungal resistance of pathogenic Candida species to existing drugs reduces the effectiveness of treatment; thus, the development of alternative antifungal agents is required. Due to the low incidence of fungal infections, the rate of development of new antifungal agents is lower than that of antibacterial agents. In addition, fungi are eukaryotic (as are their human hosts) antifungal targets. However, detailed information on the nature of fungal cells contributed to the understanding of the mechanisms of action of many antifungal agents in addition to a variety of fungal infections. Efforts are expected to reduce toxicity, increase bioavailability, improve the antifungal spectrum and combat resistance, and enhance the efficacy of existing antifungals. In fact, elucidation of the mechanism of action of a potential antifungal compound can reduce the time period from lead compound to drug candidate agent. Currently, the available antifungal agents to treat Candida infections can be divided into four categories based on their mode of action, including azoles, polyenes, echinocandins, and antimetabolites. Among these agents, azole groups are most widely used in antifungal therapy. The 1,3,4-thiadiazole compound is a five-membered heterocyclic scaffold including diverse physicochemical properties. It is a mesoionic system associated with the discrete regions of positive and negative charges leading to ? and ? electrons and highly polarizable derivatives. This distinguishing feature allows mesoionic compounds to effectively cross cellular membranes and interact with biological molecules in unique ways, which highlights the high potential of this ring system in medicinal chemistry. Furthermore, it was reported that the toxophoric –N–C–S moiety of the 1,3,4-thiadiazoles is probably responsible for a broad range of biological activity. For instance, 1,3,4-thiadiazole derivatives were extensively used as pesticides with a wide range of biocidal properties, among which antifungal activities attracted particular attention as part of a comprehensive project for the development of agricultural fungicides. The good liposolubility of the sulfur atom in the thiadiazole compounds might also have a positive effect on the biological activity. Thiadiazole is a bioisoster of triazole and imidazole, which are members of the azole group with antifungal importance. Imidazole- and triazole-based synthetic antifungal drugs prevent the conversion of lanosterol to ergosterol and inhibit the enzyme cytochrome P450 14-?-demethylase enzyme. Based on the bioisosterism and antifungal potency of azole-type compounds, researchers reported several studies including thiadiazole derivatives and their antifungal activity. From this point of view, in our previous paper, we reported the synthesis and antifungal activity evaluation of some oxadiazole–thiadiazole derivatives, which target the P450 14-?-demethylase enzyme. Results of our study encouraged us to synthesize more compounds including thiadiazole nuclei. Thus, we prepared a new series of thiadiazole derivatives, and tested their antifungal activities with in vitro and in silico studies.[46] Candida albicans is the most prevalent fungal human pathogen. The entire life cycle of this polymorphic fungus occurs in mammalian hosts and involves switches between distinct single-celled yeast and multicellular mycelial forms. As a yeast form, C. albicans exists as a ubiquitous commensal of human microbiome, colonizing the skin, mouth, gastrointestinal tract, and female reproductive tract of healthy adults. In weakened hosts, however, particularly in immunocompromised or critically ill patients or those who developed microbial dysbiosis, the C. albicans life style changes from commensal to pathogenic. The change involves a cell-type transition between benign yeast and invasive, e.g. hyphal morphology, causing diseases varying from relatively easily treatable topical (e.g. athlete’s foot and oral thrush) to life-threatening systemic infections (such as disseminated (deep-seated) candidiasis, particularly candidemia), which result in up to 400,000 human deaths annually. With the availability of modern medical treatments, including anticancer chemotherapy, organ transplantation, use of different types of implantable medical devises, and administration of broad-spectrum antibiotics and immunosuppressive agents, the number of vulnerable individuals has risen over the past several decades and so has the incidence of candidiasis. Candidemia is one of the most common healthcare-associated bloodstream infections in hospitals, especially in intensive care units, in the United States and worldwide, with a mortality of 30–55%. The clinical treatment includes three types of drugs, azoles (1,3-imidazole and 1,2,4-triazole derivatives), amphotericin B, and echinocandins. Echinocandins inhibit (133)-Dglucan synthase and thus damage the fungal cell wall. Amphotericin B removes ergosterol from fungal plasma membranes, disrupting their structure. Azoles (the largest class of antimycotic drugs in clinical use block biosynthesis of ergosterol de novo, not only depleting the source of ergosterol for the membranes but also preventing the formation of physiologically important (hormonal) intracellular sterols, which are required for the cell cycle regulation, cell development and multiplication.[48] The chemistry of oxazolone has received important attraction due to their uses as intermediate for the synthesis of some heterocyclic synthesis?1;. Imidazolidinones have been reported to possess potent CNS depressant activity 2,3. Anticonvulsant activity has also been shown with some imidazole derivatives4,5. 1,2,4-trisubstituded5-imidazolones have been reported to possess monoamine oxidase (MAO) inhibitory and anticonvulsant activity6 . The increasing popularity of Mannich reaction has been fueled by the ubiquitous nature of nitrogen in drugs, natural products and biologically active compounds as well as by its multicomponent reaction to generate diversity7. Nitrogen containing N-Mannich bases which have been evaluated for their pharmacological action. A Chemical substance produced by chemical synthesis, which inhibited the growth of organisms and hence act as antimicrobial agents. These chemical substances interfere the life cycle of organisms with specific processes that are essential for growth and/or division of the cell and many of them are widely used for chemotherapy. Most of the pathogenic bacteria are highly sensitive and susceptible to a new antibiotic or chemical, and thus microorganisms acquire chemical substance/ drug resistance. In view of the advantage offered by applications of Mannich bases in the field of medicine and biology10, we made an attempt to synthesized novel heterocyclic N-Mannich bases of imidazol-5-one and evaluation of their antimicrobial activity against bacterial and fungal microorganisms. Hence, it was thought interesting to undertake the synthesis, characterization and bactericidal activity of trisubstituted imidazol-5-one moiety. The whole work is represented.[49]
There is an important medical need for new antifungal agents with novel mechanisms of action to treat the increasing number of patients with life-threatening systemic fungal disease and to overcome the growing problem of resistance to current therapies. F901318, the leading representative of a novel class of drug, the orotomides, is an antifungal drug in clinical development that demonstrates excellent potency against a broad range of dimorphic and filamentous fungi. In vitro susceptibility testing of F901318 against more than 100 strains from the four main pathogenic Aspergillus spp. revealed minimal inhibitory concentrations of ?0.06 ?g/mL— greater potency than the leading antifungal classes. An investigation into the mechanism of action of F901318 found that it acts via inhibition of the pyrimidine biosynthesis enzyme dihydroorotate dehydrogenase (DHODH) in a fungal-specific manner. Homology modeling of Aspergillus fumigatus DHODH has identified a predicted binding mode of the inhibitor and important interacting amino acid residues. In a murine pulmonary model of aspergillosis, F901318 displays in vivo efficacy against a strain of A. fumigatus sensitive to the azole class of antifungals and a strain displaying an azole-resistant phenotype. F901318 is currently in late Phase 1 clinical trials, offering hope that the antifungal armamentarium can be expanded to include a class of agent with a mechanism of action distinct from currently marketed antifungals. Antifungal drug | Aspergillus fumigatus | mechanism of action | dihydroorotate dehydrogenase Arecent estimate puts the annual death toll from serious fungal infections at 1.5 million. As one of the four greatest killers, Aspergillus species are opportunistic human pathogens, particularly affecting immunocompromised persons, such as transplant recipients and those with hematologic malignancies. Invasive aspergillosis has a high mortality (30–90%) and is estimated to affect more than 200,000 people annually. Other diseases caused by Aspergillus species, including allergic bronchopulmonary aspergillosis and chronic pulmonary aspergillosis, have a significant global impact, affecting millions of patients. There has been a dearth of new drug classes developed to treat systemic fungal infections arriving in the clinic, with the most recent being the echinocandins, introduced in 2001. Only three other classes of antifungal drugs are currently available for the treatment of invasive fungal disease: polyenes (amphotericin B), azoles (e.g., voriconazole, posaconazole, and the recently licensed isavuconazole), and flucytosine. These agents work via a limited range of cellular targets. Echinocandins, such as caspofungin, inhibit ?- glucan synthase, exploiting the most striking difference between the fungal cell and its human counterpart—the cell wall. Two antifungal drug classes target the cell membrane: azoles inhibit ergosterol biosynthesis, and polyenes disrupt fungal membranes via ergosterol binding. Flucytosine is a pyrimidine analog, converted to 5-fluorouracil within fungal cells, that disrupts DNA and RNA synthesis; however, owing to rapid development of resistance, it is used primarily in combination therapy. Issues exist with current therapies, including overt toxicity, drug– drug interactions, variable pharmacokinetics, and increasing levels of drug resistance. In particular, the development of resistance to the azole class of antifungals is concerning, because they are currently the sole orally available antifungal agent for the treatment of aspergillosis. Azole-resistant clinical isolates of Aspergillus fumigatus have been observed and isolated from patients worldwide, including Europe, the United States, Asia, Africa, Australia, and the Middle East Apparently exacerbated by the environmental use of azole fungicides in agriculture, reported rates of azole resistance have approached 30% at certain sites in Europe, with rates outside Europe varying between 0.6% and 11.2% Fungi are causing cutaneous, sub-cutaneous or systemic infections, such as oral thrush, Tenia pedis, Tenia corporis or Tenia capidis. The azoles (imidazoles and triazoles) represent a class of versatile antifungal agents which are used to treat generalized systemic fungal infections. The mechanism of their antifungal action includes the inhibition of cytochrome P450 51 (CYP51), which is essential for ergosterol biosynthesis at the step of lanosterol-14- demethylation.1 The high affinity of the azole antifungals for CYP51 isozymes appears to be determined primarily by their bulky, polycyclic structure, giving favourable interaction with the hydrophobic residues in the largely nonpolar active site of the enzyme.2 In recent years, the widespread use of imidazoles and triazoles antifungal agents has resulted in the development of resistance to these drugs by pathogenic microorganisms, causing an increase in morbidity and mortality. Therefore, new trend of antifungal related to phenylpyrazole is developed in order to get an effective antifungal agent without known resistance.3–17 Although many reported phenylpyrazoles showed significant activity against pathogenic yeast (Candida), unfortunately, no significant effect was obtained against pathogenic moulds such as Aspergillus. 3–17 As part of our ongoing research programme aiming at the synthesis of variety of heterocyclic systems for biological and pharmacological evaluation,18–27 we report here the synthesis of several phenylpyrazoles having different aromatic bulky structures at position 4 in order to increase the selectivity of this promising new group towards true pathogenic fungi.[51]
Mannich Base process:
Mannich reaction is used widely in the synthesis of secondary and tertiary amine derivatives, and it is a key step in the synthesis of various bioactive molecules. The N?Mannich bases derived from NH?heterocycles and related compounds display a broad spectrum of potential pharmacological activities such as antimicrobial, antifungal, anti?HIV, antitubercular, neuroprotection, and anticancer activities. N?Substituted isoindolin?1,3?diones belong to an important class of compounds, and their interesting biological activities have been reviewed. They exhibit antifungal, anti?inflammatory and analgesic, anticonvulsant, and antibacterial, antioxidant, and hemolytic activities. In particular, N?Mannich bases of isoindolin?1,3? dione (phthalimide) and Mannich bases incorporating a phthalimide moiety have shown to be effective antimicrobial, anthelmintic, and insecticidal agents and are of synthetic value and promising as biologically active compounds. In view of this, and in connection of our studies in the area of N?Mannich bases of NH?heterocycles such as saccharin, isatin and its derivatives, succinimide, and 2?pyrazolin?5?ones, the present work is largely concerned with attempts to extend the scope of N?Mannich reaction with isoindolin?1,3?dione. Accordingly, the synthesis of some new N?Mannich bases, and hybrid Mannich bases related to 1, which have not been reported hitherto, and possess considerable synthetic and pharmaceutical interest, was investigated. [57]
1. Lamya H. Al-Wahaibi et al (2021) [1]
1,3,4-Oxadiazole N-Mannich Bases:
Synthesis, Antimicrobial, and Anti-Proliferative Activities bacterial stain: Gram-positive, Gram-negative bacteria, and the yeast-like pathogenic fungus Candida albicans.
Reference drug:
1,3,4-Oxadiazole
Method:
agar-disc diffusion method using Müller-Hinton agar medium culture collection (ATCC) 6571, Bacillus subtilis ATCC 5256 and Micrococcus luteus ATCC 27141, Gram-negative bacterial strains Escherichia coli ATCC 8726, and Pseudomonas aeruginosa ATCC 27853, and the yeast-like pathogenic fungus Candida albicans MTCC 227. The initial screening was performed by the semi-quantitative
Derivative:
3-Anilinomethyl-5-(3,4-dimethoxyphenyl)-1,3,4-oxadiazole-2(3H)-thione (4a), 3-[(4-Fluorophenylamino) methyl]-5-(3,4-di methoxy phenyl)-1 ,3,4-oxadia zole-2(3H)-thione 4b,3-[(3-Chlorophenylamino) methyl]-5 -(3,4-dimethox yphenyl)-1, 3,4-oxadiazole-2(3H)-thione 4c3-[(4-Chlorophenylamino) methyl]-5-(3,4-dimethoxyphenyl)-1,3,4-oxadiazole-2(3H)-thione 4d3-[(2-Nitrophenylamino) methyl]-5-(3,4-dimethoxyphenyl)-1,3,4-oxadiazole-2(3H)-thione 4e3-[(3-Nitrophenylamino) methyl]-5-(3,4-dimethoxyphenyl)-1,3,4-oxadiazole-2(3H)-thione 4f3-[(4-Nitrophenylamino) methyl]-5-(3,4-dimethoxyphenyl)-1,3,4-oxadiazole-2(3H)-thione 4g.3-[(2-Trifluoromethylphenylamino) methyl]-5-(3,4-dimethoxyphenyl)-1,3,4-oxadiazole-2(3H)- thione 4h
3-[(3-Trifluoromethylphenylamino) methyl]-5-(3,4-dimethoxyphenyl)-1,3,4-oxadiazole-2(3H)- thione 4i.3-[(2,4-Difluorophenylamino) methyl]-5-(3,4-dimethoxyphenyl)-1,3,4-oxadiazole-2(3H)-thione 4j.3-[(2,5-Difluorophenylamino) methyl]-5-(3,4-dimethoxyphenyl)-1,3,4-oxadiazole-2(3H)-thione 4k.3-[(2,4-Dichlorophenylamino) methyl]-5-(3,4-dimethoxyphenyl)-1,3,4-oxadiazole-2(3H)-thione 4l.5-(3,4-Dimethoxyphenyl)-3-[(4-phenylpiperazin-1-yl) methyl]-1,3,4-oxadiazole-2(3H)-thione 5a.5-(3,4-Dimethoxyphenyl)-3-{4-[(4-fluorophenyl)piperazin-1-yl]methyl}-1,3,4-oxadiazole-2(3H)- thione 5b.5-(3,4-Dimethoxyphenyl)-3-[(4-benzylpiperazin-1-yl) methyl]-1,3,4-oxadiazole-2(3H)-thione 5c, 5-(3,4-Dimethoxyphenyl)-3-{4-[(2-trifluorobenzyl)piperazin-1-yl]methyl}-1,3,4-oxadiazole-2(3H)- thione 5d
Result:
The piperazinomethyl derivatives 5c and 5d displayed broad-spectrum antibacterial activities the minimal inhibitory concentration (MIC) 0.5–8 µg/mL) and compounds 4j, 4l, 5a, and 5b showed potent activity against the tested Gram-positive bacteria
Structure-
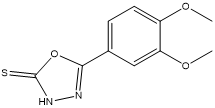
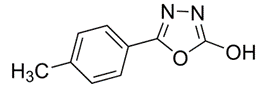
5-(3,4-dimethoxyphenyl)-1,3,4-oxadiazole-2(3H)-thione 3
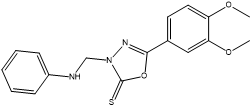
3-Anilinomethyl-5-(3,4-dimethoxyphenyl)-1,3,4-oxadiazole-2(3H)-thione (4a).
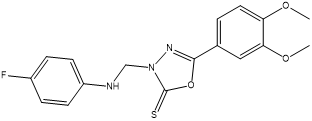
3-[(4-Fluorophenylamino) methyl]-5-(3,4-dimethoxyphenyl)-1,3,4-oxadiazole-2(3H)-thione 4b
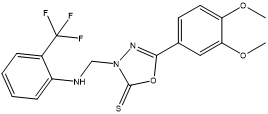
3-[(2-Trifluoromethylphenylamino) methyl]-5-(3,4-dimethoxyphenyl)-1,3,4-oxadiazole-2(3H)- thione
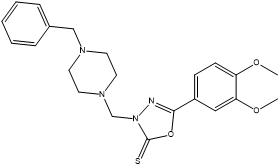
3-[(2,4-Difluorophenylamino) methyl]-5-(3,4-dimethoxyphenyl)-1,3,4-oxadiazole-2(3H)-thione
3-((4-benzylpiperazin-1-yl) methyl)-5-(3,4-dimethoxyphenyl)-1,3,4-oxadiazole-2(3H)-thione
2. Teresa Glomb et al in 2021 [2] On Antimicrobial Activity of 1,3,4-Oxadiazole Derivatives
Antifungal stain:
C. albicans strain, Aspergillus, Gram-positive strains S. aureus, Bacillus cereus and Gram-negative strains Escherichia coli,
Reference drug: fluconazole, Voriconazole, ketoconazole
Method:
IZD) using the filter paper disc-diffusion method, cyclization oxidative reactions of N-acylhydrazones, cyclodehydration reactions of diacylhydrazines or hydrazide reactions
ATCC No .M tuberculosis H37Ra, tuberculosis H37Rv (CYP51) enzyme
Derivative:
-(4-methyl-thiazol-5-yl)-1,3,4-oxadiazole-2-amine, N-(1,3,4-oxadiazol-2-yl)-benzamide
5-(4-methyl-thiazol-5-yl)-1,3,4-oxadiazole-2-amine, (5-(4-fluorophenyl)-1,3,4-oxadiazole-2-thiol)
5-(3-chlorophenyl)-2-((N- (substituted)-2-acetamoyl)mercapto)-1,3,4-oxadiazole derivatives.
2,5-disub situted- thiadi azoles., Pyridin e-4-yl -1,3,4-oxadi azol-2-yl- thio-et hylidene-hydrazi necarbothioamide. 5-(3,4-dimethoxyphenyl)-1,3,4-oxadiazole-2-thione derivatives.5-aryl-1,3,4-oxadiazo le-2-thioles structures.1,3,4-oxadiazole-1,3,4-thiadiazole.5-mercapto-1,3,4-oxadiazolepyrazole-vinyl-1,3,4-oxidazole.1,3,4-oxadiazole-quinoxalinepyrazolo[3,4-b]pyridine scaffold

2-(4-chloro-3-nitrophenyl)-5-(p-tolyl)-1,3,4-oxadiazole
3.Christina Zalaru et al in 2022 [3]
on synthesized series of pyrazolo-benzimidazole hybrid Mannich bases and assayed antibacterial and antibiofilm activity on Gram-positive Staphylococcus aureus ATCC25923, Enterococcus faecalis ATCC29212, and Gram-negative Pseudomonas aeruginosa ATCC27853, Escherichia coli ATCC25922 strains. All Pyrazolo-benzimidazole hybrids Mannich bases were reported be more active than standards either Metronidazole, Nitrofurantoin. Compounds 5a, 5b, 5c, 5e, and 5f were reported to show more activity in comparision to the standards Metronidazole, Nitrofurantoin while 5d and 5g were less active compared to Nitrofurantoin. It was also reported that all the compounds exhibited more activity against Pseudomonas aeruginosa compared to both Metronidazole, Nitrofurantoin. Compound 5f showed the best activity against Staphylococcus aureus, with a MIC of 150 g/mL. It has also inhibited the biofilm formed by all the bacterial strains, having an MBIC of 310 g/mL compared to both the reference drugs.
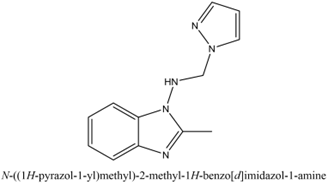
N-[(1H-pyrazol-1-yl) methyl]-1-amino-1H-benzimidazoles,
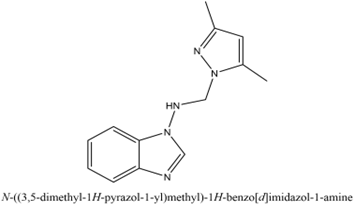
N-[(1H-3,5-dimethylpyrazol-1-yl) methyl]-1-amino-1H-benzimidazole
N-[(1H-3,5-dimethyl-4-nitropyrazol-1-yl) methyl]-1-amino-1H-benzimidazole
N-[(1H-3,5-dimethyl-4-iodopyrazol-1yl) methyl]-1-amino-1H-benzimidazole
N-[(1H-pyrazol-1yl) methyl]-1-amino-2-methyl-1H-benzimidazole
N-[(1H-3,5-dimetyl-pyrazol-1-yl) methyl]-1-amino-2-methyl-1H-benzimidazole
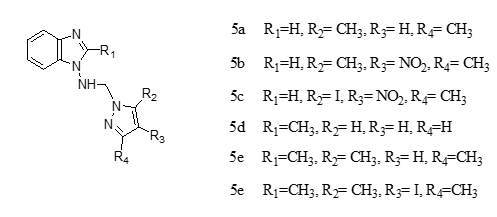

4. Dan Liu et al in (2022) [6]
On Design, Synthesis and Antifungal/Nematicidal Activity of Novel 1,2,4-Oxadiazole Derivatives Containing Amide Fragments.
Antifungal stain:
Sclerotinia sclerotiorum (S. sclerotiorum) Currently, more than 8000 species of fungi are known to cause plant diseases, including Fusarium graminearum, Botrytis cinerea (B. cinerea), Rhizoctonia solani, Blumeria spp., Pythium spp., Colletotrichum spp., Fusarium spp., Puccinia spp., Phytophthora spp., etc., and these fungi have brought about 85% plant diseases
Reference drug:
thifluzamide and fluopyram.
Method:
The methodology used for the nematocidal bioassays of these target compounds was modified in the light of the conventional method. Showed excellent nematicidal activity.
Derivative:
3-(4,5-dimethylthiazol-2- yl)-2,5-diphenyltetrazoliumbromide
Result :-
1,2,4-oxadiazole derivatives containing an amide substructure were designed and synthesized, and then, their fungicidal and nematicidal activity were determined. The antifungal results revealed that compound F15 displayed the highest in vitro antifungal activity against S. sclerotiorum, with an EC50 value of 2.9 µg/mL. In vivo test indicated that compound F15 could control the disease caused by S. sclerotiorum that infected cole leaves, demonstrating curative effect and protective effects of 62.3% and 71.0% at 100 µg/mL
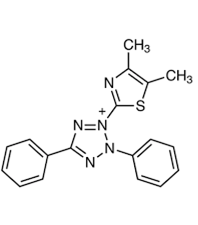
3-(4,5-dimethylthiazol-2- yl)-2,5-diphenyltetrazoliumbromide
5. Atukuri Dorababu et.al in (2020) [36]
Recent update on antibacterial and antifungal activity of quinoline scaffolds properties are far better than methicillin. In particular, in the case of S. aureus ATCC BAA?41, S. aureus ATCC 33591, and S. aureus ATCC BAA 1720, with an identical inhibitory value of 1 µg/ml, and S. aureus ATCC 33592 (MIC = 0.5 µg/ml), compound 26 is said to be an excellent antibacterial drug molecule with a 1024?fold higher activity as compared with methicillin. Time? killing curves show that compound 25 can inhibit E. coli ATCC 25922 and S. aureus ATCC 29213 completely at 1× MIC
against B. subtilis 168, S. aureus ATCC 25923, S. aureus ATCC 29213, S. aureus ATCC BA41, S. aureus ATCC 43300, E. faecium ATCC 700221, E. faecium ATCC 49624, and E. faecium ATCC 29212 bacterial strains, respectively Reference drug quinoline scaffolds, pyrazole, benzofuran, and thiazolidinone.
Derivatives:
N??s?Benzylidene?2?propylquinoline?4?carbohydrazide N??((s? 1H?pyrrol?2?yl) methylene)?2?propylquinoline?4?carbohydrazides, 2? chloroquinoline?3?carboxaldehydes 5?Amino?7?bromoquinoline, 3,5?dichlorophenyl analog 3, 4?trifluorophenyl analog, 2?methyl?6?bromoquinoline, 5?Chloro?8?hydroxyquinoline, 6?chloro?2?methylquinoline, 2?chloro? 6?methoxyquinoline scaffolds with pyrimidine 2?thiopropanenitril, 2?Chloro?6?methoxyquinolines, 5?amino?7?bromoquinolin?8?yl sulfonate, 5?nitro?8?hydroxyquinoline and 5?chloro?8?hydroxyquinoline
Result –
The antifungal evaluation of the pyrazole amine attached with quinoline scaffolds has revealed noteworthy results.Out of the potent fungal inhibitors, the selective Gram?negative antibacterial agent 82 has also been observed as the best antifungal agent. In this regard, the MIC values for determining the fungal inhibitory properties are found to be 0.98, 0.49, and 0.12 µg/ml against Aspergillus fumigatus, A. clavatus, and C. albicans strains, respectively.

4?(2?heterylidenehydrazinyl) ?7?chloroquinolin 5?Chloro?8?hydroxyquinoline
6. Gaber Moustafa, Khalaf H. et. al. in (2018) Synthesis, Molecular Docking Studies, In Vitro Antimicrobial and Antifungal Activities of Novel Dipeptide Derivatives Based on N-(2-(2Hydrazinyl-2-oxoethylamino)- 2-oxoethyl)-Nicotinamide. The strain used and test organisms: antibacterial and antifungal. such as E. coli (ATCC 25922) and B-Test fungi such as A. Niger (ATCC 16888) and C. albicans (ATCC 10231). The antimicrobial activity of the candidates was evaluated by use of an agar well diffusion method. The samples were dissolved in DMSO. Briefly, a one-day-old culture of bacteria and a two-day-old culture of fungi were then mixed with sterile physiological saline (NaCl 0.9%) Gram-positive, Gram-negative, and fungi. The results for the inhibitory activity represented a promising indication in Gram-positive, Gramnegative, and fungi pathogens. The minimum inhibitory concentration (MIC) of the newly synthesized peptide derivatives are presented. The MIC was 160 µg/mL.

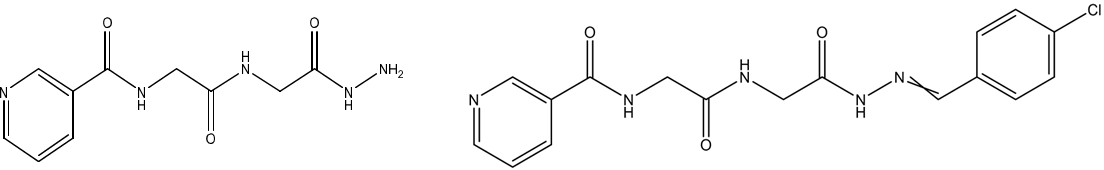
Methyl-2-(2-(nicotinamido)-acetamido)-acetate, N-(2-(2-Hydrazinyl-2-oxoethylamino)-2-oxoethyl)-nicotinamide,
N-(2-(2-(2-(4 -Chloro benzylidene) -hydra zinyl)-2-oxoethylamino)-2-oxoethyl)-nicotinamide,
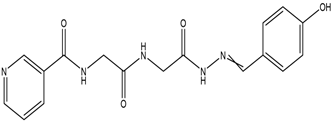
N-(2-(2-(2 -(4-Hydro xybenzyl idene)-hydraz inyl)-2-oxo ethylamino)-2-oxoethyl)-nicotinamide,

N-(2-(2 -(2-(Methylc arbam othio yl)-hydraz inyl)-2-o xoeth ylam ino)-2-oxoethyl)-nicotinamide, N-(2-(2-( 2-(Met hylimino) -4-oxothi azolidin-3 -ylamino )-2-oxo ethyl amino)-2-oxoethyl amino)-2-o xoethyl),
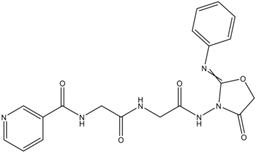
N-(2-oxo-2-(2-oxo-2-(4-oxo-2-(Phenylimino)-oxazolidin-3-ylamino)-ethylamino)-ethyl) nicotinamide
7. Zalaru C, Dumitrascu F et. al (2022)
New Pyrazolo-Benzimidazole Mannich Bases with Antimicrobial and Antibiofilm Activities. Gram-positive Staphylococcus aureus ATCC25923, Enterococcus faecalis ATCC29212, and All strains Staphylococcus aureus ATCC25923, Enterococcus faecalis ATCC29212, and Escherichia coli ATCC25922 than reference drugs with the exception of compounds which are less active compared to Nitrofurantoin, and all synthesized compounds are more active against Pseudomonas aeruginosa ATCC27853 compared to reference drugs (Metronidazole, Nitrofurantoin). Compound showed the best activity against Staphylococcus aureus ATCC 25923, with a MIC of 150 ?g/mL. This work reports on a new class of pyrazolobenzimidazole. Mannich bases that have been characterized using different methods.. Assessment against bacterial strains shown improved efficacy in comparison to reference medications, especially against Pseudomonas aeruginosa and Staphylococcus aureus. Compound 5f successfully reduced the formation of biofilms in all tested strains of Staphylococcus aureus, with a minimum inhibitory concentration (MIC) of 150 µg/mL.

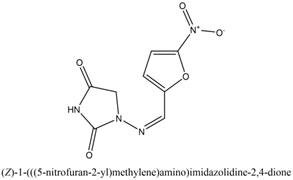
8. K. K. Sivakumar et. al. (2013)
Synthesised and characterised a series of Mannich bases generated.The goal of this study is to synthesise and characterise a set of 12 moieties that contain by spectral and physicochemical methods. synthesised Mannich was evaluated for early antibacterial activity against strains of both Gram-positive and Gram negative bacteria as well as fungi. antimicrobial activity against Gram-positive and Gram-negative bacterial as well as fungal strains by the determination of zone of inhibition. Mannich bases were found to be more potent antibacterial agents against bases have more effectiveness against fungus and bacteria that are Gram-negative. The minimum inhibitory concentration finding proved that the compound.(3)
3-(hydrazinyl)-2-phenylquinazolin-4(3H)–one
Pyrazol-5(4H)-one moiety containing 3-(hydrazinyl)-2-phenylquinazolin-4(3H)-one 3-
(hydrazinyl)-2-phenylquinazolin-4(3H)–one
9. Janowska S, Andrzejczuk S et. al. (2023) synthesised, and tested new Mannich bases for antifungal and antibacterial activity and various piperazine derivatives were used to synthesise the target compounds. Strains of Gram-negative bacteria, such as Enterobacteriaceae family bacteria (PG7), while all compounds examined shown high fungistatic activity against yeasts of the Candida spp., particularly C. Para psilosis, with minimum inhibitory concentrations (MICs) ranging from 0.49 µg/mL (PG7) to 0.98 µg/mL (PG8). piperazine derivatives.
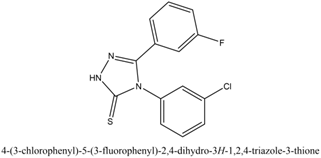
4-(3-chlorophenyl)-5-(3-fluorophenyl)-2,4-dihydro-3H-1,2,4-triazole-3thione
10. Hozien ZA, El-Mahdy AF, et. al. (2020) Synthesis of Schiff and Mannich bases of new striazole derivatives and their potential applications for removal of heavy metals from aqueous solution and as antimicrobial agents. Antimicrobial activity was evaluated in vitro using three strains of bacteria (Bacillus subtilis, Escherichia coli, and Pseudomonas aeruginosa) and three strains of fungi (Aspergillus niger, fumigatus). The inhibition zones (mm).r Gram (ve) and Gram (+ve) bacteria, respectively. Compound 3b showed the highest activity, with inhibition zones of 29 mm, Mannich reactions that are effective and cyclize Formaldehyde, primary aliphatic amines.(5)
2- methyl-6-substituted-6,7-dihydro-5H-s-triazolo[5,1-b]-1,3,5-thiadiazines via a reaction of 5methyl-1Hs-triazole-3-thiol
2-methyl-6-dihydro-6,7-triazolo-5H-s-triazolo
5-methyl-1H-s-triazole-3-thiol
3-methyl-1-((substituted-amino) methyl)-1H-s-triazole-5-thiols
3-methyl-1-((substituted-amino) methyl)-1H-s-triazol
4-amino-3-methyl-s-tria
4-amino-3-methyl-s-triazole-5-thio
11. Maria Marinescu, et. al. (2020)
on Synthesis, density functional theory study and in vitro antimicrobial evaluation of new benzimidazole Mannich bases the tri-component synthesis of chiral benzimidazole Mannich bases utilising benzimidazole, a 30% aqueous formaldehyde, and an amine is reported in this work. Elemental analysis, compounds containing saturated heterocycles, such as 1methylpiperazine and morpholine, when in vitro antibacterial screening was performed against four strains. Qualitative and quantitative methods for their in vitro software and HOMO–LUMO analysis allowed the calculation of electronic and structural parameters of the chiral Mannich bases. The geometry of 1-meth- ylpiperazine, the cumulated Mullikan atomic charges of the two heteroatoms and of the methyl, and the value of the global electrophilicity index (?=0.0527.(10)
(S)-1- (1H-Benzo[d]imidazole-2-yl) ethanol.
(S)-1- (1- ((4-Methylpiperazin-1- yl) methyl)-1H- benzo
(S)-1- (1- ((4-Morpholin-1-yl) methyl)-1H- benzo[d]imida- zole-2- yl)ethanol
(S)-1- (1- ((Diphenylamino) methyl)-1H- benzo[d]imida- zole-2- yl)ethanol
(S)-1- (1- ((4-nitrophenylamino) methyl)-1H- benzo

(S)-1- (1-((Diphenylamino) methyl)-1H- benzo[d]imida- zole-2- yl) ethanol
12. Bala S, Sharma N, et. al. (2014)
Mannich Bases An Important Pharmacophore in Present Scenario beta-amino ketone molecules, are produced by the Mannich reaction and are essential for the synthesis of many natural goods and medications. Activity as compared to ciprofloxacin against K. pneumonia and nonhemolytic streptococcus, respectively. The compounds 2d and 2e were found to have equipotent antifungal activity against M. audouinii and C. albicans as compared to clotrimazole.These bases that include aminoalkyl chains, like fluoxetine, have a strong curative effect. These properties are due to the , -unsaturated ketone that is produced by amine group deamination. The nucleophilic addition process that forms carbon-carbon bonds is essential for the construction of nitrogen-containing molecules.(17)
2-(phenyl)-2-(morpholine-4-yl)-Nphenylacetamide
3-(4-chlorophenyl)-3-(morpholin-4-yl)-N-phenylpropanamide
3-(morpholin-4-yl)-3-(4-nitrophenyl)-N-phenylpropanamide,
3-(4-methoxyphenyl)-3-(morpholin-4-yl)-N-phenylpropanamide
3-(4,6-disubstituted-2- thiomethylpyrimidyl)-4-amino-5-mercapto-1,2-4-triazoles
N-[(1-piperidinobenzyl)benzamide]

2-(phenyl)-2-(morpholine-4-yl)-Nphenylacetamide
13. Dorababu Atukuri et.al in (2020)
Identification of quinoline-chalcones and heterocyclic chalconeappended quinolines. Antifungal activity of quinoline scaffolds properties are far better than methicillin. In particular, in the case of S. aureus ATCC BAA?41, S. aureus ATCC 33591, and S. aureus ATCC BAA 1720, with an identical inhibitory value of 1 µg/ml, compound 26 is said to be an excellent. Time? killing curves show that compound. against B. subtilis 168, S. aureus ATCC 25923, S. aureus ATCC 29213, S. aureus ATCC BA41, S. aureus ATCC 43300, E. faecium ATCC 700221, E. faecium ATCC 49624, and E, respectively. Reference drug quinoline scaffolds, pyrazole, benzofuran, and thiazolidinone.
Out of the potent fungal inhibitors, the selective Gram?negative antibacterial has also been observed as the best antifungal agent. In this regard, the MIC values for determining the fungal inhibitory properties are found to be 0.12 µg/ml against Aspergillus fumigatus, A. clavatus, and C. albicans strains, respectively.
N??s?Benzylidene?2?propylquinoline?4?carbohydrazide, N??((s1H?pyrrol?2?yl) methylene)?2?propylquinoline?4?carbohydrazides , 2?
chloroquinoline?3?carboxaldehydes, 5?Amino?7?bromoquinoline, 3,5?dichlorophenyl analog 3
4?trifluorophenyl analog, 2?methyl?6?bromoquinoline
5?Chloro?8?hydroxyquinoline, 6?chloro?2?methylquinoline, 2?chloro? 6?methoxyquinoline scaffolds with pyrimidine 2?thiopropanenitril, 2?Chloro?6?methoxyquinolines, 5?amino?7?bromoquinolin?8?yl sulfonate, 5?nitro?8?hydroxyquinoline and
5?chloro?8?hydroxyquinoline.(17)
14. Al-Abdullah et. al. (2014)
[20] Antimicrobial and Hypoglycemic Activities of Novel N-Mannich Bases Derived from The appropriate N- with formaldehyde solution and different 1-substituted piperazines. The in vitro inhibitory effects of newly synthesised N Mannich bases were evaluated against a panel of (Gram-negative bacteria), and the yeast-like pathogenic fungus Candida albicans IFO 0583. The primary screening was carried out using the agar disc-diffusion method using Müller-Hinton agar medium It was determined that none of the recently created compounds had significant anti-Candida albicans activity. standard strains of the Institute of Fermentation of Osaka Staphylococcus a, Bacillus subtilis IFO 3007, Micrococcus luteus IFO 3232 (Grampositive bacteria), Pseudomonas aeuroginosa Six different substances were tested their oral hypoglycemic action using streptozotocin (STZ) 5(1-adamantyl)-4-ethyl or allyl-1,2,4-triazoline-3-thione, 5-(1-Adamantyl)-4-substituted-1,2,4- triazoline-3-thiones, 5-(1-Adamantyl)-4-substituted-1,2,4- triazoline-3-thiones, 1-(1-adamantylcarbonyl)-4-substituted thiosemicarbazides5-(1-adamantyl)-4-substituted-1,2,4-triazoline-3-thiones5(1-adamantyl)-4-ethyl or allyl-1,2,4-triazoline-3-thione5-(1-Adamantyl)-4-substituted-1,2,4- triazoline-3-thiones
15. Zafer Asim Kaplancikli et.al (2011) [7]Synthesis of Some Oxadiazole Derivatives as New
Anticandidal Agents tested in vitro by using a microbroth dilution method against with carbon disulphide. New oxadiazole derivatives 4a-f were obtained by the nucleophilic substitution reaction of compound C. albicans C. albicans (ATCC 90028), C. glabrata, C. tropicalis (NRRL Y-12968), C. krusei (NRRL Y-7179), C. parapsilosis (NRRL Y- 12696), ketoconazole: 0.001–0.007 mg/mL) against Candida species
5-[(pyrimidin-2-ylthio) methyl]-1,3,4-oxadiazole-2(3H)-thione
2-(pyrimidin-2-ylthio)acetohydrazide
2-[5-[(Pyrimidin-2-ylthio) methyl]-1,3,4-oxadiazol-2-ylthio]acetophenone
2-[5-[(Pyrimidin-2-ylthio) methyl]-1,3,4-oxadiazol-2-ylthio]-4?-chloroacetophenone
2-[5-[(Pyrimidin-2-ylthio) methyl]-1,3,4-oxadiazol-2-ylthio]-4?-nitroacetophenone
2-[5-[(Pyrimidin-2-ylthio) methyl]-1,3,4-oxadiazol-2-ylthio]-3?-nitroacetophenone
2-[5-[(Pyrimidin-2-ylthio) methyl]-1,3,4-oxadiazol-2-ylthio]-3?-chloroacetophenone
2-[5-[(Pyrimidin-2-ylthio) methyl]-1,3,4-oxadiazol-2-ylthio]-2?,4?-dichloroacetophenone
16. M. Mahesh, G. Bheemaraju et.al in (2014) [6]
on Synthesis of new oxadiazole, pyrazole and analogs as potential antibacterial and antifungal agents. Gram-negative bacteria, Escherichia coli, Pseudomonas aeruginosa, Salmonella typhi and screened for antifungal activity against Two series of diversely substituted. phenyldiazenyl(2-(4-methyl-2-oxo-2H-chromen7-yloxy)acetyl)3,5-dimethyl-1H-pyrazolepyrazolin-5-one bearing 2-((4-methyl-2- oxo-2H-chromen-7-yl)oxy)acetohydrazide phenyldiazenyl-1-(2-(4-methyl-2-oxo-4- chromen-7-yloxy)acetyl)-3-methyl-1H-pyrazol-5(4)H-one
(2-(4-méthyl-2-oxo-2Hchromène-7-yloxy)acétyl)3,5-diméthyl-1H-pyrazole
phenyldiazenyl-1-(2-(4-méthyl-2- oxo-4-chromén-7-yloxy)acétyl)-3-méthyl-1H-pyrazole-5(4)H
REFERENCES
- Al-Wahaibi LH, Mohamed AA, Tawfik SS, Hassan HM, El-Emam AA. 1, 3, 4-Oxadiazole N-Mannich bases: synthesis, antimicrobial, and anti-proliferative activities. Molecules. 2021 Apr 7;26(8):2110.
- Glomb T, ?wi?tek P. Antimicrobial activity of 1, 3, 4-oxadiazole derivatives. International journal of molecular sciences. 2021 Jun 29;22(13):6979.
- Zalaru, C., Dumitrascu, F., Draghici, C., Tarcomnicu, I., Marinescu, M., Nitulescu, G.M., Tatia, R., Moldovan, L., Popa, M. and Chifiriuc, M.C., 2022. New pyrazolo-benzimidazole Mannich bases with antimicrobial and antibiofilm activities. Antibiotics, 11(8), p.1094.
- Liu D, Luo L, Wang Z, Ma X, Gan X. Design, synthesis and antifungal/nematicidal activity of novel 1, 2, 4-oxadiazole derivatives containing amide fragments. International Journal of Molecular Sciences. 2022 Jan 29;23(3):1596.
- Kaplancikli ZA. Synthesis of some oxadiazole derivatives as new anticandidal agents. Molecules. 2011 Sep 7;16(9):7662-71.
- Mahesh M, Bheemaraju G, Manjunath G, Ramana PV. Synthesis of new oxadiazole, pyrazole and pyrazolin-5-one bearing 2-((4-methyl-2-oxo-2H-chromen-7-yl) oxy) acetohydrazide analogs as 1potential antibacterial and antifungal agents. InAnnales Pharmaceutiques Francaises 2016 Jan 1 (Vol. 74, No. 1, pp. 34-44). Elsevier Masson.
- Synthesis of Some Oxadiazole Derivatives as New Anticandidal Agents Zafer Asim Kaplancikli.
- Lichon V, Khachemoune A. Mycetoma: a review. American Journal of Clinical Dermatology. 2006 Oct; 7:315-21.
- Zarakas MA, Desai JV, Chamilos G, Lionakis MS. Fungal infections with ibrutinib and other small-molecule kinase inhibitors. Current fungal infection reports. 2019 Sep 15; 13:86-98.
- Marinescu M, Cintez? LO, Marton GI, Chifiriuc MC, Synthesis, density functional theory study and in vitro antimicrobial evaluation of new benzimidazole Mannich bases. BMC chemistry. 2020 Dec;14(1):1-6.
- Nierode G, Kwon PS, Dordick JS, Kwon SJ. Cell-based assay design for high-content screening of drug candidates. Journal of microbiology and biotechnology. 2016 Feb;26(2):213.
- Kaplancikli ZA. Synthesis of some oxadiazole derivatives as new anticandidal agents. Molecules. 2011 Sep 7;16(9):7662-71.
- Scorzoni L, Fuchs BB, Junqueira JC, Mylonakis E. Current and promising pharmacotherapeutic options for candidiasis. Expert opinion on pharmacotherapy. 2021 May 3;22(7):887-8.
- Pishawikar SA, More HN. Synthesis, docking and in-vitro screening of mannich bases of thiosemicarbazide for anti-fungal activity. Arabian Journal of Chemistry. 2017 May 1;10: S2714-22.
- Atukuri D, Vijayalaxmi S, Sanjeev Murthy R, Vidya L, Prasannakumar R, Raghavendra MM. Identification of quinoline-chalcones and heterocyclic chalcone-appended quinolines as broad-spectrum pharmacological agents. Bioorganic Chemistry. 2020 Dec 1; 105:104419.
- Biersack B, Ahmed K, Padhye S, Schobert R. Recent developments concerning the application of the Mannich reaction for drug design. Expert Opinion on Drug Discovery. 2018 Jan 2;13(1):39-49.
- Bala S, Sharma N, Kajal A. Mannich bases: an important pharmacophore in present scenario. International journal of medicinal chemistry. 2014
- Marinescu M, Popa CV. Pyridine compounds with antimicrobial and antiviral activities. International Journal of Molecular Sciences. 2022 May 18;23(10):5659.
- Al-Abdullah ES, Al-Tuwaij. Antimicrobial and hypoglycemic activities of novel N Mannich bases derived from 5-(1-adamantyl)-4-substituted-1, 2, 4-triazoline-3-thiones. International journal of molecular sciences. 2014 Dec 11;15(12):22995-3010.
- Baugh SD. Guanidine-containing antifungal agents against human-relevant fungal pathogens (2004–2022)—a review. Journal of Fungi. 2022 Oct 15;8(10):1085.
- Andriole VT. Current and future antifungal therapy: new targets for antifungal therapy.
- International journal of antimicrobial agents. 2000 Nov 1;16(3):317-21. Pishawikar SA, More HN. Synthesis, docking and in-vitro screening of mannich bases of thiosemicarbazone for anti-fungal activity. Arabian Journal of Chemistry. 2017 May 1;10: S2714-22.
- Mathapati SR, Suryawanshi VB. Synthesis of Some New N-Mannich Bases Derivatives of Phenytoin. Asian Journal of Research in Chemistry. 2018;11(2):236-40
- Liu D, Luo L, Wang Z, Ma X, Gan X. Design, synthesis and antifungal/nematicidal activity of novel 1, 2, 4-oxadiazole derivatives containing amide fragments. International Journal of Molecular Sciences. 2022 Jan 29;23(3):1596.
- Verma G, Khan MF, Akhtar W, Alam MM, Akhter M, Shaquiquzzaman M. A review exploring therapeutic worth of 1, 3, 4-oxadiazole tailored compounds. Mini Reviews in Medicinal Chemistry. 2019 Apr 1;19(6):477-509.
- Goswami BN, Kataky JC, Baruah JN, Nath SC. Synthesis of 3, 5?disubstituted 1, 3, 4?oxadiazole?2?thiones as potential fungicidal agents. Journal of heterocyclic chemistry. 1984 Jan;21(1):205-8.
- Roman G. Mannich bases in medicinal chemistry and drug design. European journal of medicinal chemistry. 2015 Jan 7; 89:743-816.
- De Oliveira CS, Lira BF, Barbosa-Filho JM, Lorenzo JG, de Athayde-Filho PF. Synthetic approaches and pharmacological activity of 1, 3, 4-oxadiazoles: a review of the literature from 2000–2012. Molecules. 2012 Aug 27;17(9):10192-231.
- Lee Y, Puumala E, Robbins N, Cowen LE. Antifungal drug resistance: molecular mechanisms in Candida albicans and beyond. Chemical reviews. 2020 May 22;121(6):3390-411.
- Zheng Z, Liu Q, Kim W, Tharmalingam N, Fuchs BB, Mylonakis E. Antimicrobial activity of 1, 3, 4-oxadiazole derivatives against planktonic cells and biofilm of Staphylococcus aureus. Future medicinal chemistry. 2018 Feb;10(3):283-96.
- Berkow EL, Lockhart SR. Fluconazole resistance in Candida species: a current perspective. Infection and drug resistance. 2017 Jul 31:237-45.
- Farooq S, Haq IU, Ullah N. Synthesis, characterization and biological evaluation of N-Mannich base derivatives of 2-phenyl-2-imidazoline as potential antioxidants, enzyme inhibitors, antimicrobials, cytotoxic and anti-inflammatory agents. Arabian Journal of Chemistry. 2021 Apr 1;14(4):103050.
- Dascalu AE, Ghinet A, Lipka E, Furman C, Rigo B, Fayeulle A, Billamboz M. Design, synthesis and antifungal activity of pterolactam-inspired amide Mannich bases. Fitoterapia. 2020 Jun 1; 143:104581.
- Dorababu A. Recent update on antibacterial and antifungal activity of quinoline scaffolds. Archiv der Pharmazie. 2021 Mar;354(3):2000232.
- Pishawikar SA, More HN. Synthesis, docking and in-vitro screening of mannich bases of thiosemicarbazide for anti-fungal activity. Arabian Journal of Chemistry. 2017 May 1;10: S2714-22.
- Aggarwal N, Kumar R, Dureja P, Khurana JM. Synthesis of novel nalidixic acid?based 1, 3, 4?thiadiazole and 1, 3, 4?oxadiazole derivatives as potent antibacterial agents. Chemical biology & drug design. 2012 Apr;79(4):384-97.
- Raza MM, Farooq M, Ahmad M, Anwar S. Analysis of Higher Education Policies of Pakistan and Suggestions for New Policy. Journal of Educational Sciences & Research. 2019 Sep 1;6(2).
- Revie NM, Iyer KR, Robbins N, Cowen LE. Antifungal drug resistance: evolution, mechanisms and impact. Current opinion in microbiology. 2018 Oct 1; 45:70-6.
- Dascalu AE, Ghinet A, Lipka E, Furman C, Rigo B, Fayeulle A, Billamboz M. Design, synthesis and antifungal activity of pterolactam-inspired amide Mannich bases. Fitoterapia. 2020 Jun 1; 143:104581.
- Kumar A. Research Output of Guru Jambheshwar University of Science and Technology during 1999–2018: A Bibliometric Study. International Journal of Information Dissemination and Technology. 2019;9(1):12-7.
- Zhang A, He H, Wang R, Shen Z, Wu Z, Song R, Song B. Synthesis, Bioactivities, and Antibacterial Mechanism of 5-(Thioether)-N-phenyl/benzyl-1, 3, 4-oxadiazole-2-carboxamide/amine Derivatives. Journal of Agricultural and Food Chemistry. 2024 Jan 11;72(3):1444-53.
- Wu YY, Shao WB, Zhu JJ, Long ZQ, Liu LW, Wang PY, Li Z, Yang S. Novel 1, 3, 4-oxadiazole-2-carbohydrazides as prospective agricultural antifungal agents potentially targeting succinate dehydrogenase. Journal of agricultural and food chemistry. 2019 Nov 27;67(50):13892-903.
- Raju SK, Vengadhajalaphathy P, Sundaram R, Periyasamy S, Chinnaraj T, Sekar P. Recent advances in biological applications of mannich bases—An overview. International Journal of Pharmaceutical Chemistry and Analysis. 2023;10(1):15-27.
- Abdulla ZN, Hussein MS. Preparation of thiourea derivatives of isoniazid and evaluation of its bacterial activity. Materials Today: Proceedings. 2023 Jan 1; 80:1101-10.
- Kapila I, Bharwal A, Sharma P, Choudhary N, Abbot V. Synthetic marvels in tuberculosis research: An in-depth review of 1, 3, 4-oxadiazole derivatives as antitubercular agents. European Journal of Medicinal Chemistry Reports. 2024 Mar 21:100150.
- Murthy YL, Durga G, Jha A. Synthesis, characterization, and antimicrobial activity of some new 2-diazo-benzimidazole derivatives and their Ni (II), Cu (II), and Ag (I) complexes. Medicinal Chemistry Research. 2013 May; 22:2266-72. Jan A. Rosier, Mark A. Martens, Josse R.Thomas."Global New Drug Development",Wiley, 2014
- Mak KK, Wong YH, Pichika MR. Artificial intelligence in drug discovery and development. Drug Discovery and Evaluation: Safety and Pharmacokinetic Assays. 2023 Sep 28:1-38.
- Perfect JR. The antifungal pipeline: a reality check. Nature reviews Drug discovery. 2017 Sep;16(9):603-16.
- K Mazu T, A Bricker B, Flores-Rozas H, Y Ablordeppey S. The mechanistic targets of antifungal agents: an overview. Mini reviews in medicinal chemistry. 2016 May 1;16(7):555-78.
- Shpatov AV, Zakharova SS, Popov SA. Synthesis of New Hybrids of Abietic Acid and 1, 3, 4-Oxadiazoles. Chemistry of Natural Compounds. 2022 Mar;58(2):290-6.
- Mekuškiené G, Vainilavi?ius P, Hetzheim A, Shematovich R. Synthesis of 5-(4, 6-dimethyl-2-pyrimidinyl)-1, 3, 4-oxadiazole-2-thione and its alkylation, aminomethylation, and acylation. Chemistry of Heterocyclic Compounds. 1993 May; 29:598-602.
- Bankaitis VA, Tripathi A, Chen XR, Igumenova TI. New strategies for combating fungal infections: Inhibiting inositol lipid signaling by targeting Sec14 phosphatidylinositol transfer proteins. Advances in biological regulation. 2022 May 1; 84:100891.
- Dilshad R, Khan KU, Saeed L, Sherif AE, Ahmad S, Ovatlarnporn C, Nasim J, Hussain M, Ghalloo BA, Basit A, Mukhtar I. [Retracted] Chemical Composition and Biological Evaluation of Typha domingensis Pers. to Ameliorate Health Pathologies: In Vitro and In Silico Approaches. BioMed Research International. 2022;2022(1):8010395.
- El-Sayed WA, El-Essawy FA, Ali OM, Nasr BS, Abdalla MM, Abdel-Rahman AA. Synthesis and antiviral evaluation of new 2, 5-disubstituted 1, 3, 4-oxadiazole derivatives and their acyclic nucleoside analogues. Monatshefte für Chemie-Chemical Monthly. 2010 Sep; 141:1021-8.
- Sara Janowska, Sylwia Andrzejczuk, Piotr Gawry?, Monika Wujec. "Synthesis and Antimicrobial Activity of New Mannich Bases with Piperazine Moiety", Molecules, 2023
- Manjunatha K, Poojary B, Lobo PL, Fernandes J, Kumari NS. Synthesis and biological evaluation of some 1, 3, 4-oxadiazole derivatives. European journal of medicinal chemistry. 2010 Nov 1;45(11):5225-33.
- Brown GD, Denning DW, Gow NA, Levitz SM, Netea MG, White TC. Hidden killers: human fungal infections. Science translational medicine. 2012 Dec 19;4(165):165rv13-.
- Antley PP, Hazen KC. Role of yeast cell growth temperature on Candida albicans virulence in mice. Infection and immunity. 1988 Nov;56(11):2884-90.
- Zhang H, Yi X, Chen M, Shi H, Tan L, Lu H, Sun Y, Yang F. Synergistic effect of chlorhexidine and azoles on candida biofilm on titanium surface. Journal of Medical Mycology. 2023 Nov 1;33(4):101417.
- Pandey OP. Synthesis and structural studies of mono-and bis-n 5-cyclopentadienyl) hafnium (IV) derivatives of heterocyclic thioketones. Transition Metal Chemistry. 1987 Dec;12(6):521-4.
- Hu Y, Li CY, Wang XM, Yang YH, Zhu HL. 1, 3, 4-Thiadiazole: synthesis, reactions, and applications in medicinal, agricultural, and materials chemistry. Chemical reviews. 2014 May 28;114(10):5572-610.
- Ziyaev AA, Galust'yan AG. Reaction of 5-aryl-1, 3, 4-oxadiazole-2 (3H)-thiones with chloromethyl alkyl ethers. Chemistry of Heterocyclic Compounds. 1999 Sep;35(9):1104-6.
- Avirdi E, Paumo HK, Kamdem BP, Singh MB, Kumari K, Katata-Seru L, Bahadur I. Imidazolium-Based Ionic Liquid-Assisted Silver Nanoparticles and Their Antibacterial Activity: Experimental and Density Functional Theory Studies. ACS omega. 2023 Nov 2;8(45):42976-86.
- Scriven EF, Turnbull K. Azides: their preparation and synthetic uses. Chemical Reviews. 1988 Mar 1;88(2):297-368.
- Vaughan J, Benson R, Vaughan K. Assessing the effectiveness of antimicrobial wound dressings in vitro. In Advanced Wound Repair Therapies 2011 Jan 1 (pp. 227-246). Woodhead Publishing.


 Rupesh ramrao shinde *
Rupesh ramrao shinde *
 Abhiman. S Jadhav
Abhiman. S Jadhav



















 10.5281/zenodo.13621814
10.5281/zenodo.13621814