Abstract
1,2,3-Triazoles:
Triazoles are renowned scaffolds that are simple to conjugate with additional heterocyclic groups. These compounds exhibit a variety of pharmacological effects, including antibacterial and anticancer activities. Researchers have targeted these triazole-conjugated structural motifs as common pharmacological targets. Additionally, a study synthesized and evaluated the antibacterial properties of 1,2,3-triazole derivatives. Some of these compounds demonstrated good antibacterial activity, making them potential candidates for further investigation.
1,2,4-Triazoles:
Compounds containing the 1,2,4-triazole ring in their structure are characterized by multidirectional biological activity. Extensive research has proven significant antibacterial activity of this heterocyclic core. Rational design and development of novel antibacterial agents incorporating 1,2,4-triazole can help address microbial resistance. Molecular docking studies also suggested their potential as inhibitors for the novel SARS-CoV-2 virus. In summary, both 1,2,3-triazoles and 1,2,4-triazoles hold promise as antimicrobial agents, and further research in this area is essential for combating drug-resistant infections
Keywords
(AMR) Antimicrobial resistance, Antifungal & Antibacterial activity, Gram positive & Gram-negative bacteria.
Introduction
The World Health Organization (WHO) has reported on drug resistance in malaria, HIV, tuberculosis, and bacteria as well as drug resistance in fungus and neglected tropical diseases (NTDs). One of the biggest risks to development and public health worldwide is AMR. Bacterial AMR is thought to have contributed to 4.95 million fatalities worldwide in 2019 in addition to directly causing 1.27 million deaths. Antimicrobials are drugs that are used to prevent and cure infectious diseases in humans, animals, and plants. They include antibiotics, antivirals, antifungals, and antiparasitic. (1) Several medications on the market, such Azoles: Fluconazole (2), itraconazole (3), posaconazole (4), and voriconazole (5) are some of these medications. Imidazole (6) The azole ring of these has two nitrogens. Imidazole examples include ketoconazole, clotrimazole, econazole, miconazole, and tioconazole. Triazoles: These azole rings contain three nitrogen. Fluconazole, Itraconazole, Posaconazole, and Voriconazole are a few triazole examples. Agar diffusion method (7), broth dilution method (8), agar well diffusion method (9) and agar plug diffusion method (10) for antibacterial activity. Test of Time Killer (11), Essay on ATP Bioluminescence (12), Flow Cytofluorometric Techniques (13). utilize as a culture medium for antifungal determination. Azides such as sodium azide (14), benzyl azide (15), phenyl azide (16), trifluromethyl azide (17), and others are utilized in synthesis.
Table No. 1. Method of antimicrobial activity:

Numerous bacterial species are harmful and have the potential to spread dangerous infectious illnesses (18), For instance, the majority of hospital-acquired and community-acquired infections are caused by Gram-positive pathogens like Staphylococcus aureus (S. aureus), Streptococcus pneumonia (S. pneumoniae), and Gram-negative organisms like Escherichia coli (E. coli), Pseudomonas aeruginosa (P. aeruginosa), and others (19–20). 1,2,3-triazole is a frequent pharmacophore in many medications since it can function as the isostere of an amide, ester, carboxylic acid, and other heterocycles (21) Numerous Gram-positive and Gram-negative bacteria that are now known to be resistant to multiple drugs have produced deadly infectious diseases that have affected the human race. Therefore, the need to create novel classes of antibacterial medicines to combat diseases resistant to multiple drugs is great. Enhancement of cross-breed atoms in the design of novel entities through the substitution of several pharmacophores within a same structure, resulting in mixtures with increased antibacterial activity (22). The most frequent fungi that cause potentially fatal human fungal infections are Aspergillus, Cryptococcus, and Candida (23), while Candida auris is a multidrug-resistant fungus (24).Sporothrix spp., Fusarium, Scedosporium, Histoplasma capsulatum, Coccidioides immitis, Candida albicans, Candida auris, Aspergillus fumigatus, Cryptococcus neoformans, and Blastomyces dermatitidis are a few examples. (25–27) Numerous bacteria coexist in biological equilibrium with the human body and its surroundings, but unchecked and unregulated microbial proliferation can result in some very serious issues (28–31). These various compounds are thought to be good starting points for future studies in antimicrobial therapy since they have the ability to suppress and control infections while having minimal cell-toxicity. Many studies have concentrated on investigating antibacterial and antiviral medications with less side effects in light of the aforementioned management strategies for the horrifying effects of the rapid growth of bacteria and viruses. (32–35) Herbal medicines were the primary way of healing in ancient Africa, Europe, the Americas, and especially Asia.(36-38) The World Health Organization recommends integrating traditionally used medications, such as phytomedicine, into the country's healthcare system if they are found to be both safe and effective (39) There is a dearth of information regarding the in vitro or in vivo efficacy of the many medicinal plants (>500 plants) that have been reported to be utilized by Nepalese people for basic healthcare for a long time(40),Researchers have created novel indole 3-substituted-[1,2,4]triazole derivatives and assessed their antibacterial efficacy, even though traditional plants may not directly contain triazole derivatives (41) Triazoles are a family of heterocyclic compounds distinguished by an aromatic ring with five members that comprises three nitrogen atoms and two carbon atoms. Due to their capacity to connect with different enzymes and receptors in the biological system, these adaptable molecules exhibit a wide range of biological activities. There are two forms of five-membered triazoles: 1,2,3-triazole and 1,2,4-triazole. Triazoles may accept a wide variety of substituents because of their structural properties, which enables the creation of a wide range of unique bioactive compounds. (42-45) Natural Deep Eutectic Solvents (NADESs) have been identified as promising solvents with antifungal and antibacterial action in current article research. They are prized for their low flammability, low volatility, simplicity in preparation, and generally cheap production costs1. Because NADESs come from natural sources and support the utilization of biologically based materials and sustainable chemistry, they are very intriguing (46).
Table No 2. General mechanism of Triazole:
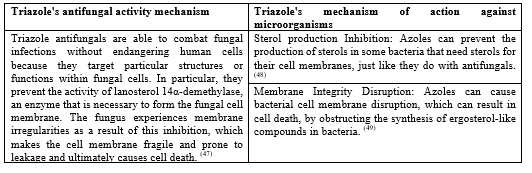
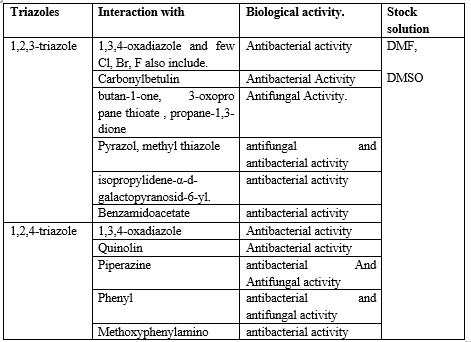
Table No 3. Table for substituted show in antimicrobial activity:
Most of the time, every antifungal medication on the market that has been observed Cl, Br, I, and F, such as fluconazole, luliconazole, itraconazole, and ketoconazole.
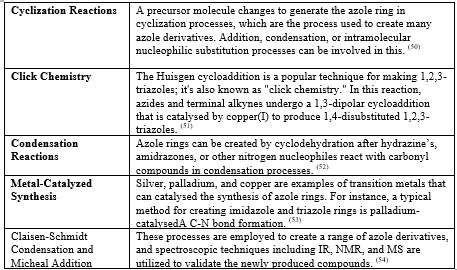
Table no 4. Triazole preparation by using following reaction:
1,2,3-TRIAZOLE AS ANTIMICROBIAL ACTIVITY
- The study conducted by Abdulraheem S. A. Almalki.(55)
focused on evaluating the antimicrobial activity of synthesized compounds against E. coli (gram negative), S. aureus (Gram positive), and C. albicans. The method employed Luria broth Agar and Sabouraud dextrose agar prepared in petri dishes, followed by incubation in a shaker, and the inhibition zones were measured in triplicate. The specific strains used were E.coli ATCC 31218, S.aureus ATCC 29213, and C.albicans ATCC 10231.A series of derivatives (1-14) were synthesized using the esterification method (2-14) and the click chemistry approach. Thymol was used as the starting material for synthesis. The compounds were then evaluated for antimicrobial activity against E. coli, S. aureus, and C. albicans. In silico studies were also conducted, involving computational analysis with thymidylate synthase (PDB ID 6QXG) and DNA gyrase (PDB ID 4URO) for antimicrobial studies. Molecular docking of all compounds with thymidylate synthase (TS) and DNA gyrase B was performed, and the Ramachandran plot was checked.

2-((2-isopropyl-5-methylphenoxy)methyl)-5-(prop-2-ynylthio)-1,3,4-oxadiazole (1), 4-((5-((2-isopropyl-5-methylphenoxy)methyl)-1,3,4-oxadiazol-2-ylthio)methyl)-1- phenyl-1H-1,2,3-triazole (2), 4-((5-((2-iso propyl-5 -methylp henoxy) methyl)-1,3, 4-oxadia zol-2-ylt hio)me thyl)-1 -(2-methoxyphenyl)-1H-1,2,3-triazole (3), 3-(4-((5-((2-isopropyl-5-methylphenoxy ) methyl)-1,3,4-oxadiazol-2-ylthio) methyl)-1H -1,2,3-triaz ol-1-yl)b enzoic acid (4), 2-(4-(5-((2-isopropyl-5-methylphenoxy)methyl)-1,3,4-oxadiazol-2-ylthio)methyl)-1H- 1,2,3-triazol-1-yl)phenol (5), 4-((5-((2-isopropyl-5-methylphenoxy)methyl)-1,3,4-oxadiazol-2-ylthio)methyl)-1-2-chlorophenyl)-1H-1,2,3-triazole (6), 4-((5-((2-isopropyl-5-methylphenoxy)methyl)-1,3,4-oxadiazol-2-ylthio)methyl)-1- (3-chlorophenyl)-1H-1,2,3-triazole (7), 4-((5-((2-isopropyl-5-methylphenoxy)methyl)-1,3,4-oxadiazol-2-ylthio)methyl)-1-(2,4dichlorophenyl)-1H-1,2,3-triazole (8), 4-((5-((2-isopropyl-5-methylphenoxy) methyl)-1,3,4-oxadiazol-2-ylthio)methyl)-1- (4-bromophenyl)-1H-1,2,3-triazole (9), 4-((5-((2-isopropyl-5-methylphenoxy) methyl)-1,3,4-oxadiazol-2-ylthio)methyl)-1- 3-bromophenyl)-1H-1,2,3-triazole (10), 4-((5-((2-isopropyl-5-methylphenoxy)methyl)-1,3,4-oxadiazol-2-ylthio)methyl)-1-4-florophenyl)-1H-1,2,3-triazole (11), 4-((5-((2-isopropyl-5-methylphenoxy)methyl)-1,3,4-oxadiazol-2-ylthio)methyl)-1-(2,4- diflorophenyl)-1H-1,2,3-triazole (12), 4-((5-((2-isopropyl-5-methylphenoxy)methyl)-1,3,4-oxadiazol-2-ylthio) methyl)-N-o-tolyl-1H-1,2,3-triazole-1-carboxamide (13), 4-((5-((2-isopropyl-5-methylphenoxy) methyl)-1-(3-bromophenyl)-1H-1,2,3-triazole (14)The results indicated that compounds 1, 2, 3, 5, 8, 9, 10, 12, and 13 showed significant antimicrobial activity against E. coli. Compounds 1, 2, 3, 4, 5, 12, and 13 demonstrated notable antimicrobial activity against S. aureus, while compounds 1, 5, and 8 were effective against C. albicans. Additionally, it was noted that novobiocin showed activity against both gram-positive and gram-negative microbial strains, while fluconazole exhibited activity against C. albicans. In summary, the study presented a series of synthesized compounds, their antimicrobial activity against specific strains, as well as in silico studies involving molecular docking and computational analysis with relevant enzymes
2. The research conducted by Ewa Bebenek et al.
(56) involved the evaluation of antimicrobial activity against various bacterial strains, including Staphylococcus aureus (gram-positive), Enterococcus faecalis (gram-positive), Escherichia coli (gram-negative), Pseudomonas aeruginosa (gram-negative), Klebsiella pneumoniae (gram-negative), and Candida albicans. The method employed for this evaluation was the broth microdilution method. The specific strains used in the study were Staphylococcus aureus ATCC 25923, Enterococcus faecalis ATCC 29212, Escherichia coli ATCC 25922, Pseudomonas aeruginosa ATCC 27853, and Candida albicans ATCC 10231.A series of derivatives were synthesized using the Huisgen cycloaddition reaction, producing compounds 5a to 5k.
28-O-(1- Benzyl- 1H-1,2, 3-triazol-4-yl)carbonyl betulin.(5a), 28-O-[1-(4-Fluorobenzyl)-1H-1,2,3-triazol-4-yl]carbonylbetulin (5b), 28-O-[1-(4-Cyanobenzyl)-1H-1,2,3-triazol-4-yl]carbonylbetulin (5c), 28-O-(1-Phenylthio methyl-1H-1,2,3-triazol-4-yl)carbonylbetulin (5d), 28-O-[1-(3' -Deoxythymidine-5' -yl)-1H-1,2,3-triazol-4-yl]carbonylbetulin (5e), 28-O-[1-(1-Deoxy-?-D-glucopyranosyl)-1H-1,2,3-triazol-4-yl]carbonyl betulin (5f), 28-O-(1-Ethylacetyl-1H-1,2,3-triazol-4-yl)carbonylbetulin (5g), 28-O-[1-(3-Hydr oxypropyl)-1H-1,2,3-triazol-4-yl]carbonylbetulin (5h), 28-O-[1-(3-Aminopropyl)-1H-1,2,3-triazol-4-yl]carbonylbetulin (5i), 2-Amino-3-[4-(28-O-betuliny lcarb onyl)-1H-1,2,3-triazol-1-yl]propanoic acid (5j), 3-Methyl-3-[4-(28-O-betulinylcarbonyl)-1H-1,2,3-triazol-1-yl]butyric acid (5k).The results indicated that the triazole 28-O-[1-(3' -Deoxythymidine-5'-yl)-1H-1,2,3-triazol-4-yl]carbonylbetulin showed antibacterial activity against Klebsiella pneumoniae [MIC (µM) = 0.95, MBC (µM) = 3.9] and Escherichia coli strains [MIC (µM) = 1.95, MBC (µM) = 7.8].In summary, the study demon started the potential antibacterial activity of a specific synthesized compound against Klebsiella pneumoniae and Escherichia coli strains, presenting valuable insights for further research and development in antimicrobial compounds.
3. The research by Attalah Rahma et al.(57) involved an in-silico study utilizing QSAR and QSPR before progressing to the synthesis and in vitro testing stage. The Molecular Operating Environment software (MOE) and Chemdraw were used for the preparation of hypothetical compounds in 3D The study focused on enzymes COX-1 (PDB ID 1EGQ), COX-2 (PDB ID 1CX2), and CYP51 (PDB ID 1E9X). Several derivatives of hypothetical compounds were synthesized, labeled as compounds a to j.The results of the study indicated the following best ligands for each enzyme, along with their respective scores:For COX-1: compounds a, c, and h showed scores of -7.8270 kcal/mol, -7.7995 kcal/mol, and -7.7872 kcal/mol, respectively, in comparison with reference ligands k, l, and m, For COX-2: compounds i, c, and a demonstrated scores of -9.3667 kcal/mol, -9.1695 kcal/mol, and -8.9740 kcal/mol, respectively, For CYP51: the best ligand showed a score of -7.9008 kcal/mol, Additionally, reference ligands were also utilized, including Ibuprofen (IBP), Protoporphyrin IX containing FE (HEM), and 4-phenyl-1H-Imidazole (PIM), with their respective RMSD and scores. In summary, the study presented promising hypothetical compounds as potential ligands for COX-1, COX-2, and CYP51, providing valuable insights for further research and development in drug design and discovery.
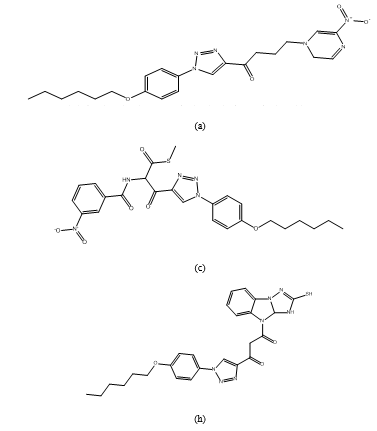
1-(1-(4-(hexyloxy)phenyl)-1H-1,2,3-tria zol-4- yl)-4-(5-nitropyrazin-1(2H)-yl)butan-l-one (a), (2-(4,4-difluorocyclohexa-1,5-dien-1-yl)-1 H- benzo[d]imidazol-1-yl)(1-(4- (hexyloxy)phenyl)-1H-1,2,3 triazol-4-yl)methanone(b), S-methyl 3-(1-(4-(hexyloxy)phenyl)-1H-1,2,3-triazol-4-yl)-2-(3-nitrobenzamido)-3-oxopro pane thioate (c), ethyl 2-2-(1-4-hexyloxy)phenyl)-1 H-1,2,3- D triazole-4-carbonyl) thio)acetamido) thiazole-4- carboxylate (d), (4-1-(2,4-dichloro-5-fluorophenoxy)ethyl)phenyl)(1-(4-(hexyloxy) phenyl)-1H-1,2,3-triazol-4-yl)methanone.(e), (1-(4-(hexyloxy)phenyl)-1H-1,2,3-triazol-4-yl) (8-(trifluoromethyl)quinolin-6- yl)methanone (f), 3-(1-(4-(hexyloxy)phenyl)-1H-1,2,3-triazole-4- carbonyl)-1,2,4-oxadiazol-5(4H)-one (g), 1-(1-(4-(hexyloxy)phenyl)-1H-1,2,3-triazol-4- yl)-3-(2-mercapto-3,3a-dihydro-4Hbenzo[4,5]imidazo[1,2-b][1,2,4] triazol-4- yl)propane-1,3-dione(h), 5-((5-(1,3-oxazepan-3-yl)oxy) furan-2-yl)thio)- 1-(1-(4-(hexyloxy)phenyl)-1H-1,2,3-triazol-4- yl)pentan-1-one (i), 1-(1-(4-(hexyloxy)phenyl)-1H-1,2,3-triazol-4-yl)- 6-(4methylpipe razin-1-yl)-6-(3- nitrophenyl) hexan-l-one (j).
4.The study by Raghavender Matta et al. (58) evaluated the in vitro antimicrobial activity of synthesized compounds against several bacterial strains, including two Gram-positive bacteria (Bacillus subtilis and Staphylococcus aureus) and two Gram-negative bacteria (Escherichia coli and Pseudomonas aeruginosa).The compounds were synthesized through a nucleophilic addition of phenylhydrazine to 1-(4-hydroxyphenyl)ethanone, followed by Vilsmeier-Haack cyclization. The evaluated derivatives,
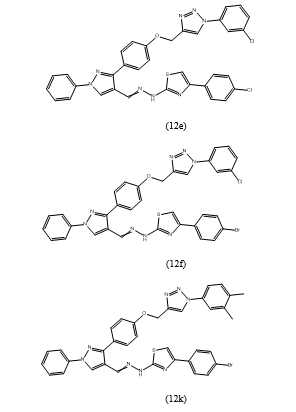
labeled 12a-l, 4-(4-bromophenyl)-2-(2-((1-phenyl-3-(4-((1-phenyl-1H-1,2,3-triazol-4-yl)methoxy)-phenyl)-1H-pyrazol-4-yl)methylene)hydrazin yl)thiazole(12a),4-(4-chlorophenyl)-2-(2-((1-phenyl-3-(4-((1-phenyl-1H-1,2,3-triazol-4-yl)methoxy)phenyl)1H-pyrazol-4-yl)methylene)hydrazinyl)thiazole (12b), 4-(4-methoxyphenyl)-2-(2-((1-phenyl-3-(4-((1-phenyl-1H-1,2,3-triazol-4-yl)methoxy)-phenyl)-1H-pyrazol-4-yl)methylene)hydrazinyl)thiazole(12c),4-phenyl-2-(2-((1-phenyl-3-(4-((1-phenyl-1H-1,2,3-triazol-4-yl)methoxy)phenyl)-1H-pyrazol-4-yl)methylene)hydrazinyl) thiazole(12d),4-(4-chlorophenyl)-2-(2-((3-(4-((1-(3-chlorophenyl)-1H-1,2,3-triazol-4-yl)methoxy)phenyl)-1-phenyl-1H-pyrazol-4-yl)methylene)hydrazinyl)thiazole(12e),4-(4-bromophenyl)-2-(2-((3-(4-((1-(3-chlorophenyl)-1H-1,2,3-triazol-4-yl)methoxy) phenyl)-1-phenyl-1H-pyrazol-4-yl)methylene)hydr azinyl)thiazole (12f),2-(2-((3-(4-((1-(3-chlorophenyl)-1H-1,2,3-triazol-4-yl)methoxy)phenyl)-1-phenyl-1H-pyrazol-4-yl)methylene)hydrazinyl)-4-(4-methoxyphenyl)thiazole(12g),2-(2-((3-(4-((1-(3-chlorophenyl)-1H-1,2,3-triazol-4-yl)methoxy)phenyl)-1-phenyl-1H-pyrazol-4-yl)methylene)hydrazinyl)-4-phenylthiazole(12h),2-(2-((3-(4-((1-(3,4-dimethylphenyl)-1H-1,2,3-triazol-4-yl)methoxy)phenyl)-1-phenyl-1H-pyrazol-4-yl)methylene) hydrazinyl)-4-(4-methoxy phenyl)thiazole(12i).4-(4-chlorophenyl)-2-(2-((3-(4-((1-(3,4-dimethylphenyl)-1H-1,2,3-triazol-4-yl)methoxyphenyl)-1-phenyl-1H-pyrazol-4-yl)methylene)hydrazinyl)thiazole(12j),4-(4-bromophenyl)-2-(2-((3-(4-((1-(3,4-dimethylphenyl)-1H-1,2,3-triazol-4-yl)methoxy)phenyl)-1-phenyl-1H-pyrazol-4-yl)methylene)hydrazinyl)thiazole(12k),2-(2-((3-(4-((1-(3,4-dimethy lphenyl)-1H-1,2,3-triazol-4-yl)methoxy)phenyl)-1-phenyl-1H-pyrazol-4-yl)methylene)hydrazinyl)-4-phenylthiazole(12l), were then tested for their antibacterial activity.The results revealed that the compounds demonstrated higher activity against Gram-positive bacteria compared to Gram-negative bacteria. Specifically, compounds 12e, 12f, and 12k exhibited the most potent antimicrobial activity with minimum inhibitory concentration (MIC) values of 4.8, 5.1, and 4.0 ?g/ml, respectively, against Staphylococcus aureus. These compounds also showed activity against Bacillus subtilis, with MIC values of 6.7, 6.1, and 6.2 ?g/ml, respectively. However, they did not exhibit activity against Escherichia coli or Pseudomonas aeruginosa.In summary, the study presented a thorough synthesis and evaluation of antimicrobial compounds, revealing promising activity against specific bacterial strains, with compounds 12e, 12f, and 12k demonstrating notable antimicrobial efficacy against Gram-positive bacteria.
5. The research conducted by Ismail Fichtali et al. (59)
investigated the antimicrobial activity of synthesized compounds against a variety of bacterial and fungal strains. The study included two Gram-negative bacteria (Escherichia coli and Pseudomonas aeruginosa), four Gram-positive bacteria (Micrococcus luteus, Bacillus cereus, Bacillus subtilis, and Staphylococcus aureus), and one fungal strain (Candida albicans).The method employed for testing antimicrobial activity was the Mueller Hinton Broth medium (MHB) well-microplate using the microdilution assay. The compounds were synthesized using the Huisgen 1,3-dipolar cycloaddition reaction between dimethylacetylene dicarboxylate and N-protected methyl azido glycinate, resulting in the creation of derivatives labeled as compounds 9-14.

Methyl 2-(4-((1H-benzo[d]imidazol-1-yl)methyl)-1H-1,2,3-triazol-1-yl)-2-benzamidoacetate (9), Methyl 2-benzamido-2-(4-(p-tolyl)-1H-1,2,3-triazol-1-yl)acetate (10), Methyl 2-benzamido-2-(4-phenyl-1H-1,2,3-triazol-1-yl)acetate (11), 1-octyl-4-phenyl-1H-1,2,3-triazole (12), 4-phenyl-1-tetradecyl-1H-1,2,3-triazole (13) , Dimethyl 1-(1-benzamido-2-methoxy-2-oxoethyl)-1H-1,2,3-triazole-4,5-dicarboxylate (14), Upon evaluation, compounds 9-14 did not exhibit any antimicrobial effects against Escherichia coli or Pseudomonas aeruginosa. However, the results showed antimicrobial activity of compounds 10, 11, 12, and 14 against Bacillus subtilis, with minimum inhibitory concentrations (MICs) of 5, 5, 5, and 2.5 mg/mL, respectively. Additionally, Bacillus cereus demonstrated antimicrobial susceptibility to compounds 8, 9, 10, 11, 12, and 14, with MICs of 5, 1.5, 5, 5, 5, and 1.5 mg/mL, respectively. Staphylococcus aureus exhibited susceptibility to compounds 10, 11, and 14, with MICs of 5, 5, and 1.5 mg/mL, while Micrococcus luteus was susceptible to compounds 10, 11, 12, and 14, with MICs of 5, 1.5, 5, 5, 5, and 1.5 mg/mL. Compound 14 displayed an intriguing inhibitory effect against all Gram-positive bacteria studied compared to the other derivatives. However, no inhibition was observed against Gram-negative bacteria or Candida albicans.
6.The research conducted by Abdulsalam AM Alkhaldi et al. (60)
involved evaluating the antibacterial activity of synthesized compounds against a diverse range of microbial strains including Klebsiella sp. (Kleb.), Listeria innocua (Lis.), Proteus vulgaris (Prot.), Micrococcus sp. (Micr.), Staphylococcus epidermidis (Staph.), Candida albicans (Ca. 1), Candida kruzi (Ca. 2), Klebsiella pneumonia (Kl. Pn.), Escherichia coli (E. coli), Acinetobacter sp. (Acin.), Enterococcus faecium (Ent.), Vancomycin Resistant Staphylococcus aureus (VRSA), Bacillus sp. (Ba.), Streptococcus pyogenes (Strep.), Escherichia coli (E. coli 2), Enterococcus faecalis (Enter.), Sarcina lutea (Sar.), and Pseudomonas aeruginosa (Ps.).The activity was focused on testing the antibacterial effect against Trypanosoma brucei bloodstream forms (BSF) in-vitro.

The derivatives assessed in the study were Chloroacetic acid N-[2-(4-phenyl-[1, 2, 3] triazol-1-yl) acetyl] hydrazide (II), 2-Phenyl-5-(4-phenyl-[1, 2, 3]-triazol-1- ylmethyl) [1, 3, 4]oxadiazole (III), (4-Phenyl-[1,2,3]-triazol-1-yl) acetic acid (2- hydroxybenzylidene) hydrazide (IVb), (4-Phenyl-[1, 2, 3]-triazol-1-yl) acetic acid (4- nitrobenzylidene) hydrazide (IVc), (4-Phenyl-[1, 2, 3] triazol-1-yl) acetic acid (3, 4-dimethoxybenzylidene) hydrazide (IVd), 1-[2-Phenyl-5-(4-phenyl [1, 2, 3]-triazol-1- ylmethyl) [1, 3, 4] oxadiazol-3-yl] ethanone (Va), 1-[2-(4-Nitrophenyl-5-(4-phenyl-[1, 2, 3] triazol-1-ylmethyl)–[1, 3, 4]–oxadiazol-3-yl] ethanone (Vb), and 1-[2-(3,4-Dimethoxyphenyl)-5-(4-phenyl- [1,2,3]triazol-1-ylmethyl)–[1,3,4]- oxadiazol-3- yl-] ethanone (Vc).The study findings highlighted the significant antibacterial activity of the oxadiazole nucleus of compound 1-[2-(3,4-Dimethoxyphenyl)-5-(4-phenyl- [1,2,3]triazol-1-ylmethyl)–[1,3,4]- oxadiazol-3- yl-] ethanone (Vc) against Staphylococcus Epidermidis (>100 mic) and Pseudomonas aeruginosa (50 mic). This compound exhibited important antibacterial activity, particularly against Pseudomonas aeruginosa.The study findings highlighted the significant antibacterial activity of the oxadiazole nucleus of compound 1-[2-(3,4-Dimethoxyphenyl)-5-(4-phenyl- [1,2,3]triazol-1-ylmethyl)–[1,3,4]- oxadiazol-3- yl-] ethanone (Vc) against Staphylococcus Epidermidis (>100 mic) and Pseudomonas aeruginosa (50 mic). This compound exhibited important antibacterial activity, particularly against Pseudomonas aeruginosa.
7.The research by Raghavender Matta et al. (61)
investigated the antimicrobial activity of synthesized derivatives against Gram-positive bacteria (Bacillus subtilis, Staphylococcus aureus) and Gram-negative bacteria (Escherichia coli, Pseudomonas aeruginosa) using the broth dilution method. Reference drugs including novobiocin and ampicillin were used as benchmarks.The derivatives, labeled 12a to 12k, were tested for their antibacterial activities. Among these derivatives, compounds 12e, 12f, and 12k exhibited significant inhibitory activity. Notably, compound 12k, which contains a para-bromo attached phenyl group directly bound to the thiazole ring, demonstrated the highest inhibitory activity against all tested bacterial strains.This study provides valuable insights into the potential antimicrobial properties of the synthesized compounds and highlights the efficacy of specific derivatives against both Gram-positive and Gram-negative bacterial strains.

4?(4?bromophenyl)?2?(2?((1?phenyl?3?(4?((1?phenyl?1H?1,2,3?triazol?4?yl)meth?oxy)?phenyl)?1H?pyrazol?4?yl)methylene)hydrazinyl)thiazole (12a,C34H25BrN8OS)4?(4?chlorophenyl)?2?(2?((1?phenyl?3?(4?((1?phenyl?1H?1,2,3?triazol?4?yl)meth?oxy)?phenyl)?1H?pyrazol?4?yl)methylene)hydrazinyl)thiazole(12b,C34H25ClN8OS)4?(4?methoxyphenyl)?2?(2?((1?phenyl?3?(4?((1?phenyl?1H?1,2,3?triazol?4?yl)meth?oxy)?phenyl)?1H?pyrazol?4?yl)methylene)hydrazinyl)thiazole(12c,C35H28N8O2S)4?phenyl?2?(2?((1?phenyl?3?(4?((1?phenyl?1H?1,2,3?tria?zol?4?yl)methoxy)phenyl)?1H?pyrazol?4?yl)methylene)hydrazinyl)thiazole(12d,C34H26N8OS), 4-(4-chlorophenyl)-2-(2-((3-(4-((1-(3-chlorophenyl)-1H-1,2,3triazol-4-yl)methoxy)phenyl)-1-phenyl-1H-pyrazol-4-yl)methylene)hydrazinyl)thiazole (12e,C34H24Cl2N8OS), 4-(4-bromophenyl)-2-(2-((3-(4-((1-(3-chlorophenyl)-1H-1,2,3-triazol-4-yl)methoxy)-phenyl)-1-phenyl-1H-pyrazol-4-yl)methylene)hydrazinyl)thiazole (12f,C34H24BrClN8OS)2?(2?((3?(4?((1?(3?chlorophenyl)?1H?1,2,3?triazol?4?yl)methoxy)phenyl)?1?phenyl?1H?pyrazol?4?yl)methyl?ene)hydrazinyl)?4?(4?methoxyphenyl)thiazole(12 g,C35H27ClN8O2S)2?(2?((3?(4?((1?(3?chlorophenyl)?1H?1,2,3?triazol?4?yl)methoxy)phenyl)?1?phenyl?1H?pyrazol?4?yl)methylene)hydrazinyl)?4?phenylthiazole (12 h, C34H25ClN8OS)2?(2?((3?(4? ((1?(3,4?dimethylphenyl)?1H?1,2,3?triazol?4?yl)methoxy)phenyl)?1?phenyl?1H?pyrazol?4?yl)methylene)hydrazinyl)?4?(4?methoxyphenyl)thiazole(12i,C37H32N8O2S)4?(4?chlorophenyl)?2?(2?((3?(4?((1?(3,4?dimethylphenyl)?1H?1,2,3?triazol?4?yl)methoxy)phenyl)?1?phenyl?1H?pyrazol?4?yl)methylene)hydrazinyl)thiazole(12j,C36H29ClN8OS)4-(4-bromophenyl)-2-(2-((3-(4-((1(3,4-dimethylphenyl)-1H-1,2,3-triazol-4-yl)methoxy)phenyl)-1-penyl-1H-pyrazol-4-yl)methylene)hydrazinyl)thiazole (12 k, C36H29BrN8OS)
1,2,4-TRIAZOLE AS ANTIMICROBIAL ACTIVITY:
1.The study conducted by Ismail Fichtali et al.(62)
assessed the antimicrobial properties of synthesized compounds against various microbial strains including Escherichia coli ATCC 35218, Klebsiella pneumoniae ATCC 13883, Yersinia pseudotuberculosis ATCC 911, Enterobacter aerogenes ATCC 13048, Pseudomonas aeruginosa ATCC 10145, Staphylococcus aureus ATCC 25923, Enterococcus faecalis ATCC 29212, Bacillus cereus 709 Roma, Candida tropicalis ATCC 13803, Candida glabrata 66032, and Candida albicans ATCC 60193.The antimicrobial and antifungal assays were performed in Mueller-Hinton broth (MH) at pH 7.3 and buffered Yeast Nitrogen Base at pH 7.0, respectively.The investigated derivatives, marked 3, 5, 6, 8, 9a, 9b, 11, 12, 13, 14, 15a, and 15b, were synthesized and evaluated for their antimicrobial effects
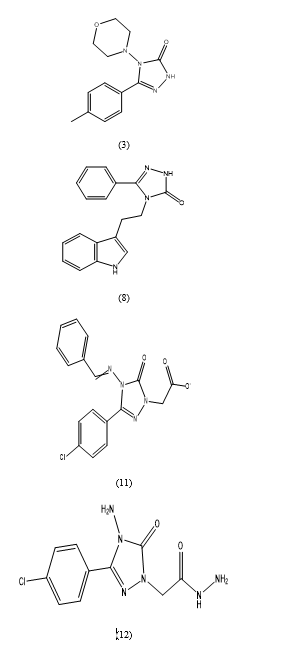

(4-Methylphenyl)-4-morpholin-4-yl-2,4-dihydro 3H-1,2,4-triazol-3-one.(3), 3-(4-Methylphenyl)[1,2,4]triazolo[3,4-b][1,3]benzoxazole.(5), 4-[3-(1H-Imidazol-1-yl)propyl]-5-methyl-2,4-dihydro-3H-1,2,4-triazol-3-one (6), 4-[2-(1H-Indol-3-yl)ethyl]-5-phenyl-2,4-dihydro-3H-1,2,4-triazol-3-one (8), 5-Methyl-4-(3-{4-[3-(3–methyl-5-oxo-1,5-dihydro-4H-1,2,4–triazol–4-yl)propyl] piperazin-1- yl}propyl)-2,4-dihydro-3H-1,2,4-triazol-3-one (9a).5-(4-Chlorobenzyl)-4-(3-{4-[3-(3-methyl-5-oxo-1,5-dihydro-4H-1,2,4-triazol-4-yl)propyl] piperazin-1- yl}propyl)-2,4-dihydro-3H-1,2,4-triazol-3-one (9b)[3-(4-chlorophenyl)-5-oxo-4-{[phenylmethylene]amino}-4,5-dihydro-1H-1,2,4-triazol-1- yl]acetate (11). 2-[4-amino-3-(4-chlorophenyl)-5-oxo-4,5-dihydro-1H-1,2,4-triazol-1-yl]aceto hydrazide (12).4-Amino-5-(4-chlorophenyl)-2-[(5-mercapto-1,3,4-oxadiazol-2-yl)methyl]-2,4-dihydro-3H-1,2,4- triazol-3-one (13).5-(4-Chlorophenyl)-2-[(5-mercapto-1,3,4-oxadiazol-2-yl)methyl]-4-[(4-methoxyben-zylidene)amino]- 2,4-dihydro-3H-1,2,4-triazol-3-one (14).4-[(4-Methoxybenzylidene)amino]-5-(4-chlorophenyl)-2-({4-[(4-methylpiperazin-1-yl)methyl]-5- thioxo-4,5-dihydro-1,3,4-oxadiazol-2-yl}methyl)-2,4-dihydro-3H-1,2,4-triazol-3-one (15a).4-[(4-Methoxybenzylidene) amino]-5-(4-chlorophenyl)-2-{[4-(morpholin-4-ylmethyl)-5-thioxo-4,5- dihydro-1,3,4-oxadiazol-2-yl}methyl)-2,4-dihydro-3H-1,2,4-triazol-3-one (15b).The study results revealed that compounds 3 and 8 demonstrated moderate activities against Escherichia coli (Ec) and Klebsiella pneumoniae (Kp), while compounds 11 and 12 exhibited moderate activities against Enterobacter aerogenes (En), Staphylococcus aureus (Sa), Enterococcus faecalis (Ef), and Bacillus cereus (Be). Compounds 13 and 14 displayed good antimicrobial activities against the test microorganisms. Furthermore, Mannich bases 15a and 15b demonstrated either good or moderate antimicrobial activities against the test microorganisms. Importantly, none of the synthesized compounds showed antimicrobial activity against Candida tropicalis (Ct) and Candida albicans (Ca).In conclusion, the study provided insights into the antimicrobial activities of synthesized compounds against a range of bacterial and fungal strains, with several compounds showing promising antimicrobial effects against specific microorganisms
- The research conducted by Asmita V et al. (63)
focuses on the in-vitro antimicrobial activities of a series of derivatives known as the triazolo quinoline (SHT series) against a variety of bacterial strains. The strains tested included Gram-positive bacteria such as Bacillus cereus (BC), Bacillus subtilis (BS), and Staphylococcus aureus (SA), as well as Gram-negative bacterial strains such as E. coli (EC), Klebsiella pneumoniae (KP), and P. aeruginosa (PA).The study employed DMSO and DMF as solvents. The antimicrobial activities of the derivatives were determined using the standard agar well diffusion assay, with Mueller Hinton agar and Sabouraud dextrose agar media used for antibacterial and antifungal activity testing, respectively.A total of 20 derivatives

SHT-1:
4-(benzo[d]thiazol-2-yl)-5-(4-hydroxy-3-methoxyphenyl)-8,8-dimethyl-2-(pyridin-4- yl)-2,7,8,9-tetrahydro-[1,2,4]triazolo[1,5-a]quinolin-6(1H)-one SHT-2:4-(benzo[d]thiazol-2-yl)-5-(2-chlorophenyl)-8,8-dimethyl-2-(pyridin-4-yl)-2,7,8,9- tetrahydro-[1,2,4]triazolo[1,5-a]quinolin-6(1H)-one SHT-3:4-(benzo[d]thiazol-2-yl)-5-(3-bromophenyl)-8,8-dimethyl-2-(pyridin-4-yl)-2,7,8,9- tetrahydro-[1,2,4]triazolo[1,5-a]quinolin-6(1H)-one SHT-4:4-(benzo[d]thiazol-2-yl)-5-(4-bromophenyl)-8,8-dimethyl-2-(pyridin-4-yl)-2,7,8,9- tetrahydro-[1,2,4]triazolo[1,5-a]quinolin-6(1H)-one SHT-5:4-(benzo[d]thiazo l-2-yl)-5-(4-fluorophenyl)-8,8-dimethyl-2-phenyl-2,7,8,9- tetrahydro-[1,2,4]triazolo[1,5-a]quinolin-6(1H)-one SHT-6:4-(benzo[d]thiazol-2-yl)-5-(3-chlorophenyl)-8,8-dimethyl-2-(pyridin-4-yl)-2,7,8,9- tetrahydro-[1,2,4]triazolo[1,5-a]quinolin-6(1H)-one SHT-7:4-(benzo[d]thiazol-2-yl)-5-(4-chlorophenyl)-8,8-dimethyl-2-(pyridin-4-yl)-2,7,8,9- tetrahydro-[1,2,4]triazolo[1,5-a]quinolin-6(1H)-one SHT-8:4-(benzo[d]thiazol-2-yl)-5-(2-hydroxyphenyl)-8,8-dimethyl-2-(pyridin-4-yl)-2,7,8,9- tetrahydro-[1,2,4]triazolo[1,5-a]quinolin-6(1H)-one SHT-9:4-(benzo[d]thia zol-2-yl) -5-(3-hydroxyphenyl)-8,8-dimethyl-2-(pyridin-4-yl)-2,7,8,9- tetrahydro-[1,2,4]triazolo[1,5-a]quinolin-6(1H)-one SHT-10:4-(benzo[d]thiazol-2-yl)-5-(4-hydroxyphenyl)-8,8-dimethyl-2-(pyridin-4-yl)-2,7,8,9- tetrahydro-[1,2,4]triazolo[1,5-a]quinolin-6(1H)-one SHT-11:4-(benzo[d]thiazol-2-yl)-8,8-dimethyl-5-(2-nitrophenyl)-2-(pyridin-4-yl)-2,7,8,9- tetrahydro-[1,2,4]triazolo[1,5-a]quinolin-6(1H)-one SHT-12:4-(benzo[d]thiazol-2-yl)-8,8-dimethyl-5-(3-nitrophenyl)-2-(pyridin-4-yl)-2,7,8,9- tetrahydro-[1,2,4]triazolo[1,5-a]quinolin-6(1H)-one SHT-13:4-(benzo[d]thiazol-2-yl)-8,8-dimethyl-5-(4-nitrophenyl)-2-(pyridin-4-yl)-2,7,8,9- tetrahydro-[1,2,4]triazolo[1,5-a]quinolin-6(1H)-one SHT-14:4-(benzo[d]thiazol-2-yl)-5-(2,5-dimethoxyphenyl)-8,8-dimethyl-2-(pyridin-4-yl)- 2,7,8,9-tetrahydro-[1,2,4]triazolo[1,5-a]quinolin-6(1H)-one SHT-15:4-(benzo[d]thiazol-2-yl)-5-(3,4-dimethoxyphenyl)-8,8-dimethyl-2-(pyridin-4-yl)- 2,7,8,9-tetrahydro-[1,2,4]triazolo[1,5-a]quinolin-6(1H)-oneSHT-16:4-(benzo[d]thiazol-2-yl)-8,8-dimethyl-2-(pyridin-4-yl)-5-(3,4,5-trimethoxyphenyl)-2,7,8,9-tetrahydro-[1,2,4]triazolo[1,5-a]quinolin-6(1H)-one SHT-17:4-(4-(benzo[d]thiazol-2-yl)-8,8-dimethyl-6-oxo-2-(pyridin-4-yl)-1,2,6,7,8,9- hexahydro-[1,2,4]triazolo[1,5-a]quinolin-5-yl)benzonitrileSHT-18:4-(benzo[d]thiazol-2-yl)-8,8-dimethyl-2-(pyridin-4-yl)-5-(p-tolyl)-2,7,8,9- tetrahydro-[1,2,4]triazolo[1,5-a]quinolin-6(1H)-one SHT-19:4-(benzo[d]thiazol-2-yl)-5-(2-methoxyphenyl)-8,8-dimethyl-2-(pyridin-4-yl)- 2,7,8,9-tetrahydro-[1,2,4]triazolo[1,5-a]quinolin-6(1H)-one SHT-20:4-(benzo[d]thiazol-2-yl)-5-(4-methoxyphenyl)-8,8-dimethyl-2-(pyridin-4-yl)- 2,7,8,9-tetrahydro-[1,2,4]triazolo[1,5-a]quinolin-6(1H)-one were evaluated in the study (SHT-1 to SHT-20). The conclusions drawn from the findings are as follows:Zone of inhibition against Gram-positive bacteria in DMSO solution:SHT-19 exhibited greater inhibition than SHT-18 against Bacillus cereus (BC),Multiple compounds showed inhibition against Bacillus subtilis (BS), and Staphylococcus aureus (SA).Zone of inhibition for compounds against Gram-positive bacterial strains in DMF:SHT-4 (containing 4-Br), SHT-10 (containing 4-OH), SHT-18 (containing 4-CH3), and SHT-19 (containing 2-OCH3) exhibited maximum inhibition against Bacillus cereus (BC).Different compounds demonstrated maximum inhibition against Bacillus subtilis (BS) and Staphylococcus aureus (SA).Zone of inhibition against Gram-negative bacterial strains in DMSO:Various compounds showed inhibition against E. coli (EC), Klebsiella pneumoniae (KP), and P. aeruginosa (PA).The final conclusion drawn from the study is that DMF is a better solvent for the studied compounds.In summary, the research provides valuable insights into the antimicrobial activities of the triazolo quinoline derivatives against a range of bacterial strains, indicating the potential of these compounds in antimicrobial application
- Raghavender Matta et al. (64)
conducted a study on the antifungal activity of synthesized derivatives using the steak plate method with YEP agar media. The study assessed the susceptibility of Aspergillus niger MTCC 404 and Saccharomyces cerevis iae MTCC 1344 to the derivatives. Reference drug Miconazole was used as a benchmark. The results indicated that compounds 12a, 12f, 12g, 12i, and 12k showed potent fungal growth inhibition against Saccharomyces cerevisiae MTCC 1344. Additionally, compounds 12a, 12f, 12g, 12i, and 12k demonstrated different levels of effectiveness at inhibiting the growth of Aspergillus niger and Saccharomyc es cerevisiae. This study provides valuable insights into the antifungal properties of the synthesized compounds, indicating their potential efficacy against the testedfungal4?(4?bromophenyl)?2?(2?((1?phenyl?3?(4?((1?phenyl?1H?1,2,3?triazol?4?yl)meth?oxy)?phenyl)?1H?pyrazol?4?yl)methylene)hydrazinyl)thiazole(12a,C34H25BrN8OS)4?(4?chlorophenyl)?2?(2?((1?phenyl?3?(4?((1?phenyl?1H?1,2,3?triazol?4?yl)meth?oxy)?phenyl)?1H?pyrazol?4?yl)methylene)hydrazinyl)thiazole(12b,C34H25ClN8OS)4?(4?methoxyphenyl)?2?(2?((1?phenyl?3?(4?((1?phenyl?1H?1,2,3?triazol?4?yl)meth?oxy)?phenyl)?1H?pyrazol?4?yl)methylene)hydrazinyl)thiazole(12c,C35H28N8O2S)4?phenyl?2?(2?((1?phenyl?3?(4?((1?phenyl?1H?1,2,3?tria?zol?4?yl)methoxy)phenyl)?1H?pyrazol?4?yl)methylene)hydrazinyl)thiazole(12d,C34H26N8OS)4?(4?chlorophenyl)?2?(2?((3?(4?((1?(3?chlorophenyl)?1H?1,2,3?triazol?4?yl)methoxy)phenyl)?1?phenyl?1H?pyrazol?4?yl)methylene)hydrazinyl)thiazole(12e,C34H24Cl2N8OS)4?(4?bromophenyl)?2?(2?((3?(4?((1?(3?chlorophenyl)?1H?1,2,3?triazol?4?yl)methoxy)?phenyl)?1?phenyl?1H?pyrazol?4?yl)methylene)hydrazinyl)thiazole(12f,C34H24BrClN8OS)2?(2?((3?(4?((1?(3?chlorophenyl)?1H?1,2,3?triazol?4?yl)methoxy)phenyl)?1?phenyl?1H?pyrazol?4?yl)methyl?ene)hydrazinyl)?4?(4?methoxyphenyl)thiazole(12g,C35H27ClN8O2S)2?(2?((3?(4?((1?(3?chlorophenyl)?1H?1,2,3?triazol?4?yl)methoxy)phenyl)?1?phenyl?1H?pyrazol?4?yl)methylene)hydrazinyl)?4?phenylthiazole (12 h, C34H25ClN8OS)2?(2?((3?(4?((1?(3,4?dimethylphenyl)?1H?1,2,3?triazol?4?yl)methoxy)phenyl)?1?phenyl?1H?pyrazol?4?yl)methylene)hydrazinyl)?4?(4?methoxyphenyl)thiazole(12i,C37H32N8O2S)4?(4?chlorophenyl)?2?(2?((3?(4?((1?(3,4?dimethylphenyl)?1H?1,2,3?triazol?4?yl)methoxy)phenyl)?1?phenyl?1H?pyrazol?4?yl)methylene)hydrazinyl)thiazole(12j,C36H29ClN8OS)4?(4?bromophenyl)?2?(2?((3?(4?((1?(3,4?dimethylphenyl)?1H?1,2,3?triazol?4?yl)methoxy)phenyl)?1?penyl?1H?pyrazol?4?yl)methylene)hydrazinyl)thiazole (12 k, C36H29BrN8OS)
- Mohamed Ellouz et al. (65)
conducted a study on the antimicrobial activity of synthesized compounds using the disc diffusion method against various bacterial strains. The study assessed the effectiveness of the compounds against two Gram-positive bacteria, Staphylococcus aureus ATCC 25923 and Staphylococcus aureus MLSB, and six Gram-negative bacteria, including Escherichia coli ATCC 25922, Pseudomonas aeruginosa (PA) ATCC 27853, Acinetobacter (Acin) ATCC 17978, Escherichia coli ESBL, Klebsiella pneumonia (KP) ESBL, and Acinetobacter ESBL.The synthesized compounds included a series of derivatives (4-16) prepared using cycloaddition reactions. Among these, compound 8a demonstrated significant antibacterial activity, showing excellent effectiveness against Pseudomonas aeruginosa (PA) ATCC 27853 and Acinetobacter (Acin) ESBL, with a minimum inhibitory concentration (MIC) of 31.2 ?g/ml. These findings highlight the potential of compound 8a as a promising candidate for further exploration in antimicrobial research.4?(Prop?2?ynyl)?3,4?dihydro?2H?1,4?benzothiazin?3?one,4(2Z)?2?Benzylidene?4?(prop?2?ynyl)?3,4?dihydro?2H?1,4?ben?zothiazin?3?one 5(Z)?2?(4?Chlorobenzylidene)?4?(prop?2?ynyl)?2H?1,4?benzo?thiazin?3?one6,4?[(1??1?,2?:3?,4??Di?O?isopropylidene???d?galactopyranosid?6??yl)?1?,2?,3??triazol?4??yl)methyl]?2H?1,4?benzothia?zin?3?one 7a, 4?[(1??1?,2?:3?,4??Di?O?isopropylidene???d?galactopyranosid?6??yl)?1?,2?,3??triazol?5??yl) methyl]?2H?1,4?benzothia?zin?3?one7b,(2Z)?2?Benzylidene?4?[(1??1?,2?:3?,4??di?O?isopropylidene???d?galactopyranosid?6??yl)?1?,2?,3??triazol?4??yl)methyl]?2H?1,4?benzothiazin?3?one.8a, (2Z)?2?Benzylidene?4?[(1??1?,2?:3?,4??di?O?isopropylidene???d?galactopyranosid?6??yl)?1?,2?,3??triazol?5??yl)methyl]?2H?1,4?benzothiazin?3?one8b,(2Z)?2?(4?Chlorobenzylidene)?4?[(1??1?,2?:3?,4??di?O?isopropylidene???d?galactopyranosid?6??yl)?1?,2?,3??triazol?4??yl)methyl]?2H?1,4?benzothiazin?3?one 9a, (2Z)?2?(4?Chlorobenzylidene)?4?[(1??1?,2?:3?,4??di?O?isopropylidene???d?galactopyranosid?6??yl)?1?,2?,3??triazol?5??yl)methyl]?2H?1,4?benzothiazin?3?one9b,4?[(1??2?,3?,4?,6??Tétra?O?acétyl?(d)?glucopyranos?1??yl)?1?,2?,3??triazol?4??yl)methyl]?2H?1,4?benzothiazin?3?one10a,4?[(1??2?,3?,4?,6??Tétra?O?acétyl?(d)?glucopyranos?1??yl)?1?,2?,3??triazol?5??yl)methyl]?2H?1,4?benzothiazin?3?one10b,(2Z)?2?Benzylidene?4?[(1??2?,3?,4?,6??tétra?O?acétyl?(d)?glucopyranos?1??yl)?1?,2?,3??triazol?4??yl)methyl]?2H?1,4?benzothi?azin?3?one11a,(2Z)?2?Benzylidene?4?[(1??2?,3?,4?,6??tétra?O?acetyl?(d)?glucopyranos?1??yl)?1?,2?,3??triazol?5??yl)methyl]?2H?1,4?benzothi?azin?3?one11b,(2Z)?2?(4?Chlorobenzylidene)?4?[(1??2?,3?,4?,6??tetra?O?acetyl?(d)?glucopyranos?1??yl)?1?,2?,3??triazol?4??yl)methyl]?2H?1,4?benzothiazin?3?one 12a,(2Z)?2?(4?Chlorobenzylidene)?4?[(1??2?,3?,4?,6??tetra?O?acetyl?(d)?glucopyranos?1??yl)?1?,2?,3??triazol?5??yl)methyl]?2H?1,4?benzothiazin?3?one12b,4?[1,2,3?Triazolylmethyl]?2H?1,4?benzothiazin?3?one-13,(2Z)?2?Benzylidene?4?[1,2,3?triazolylmethyl]?2H?1,4?benzo?thiazin?3?one14, (2Z)?2?(4?Chlorobenzylidene)?4?[1,2,3?tria?zolyl?methyl]?2H?1,4?benzothiazin?3?one15, Ethyl?n?(benzoyl)?2?ethoxylglycinate 16
- In the research conducted by Ismail Fichtali et al. (66)
the antimicrobial activity of synthesized compounds was evaluated against a range of bacterial and fungal strains. The testing encompassed two Gram-negative bacteria (Escherichia coli and Pseudomonas aeruginosa), four Gram-positive bacteria (Micrococcus luteus, Bacillus cereus, Bacillus subtilis, and Staphylococcus aureus), as well as one fungal strain (Candida albicans).The method of assessment involved the use of a 96 well-microplate and the microdilution assay. The compounds were synthesized through Huisgen 1,3-dipolar cycloaddition reactions, resulting in the creation of several derivatives, namely compounds 8 to 14. Methyl 2-(4-((1H-1,2,4-triazol-1-yl)methyl)-1H-1,2,3-triazol-1-yl)-2-benzamidoacetate (8), Methyl 2-(4-((1H-benzo[d]imidazol-1-yl)methyl)-1H-1,2,3-triazol-1-yl)-2-benzamidoacetate (9), Methyl 2-benzamido-2-(4-(p-tolyl)-1H-1,2,3-triazol-1-yl)acetate (10), Methyl 2-benzamido-2-(4-phenyl-1H-1,2,3-triazol-1-yl)acetate (11), 1-octyl-4-phenyl-1H-1,2,3-triazole (12), 4-phenyl-1-tetradecyl-1H-1,2,3-triazole (13)
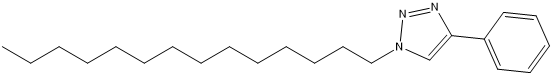
Among these derivatives, compound 13 demonstrated intriguing inhibitory effects against all Gram-positive bacteria studied, with specific minimum inhibitory concentrations (MICs) of 2.5, 1.25, 1.25, and 1.25 mg/mL for Bacillus subtilis, Bacillus cereus, Staphylococcus aureus, and Micrococcus luteus, respectively. However, it's important to note that no inhibitory effect on the fungal strain Candida albicans was observed in the study
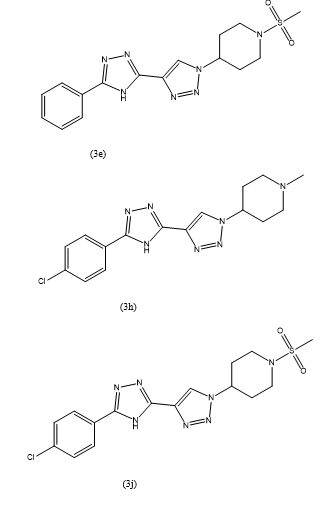
- In the investigation led by Bhimagouda S. Patil et al. (67),
a series of novel [1,2,4]-triazolo piperidine derivatives were examined for their antibacterial and antifungal activities. The antibacterial assessment targeted Gram-positive organisms (Bacillus subtilis, Staphylococcus aureus, Staphylococcus epidermidis) and Gram-negative organisms (Escherichia coli and Pseudomonas aeruginosa). The antifungal activity was evaluated against R. oryzae, A. niger, A. Flavus, C. albicans, and S. cerevisiae.The two fold serial dilution method employing 64-well microtest plates was utilized for the assessment, with dimethylformamide (DMF) as the solvent. Streptomycin and Penicillin served as reference drugs. Compound derivatives 8 to 11c were synthesized as part of the study.The results indicated that compounds 11b and 9a exhibited notable antibacterial activity. Specifically, compound 11b demonstrated effectiveness against S. aureus, S. epidermidis, E. coli, and P. aeruginosa with respective MIC values of 18.75, 9.75, 18.75, and 9.75 µg/mL. Compound 9a was effective against S. aureus, S. epidermidis, E. coli, and P. aeruginosa with MIC values of 18.75, 18.75, 18.75, and 18.75 µg/mL, respectively. Additionally, it was observed that almost all newly synthesized compounds exhibited poor activity against the tested fungal strains.
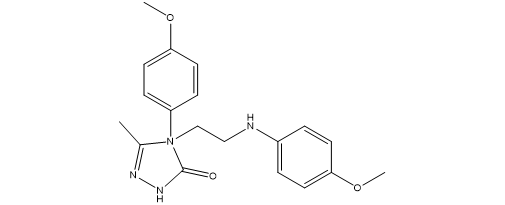
4-[2-(4-Acetyl-4-phenylpiperidin-1-yl)-ethyl]-2-(4-methoxyphenyl)-5-methyl-2,4-dihydro[1, 2, 4]triazol-3-one (8)4-{2-[4-(2,4-Dichlorophenyl)piperazin-1-yl]ethyl}-2-(4-methoxyphenyl)-5-methyl-2,4-dihydro[1, 2, 4]triazol-3-one (9a)4-{2-[4-(2-Chloro-4-fluorophenyl)piperazin-1-yl]ethyl}-2-(4-methoxyphenyl)-5-methyl-2,4-dihydro[1, 2, 4]triazol-3-one (9b).4-{2-[4-(2,4-Difluorophenyl)piperazin-1-yl]ethyl}-2-(4-methoxyphenyl)-5-methyl-2,4-dihydro[1, 2, 4]triazol-3-one(9c).4-[2-(2-Fluorophenoxy)ethyl]-2-(4-methoxyphenyl)-5-methyl-2,4-dihydro[1, 2, 4]triazol-3-one (10a)4-[2-(4-Fluorophenoxy)ethyl]-2-(4-methoxyphenyl)-5-methyl-2,4-dihydro[1, 2, 4]triazol-3-one (10b).4-[2-(Pentafluorophenoxy)ethyl]-2-(4-methoxyphenyl)-5-methyl-2,4-dihydro[1, 2, 4]triazol-3-one (10c).4-[2-(4-Trifluoromethoxyphenoxy)ethyl]-2-(4-methoxyphenyl)-5-methyl-2,4-dihydro[1, 2, 4]triazol3-one (10d).4-[2-(4-Phenylphenoxy)ethyl]-2-(4-methoxyphenyl)-5-methyl-2,4-dihydro[1, 2, 4]triazol-3-one (10e).4-[2-(4-sec-Butylphenylamino)ethyl]-2-(4-methoxyphenyl)-5-methyl-2,4-dihydro[1, 2, 4]triazol-3-one (11a)4-(4-Methoxyphenyl)-4-[2-(4-methoxyphenylamino)ethyl]-5-methyl-2,4-dihydro[1, 2, 4]triazol-3one (11b).4-[2-(4-Fluorophenylamino)ethyl]-2-(4-methoxyphenyl)-5-methyl-2,4-dihydro[1, 2, 4]triazolo-3-one (11c)
- The research conducted by Ebtehal S Al-Abdullah et al. (68)
involved testing new compounds for their activity against a panel of Gram-positive (Bacillus subtilis, Staphylococcus aureus, Micrococcus luteus) and Gram-negative bacteria (Escherichia coli and Pseudomonas aeruginosa), as well as the pathogenic fungus Candida albicans.The primary screening was carried out using the agar disc diffusion method with Müller–Hinton agar medium. Reference antibiotics used in the study included Gentamicin, Ampicillin, and Clotrimazole. The derivatives tested in the study include 5-(1-Adamantyl)-4-phenyl-2-(4-substituted piperazine-1-ylmethyl)-1,2,4-triazoline-3-thiones (6a–f), 5-(1-Adamantyl)-4-methyl-3-(2-aminoethylthio)-1,2,4-triazoles (9a–d), 5-(1-adamantyl)-4-methyl-2-(2-aminoethyl)-1,2,4-triazoline-3-thiones (10a–d), 5-(1-Adamantyl)-3-[(2-methoxyethyl) sulfanyl]-4-phenyl-1,2,4-triazole (11), 5-(1-Adamantyl)-3-arylmethylsulfanyl-4-phenyl-1,2,4-triazoles (12a–e), Ethyl 2-[5-(1-adamantyl)-4-phenyl-1,2, 4-triazol-3-ylsulfanyl]acetate (13), and 2-[5-(1-Adamantyl)-4-phenyl-1,2, 4-triazol-3-ylsulfanyl]acetic acid (14).The conclusion drawn from the study indicated that the new compounds, specifically compounds 6b, 6c, 6d, 6e, 6f, 10b, 10c, 10d, 12c, 12d, 12e, 13, and 14, displayed potent antibacterial activity against the tested panel of Gram-positive and Gram-negative bacteria, as well as the pathogenic fungus Candida albicans.
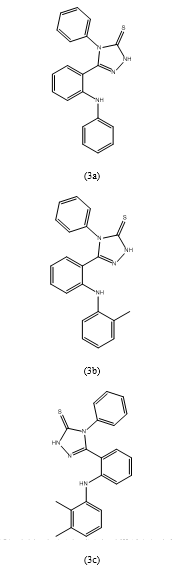
- Vaibhav Saxena et al. (69)
conducted an antimicrobial study using the cup plate method, with DMSO as the solvent control and nutrient broth and Sabourd dextrose broth as the culture media for bacteria and fungi, respectively.
The derivatives tested included 4-Phenyl-1-(2-(phenylamino)benzoylthiosemicarb azide (2a), 1-(2-(o-Toluidino)benzoyl)-4-phenylthiosemicarbazide (2b), 1-(2-(2,3-Dimethylphenylamino)benzoyl)-4-phenylthiosemicarbazide (2c), 4-Phenyl-1-(2-(o-tolyloxy)benzoyl)thiosemicarbazide (2e), 4-Phenyl-5-(2-(phenylamino)phenyl)-2H-1,2,4-triazole-3(4H)-thione (3a), 5-(2-(o-Toluidino)phenyl)-4-phenyl-2H-1,2,4-triazole-3(4H)-thione (3b), 5-(2-(2,3-Dimethylphenylamino)phenyl)-4-phenyl-2H-1,2,4-triazole-3(4H)-thione (3c), 5-(2-Phenoxyphenyl)-4-phenyl-2H-1,2,4-triazole-3(4H)-thione (3d), 4-Phenyl-5-(2-(o-tolyloxy)phenyl)-2H-1,2,4-triazole-3(4H)-thione (3e), and 5-(2-(2,3-Dimethylphenoxy)phenyl)-4-phenyl-2H-1,2,4-triazole-3(4H)-thione (3f).The study evaluated the antibacterial activity against Staphylococcus aureus, Bacillus anthracis, Pseudomonas aeruginosa, Escherichia coli, and the antifungal activity against Candida albicans and Aspergillus niger. It was observed that the zone of inhibition for antibacterial activity ranged from 10 to 25 mm for gram-positive bacteria and 11 to 23 mm for gram-negative bacterial strains. Compounds 3a, 3b, and 3e exhibited mild to moderate activity, whereas compounds 3c and 3d demonstrated significant activity against gram-positive bacteria. In terms of antifungal activity, the zone of inhibition ranged from 7 to 21 mm, with compounds 3b and 3c showing mild to moderate activity against the fungal strains compared to fluconazole.
- Gupta et al. (70)
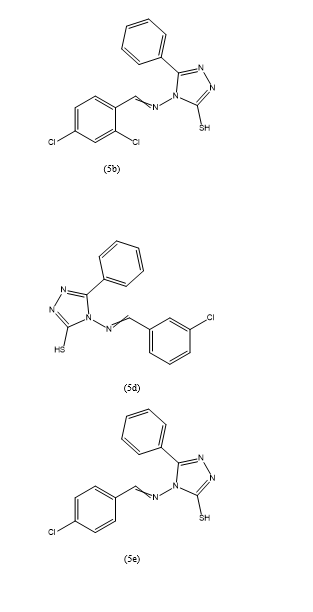
Conducted an antibacterial Activity The study evaluated the antibacterial efficacy of various 1,2,4-triazole derivatives against Staphylococcus aureus using the cup-plate method. Among the derivatives tested, 4-(4-chlorobenzylideneamino)-5-phenyl-4H-1,2,4-triazole-3-thiol demonstrated an antibacterial potency at a concentration of 6.25 µg/ml, surpassing that of the reference antibiotic streptomycin.
They also conducted antifungal Activity The antifungal potential of the same set of triazole derivatives was assessed against Candida albicans, Aspergillus niger, and Microsporum gypseum. The results indicated that M. gypseum was particularly susceptible to these compounds. Notably, six derivatives,

Specifically 4-(2,4-dichlorobenzylideneamino)-5-phenyl-4H-1,2,4-triazole-3-thiol (5b), 4-(4-fluorobenzylideneamino)-5-phenyl-4H-1,2,4-triazole-3-thiol (5c), 4-(3-chlorobenzylideneamino)-5-phenyl-4H-1,2,4-triazole-3-thiol (5d), 4-(4-chlorobenzylideneamino)-5-phenyl-4H-1,2,4-triazole-3-thiol (5e), 4-(2-chlorobenzylideneamino)-5-(4-fluorophenyl)-4H-1,2,4-triazole-3-thiol (5m), and 4-(3-chlorobenzylideneamino)-5-(4-fluorophenyl)-4H-1,2,4-triazole-3-thiol (5n), exhibited significant activity at concentrations ranging from 3 to 6.25 µg/ml.These findings suggest that 1,2,4-triazole derivatives could be promising candidates for the development of new antibacterial and antifungal agents. The superior activity of certain derivatives against M. gypseum highlights their potential in treating fungal infections caused by this pathogen.
10. Jaiprakash et al. (71) In this study, we evaluated the antifungal activity of novel derivatives against various fungal strains, including C. albicans, Fusarium oxysporum, Aspergillus flavus, Aspergillus niger, and Cryptococcus neoforman, using the standard agar method. Miconazole and Fluconazole were employed as reference drugs. Among the synthesized derivatives, compound 3j displayed notable inhibitory activity against all tested fungal strains, with minimum inhibitory concentrations (MIC) ranging from 20 to 30 µg/ml. Tert-butyl-(4-(5-phenyl-4H-1,2,4-triazol-3-yl)-1H-1,2,3-triazol-1-yl)piperidine-1-carboxylate (3a), 4-(4-(5-phenyl-4H-1,2,4-triazol-3-yl)-1H-1,2,3-triazol-1-yl)piperidine (3b), 1-methyl-4-(4-(5-phenyl-4H-1,2,4-triazol-3-yl)-1H-1,2,3-triazol-1-yl)piperidine (3c), 1-ethyl-4-(4-(5-phenyl-4H-1,2,4-triazol-3-yl)-1H-1,2,3-triazol-1-yl)piperidine (3d), 1-(methylsulfonyl)-4-(4-(5-phenyl-4H-1,2,4-triazol-3-yl)-1H-1,2,3-triazol-1-yl)piperidine (3e), Phenyl(4-(4-(5-phenyl-4H-1,2,4-triazol-3-yl)-1H-1,2,3-triazol-1-yl)piperidine-1-yl)methanone (3f), (4-chlorophenyl)(4-(4-(5-phenyl-4H-1,2,4-triazol-3-yl)-1H-1,2,3-triazol-1-yl)piperidine-1-yl)methanone (3g), (4-chlorophenyl)(4-(4-(5-phenyl-4H-1,2,4-triazol-3-yl)-1H-1,2,3-triazol-1-yl)piperidine-1-yl)methanone (3g), 4-(4-(5-(4-chlorophenyl)-4H-1,2,4-triazol-3-yl)-1H-1,2,3-triazol-1-yl)-1-methylpiperidine(3h), 1-(4-(4-(5-(4-chlorophenyl)-4H-1,2,4-triazol-3-yl)-1H-1,2,3-triazol-1-yl)piperidine-1-yl)ethanone(3i), 4-(4-(5-(4-chlorophenyl)-4H-1,2,4-triazol-3-yl)-1H-1,2,3-triazol-1-yl)-1-(methylsulfonyl)piperidine(3j), 4-(4-(5-(4-methoxyphenyl)-4H-1,2,4-triazol-3-yl)-1H-1,2,3-triazol-1-yl)-1-methylpiperidine (3k), 1-ethyl-4-(4-(5-(4-methoxyphenyl)-4H-1,2,4-triazol-3-yl)-1H-1,2,3-triazol-1-yl)piperidine (3l), 1-(methylsulfonyl)-4-(4-(5-(4-nitrophenyl)-4H-1,2,4-triazol-3-yl)-1H-1,2,3-triazol-1-yl)piperidine(3m), 1-(4-(4-(5-p-tolyl-4H-1,2,4-triazol-3-yl)-1H-1,2,3-triazol-1-yl)piperidine-1-yl)ethanone (3n), Phenyl(4-(4-(5-p-tolyl-4H-1,2,4-triazol-3-yl)-1H-1,2,3-triazol-1-yl)piperidine-1-yl)methanone (3o).
Additionally, compounds 3e and 3h exhibited moderate antifungal activity, whereas others showed varying degrees of effectiveness compared to the reference drugs. These findings suggest the potential of these derivatives as candidates for further development as antifungal agents.
REFERENCES
- Purssell, E., 2020. Antimicrobials. Understanding pharmacology in nursing practice, pp.147-165.
- Zarf, S. and Arman, I., 2020. In vitro and in silico analysis of the effect of fluconazole, an antifungal drug, on DNA. Journal of the Serbian Chemical Society, 85(9), pp.1137-1147.
- Van Damme, E., De Meyer, S., Bojkova, D., Ciesek, S., Cinatl, J., De Jonghe, S., Jochmans, D., Leyssen, P., Buyck, C., Neyts, J. and Van Loock, M., 2021. In vitro activity of itraconazole against SARS?CoV?2. Journal of medical virology, 93(7), pp.4454-4460.
- Ellepola, A.N.B., Dassanayake, R.S. and Khan, Z., 2019. In vitro post-antifungal effect of posaconazole and its impact on adhesion-related traits and hemolysin production of oral candida dubliniensis isolates. Medical Principles and Practice, 28(6), pp.552-558.
- De Sagun-Bella, K.Q. and Agahan, A.L.D., 2013. Antifungal Activity of Voriconazole on Local Isolates: an In-vitro Study. Philipp J Ophthalmol, 38, pp.29-34.
- Shojaei, P., Mokhtari, B. and Ghorbanpoor, M., 2019. Synthesis, in vitro antifungal evaluation and docking studies of novel derivatives of imidazoles and benzimidazoles. Medicinal Chemistry Research, 28, pp.1359-1367.
- Fitzgerald, C., Stapleton, P., Phelan, E., Mulhare, P., Carey, B., Hickey, M., Lynch, B. and Doyle, M., 2016. Rapid identification and antimicrobial susceptibility testing of positive blood cultures using MALDI-TOF MS and a modification of the standardised disc diffusion test: a pilot study. Journal of Clinical Pathology, 69(11), pp.1025-1032.
- Clinical and Laboratory Standards Institute, 1999. Methods for determining bactericidal activity of antimicrobial agents; approved guideline. CLSI document M26-A.
- Magaldi, S., Mata-Essayag, S., De Capriles, C.H., Pérez, C., Colella, M.T., Olaizola, C. and Ontiveros, Y., 2004. Well diffusion for antifungal susceptibility testing. International journal of infectious diseases, 8(1), pp.39-45.
- Ortlieb, N., Klenk, E., Kulik, A. and Niedermeyer, T.H.J., 2021. Development of an agar-plug cultivation system for bioactivity assays of actinomycete strain collections. PLoS One, 16(11), p.e0258934.
- Öz, Y., Özdemir, H.G., Gökbolat, E., Kiraz, N., Ilkit, M. and Seyedmousavi, S., 2016. Time-kill kinetics and in vitro antifungal susceptibility of non-fumigatus Aspergillus species isolated from patients with ocular mycoses. Mycopathologia, 181, pp.225-233.
- Finger, S., Wiegand, C., Buschmann, H.J. and Hipler, U.C., 2012. Antimicrobial properties of cyclodextrin–antiseptics-complexes determined by microplate laser nephelometry and ATP bioluminescence assay. International journal of pharmaceutics, 436(1-2), pp.851-856.
- Test, N.B.M., Flow Cytometry Antifungal Susceptibility.
- Natasha McStay, Creina Slator, Vandana Singh, Alex Gibney, Fredrik Westerlund, Andrew Kellett, Click and Cut: a click chemistry approach to developing oxidative DNA damaging agents, Nucleic Acids Research, Volume 49, Issue 18, 11 October 2021, Pages 10289–10308, https://doi.org/10.1093/nar/gkab817
- Li, X., Jin, B., Guo, Z., Chu, S. and Peng, R., 2019. Thermodynamics and Kinetics of Click Reaction between Benzyl Azide and Different Alkynes by Microcalorimetry. Organic Process Research & Development, 24(2), pp.163-171.
- Levandowski BJ, Graham BJ, Houk KN, Raines RT. Click Organocatalysis: Acceleration of Azide-Alkyne Cycloadditions with Mutually Orthogonal Click Reactions. J Org Chem. 2024 Feb 16;89(4):2232-2237. doi: 10.1021/acs.joc.3c02182. Epub 2024 Jan 26. PMID: 38275285; PMCID: PMC10922906.
- Wang, S., Zhang, S., Feng, C., Lu, G., Theato, P. and Huang, X., 2021. Modification of polybutadiene with trifluoromethyl and clickable azide groups in one shot. Polymer Chemistry, 12(39), pp.5589-5597.
- C. Gao, Y.L. Fan, F. Zhao, Q.C. Ren, X. Wu, L. Chang, F. Gao, Quinolone derivatives and their activities against methicillin-resistant Staphylococcus aureus (MRSA), Eur. J. Med. Chem. 157 (2018) 1081e1095.
- Z. Xu, S.J. Zhao, Z.S. Lv, F. Gao, Y.L. Wang, F. Zhang, L.Y. Bai, J.L. Deng, Fluoroquinolone-isatin hybrids and their biological activities, Eur. J. Med. Chem. 162 (2019) 396e406.
- F. Gao, P. Wang, H. Yang, Q. Miao, L. Ma, G.M. Lu, Recent developments of quinolone-based derivatives and their activities against Escherichia coli, Eur. J. Med. Chem. 157 (2018) 1223e1248.
- E. Bonandi, M.S. Christodoulou, G. Fumagalli, D. Perdicchia, G. Rastelli, D. Passarella, The 1,2,3-triazole ring as a bioisostere in medicinal chemistry, Drug Discov. Today 22 (2017) 1572e1581
- H. Bayrak, A. Demirbas, N. Demirbas, S.A. Karaoglu, Synthesis of some new 1, 2, 4-triazoles starting from isonicotinic acid hydrazide and evaluation of their antimicrobial activities. Eur. J. Med. Chem. 44(11), 4362–4366 (2009)
- Boral H., Metin B., Dogen A., Seyedmousavi S., Ilkit M. Overview of selected virulence attributes in Aspergillus fumigants, Candida albicans, Cryptococcus neoformans, Trichophyton rubrum, and Exophiala dermatitidis. Fungal Genet. Biol. 2018;111:92–107. doi: 10.1016/j.fgb.2017.10.008
- Du H., Bing J., Hu T.R., Ennis C.L., Nobile C.J., Huang G.H. Candida auris: Epidemiology, biology, antifungal resistance, and virulence. PLoS Pathog. 2020;16:e1008921. doi: 10.1371/journal.ppat.1008921.
- Fernandez de Ullivarri M, Arbulu S, Garcia-Gutierrez E, Cotter PD. 2020. Antifungal peptides as therapeutic agents. Front Cell Infect Microbiol 10:105. doi: 10.3389/fcimb.2020.00105
- Perfect JR 2017. The antifungal pipeline: a reality check. Nat Rev Drug Discov 16:603–616. doi: 10.1038/nrd.2017.46
- Mazu TK, Bricker BA, Flores-Rozas H, Ablordeppey SY. 2016. The mechanistic targets of antifungal agents: an overview. Mini Rev Med Chem 16:555–578. doi: 10.2174/1389557516666160118112103
- Scheepmaker J.W., Busschers M., Sundh I., Eilenberg J., Butt T.M. Sense and nonsense of the secondary metabolites data requirements in the EU for beneficial microbial control agents. Biol. Control. 2019;136:104005. doi: 10.1016/j.biocontrol.2019.104005.
- Dastjerdi R., Montazer M. A review on the application of inorganic nano-structured materials in the modification of textiles: Focus on anti-microbial properties. Colloids Surf. B. 2010;79:5–18. doi: 10.1016/j.colsurfb.2010.03.029.
- Yang Z.C., Wang B.C., Yang X.S., Wang Q., Ran L. The synergistic activity of antibiotics combined with eight traditional Chinese medicines against two different strains of Staphylococcus aureus. Colloids Surf. B. 2005;41:79–81. doi: 10.1016/j.colsurfb.2004.10.033.
- Parham S., Wicaksono D.H., Bagherbaigi S., Lee S.L., Nur H. Antimicrobial treatment of different metal oxide nanoparticles: A critical review. J. Chin. Chem. Soc. 2016;63:385–393. doi: 10.1002/jccs.201500446.
- Abu-Shanab B., Adwang M., Abu-Safiya D., Jarrar N., Adwan K. Antibacterial activities of some plant extracts utilized in popular medicine in Palestine. Turk. J. Biol. 2005;28:99–102.
- Sharma A., del Carmen Flores-Vallejo R., Cardoso-Taketa A., Villarreal M.L. Antibacterial activities of medicinal plants used in Mexican traditional medicine. J. Ethnopharmacol. 2017;208:264–329. doi: 10.1016/j.jep.2016.04.045.
- Waris K., Saleem S., Arshad M.U., Iqbal J. A novel complementary alternative medicine: An In-Vitro evaluation of efficacy of Nigella sativa extract as an antibacterial agent against Porphyromonas gingivalis. Ann. Punjab. Med. Coll. 2017;11:247–251. doi: 10.29054/APMC/17.411.
- Pourghanbari G., Nili H., Moattari A., Mohammadi A., Iraji A. Antiviral activity of the oseltamivir and Melissa officinalis L. essential oil against avian influenza A virus (H9N2) Virus Dis. 2016;27:170–178. doi: 10.1007/s13337-016-0321-0.
- Shim J.M. The relationship between the use of complementary and alternative medicine and the use of biomedical services: Evidence from East Asian medical systems. Asia Pac. J. Public Health. 2016;28:51–60. doi: 10.1177/1010539515613411
- Abdullahi A.A. Trends and challenges of traditional medicine in Africa. Afr. J. Tradit. Complement. Altern. Med. 2011;8:115–123. doi: 10.4314/ajtcam.v8i5S.5.
- Upton R., Graff A., Jolliffe G., Länger R., Williamson E. American Herbal Pharmacopoeia: Botanical Pharmacognosy-Microscopic Characterization of Botanical Medicines. CRC Press; Boca Raton, FL, USA: 2016
- World Health Organization. WHO Traditional Medicine Strategy 2002–2005. Geneva, Switzerland: World Health Organization; 2002
- Kunwar R. M., Bussmann R. W. Ethnobotany in the Nepal Himalaya. Journal of Ethnobiology and Ethnomedicine. 2008;4, article 24 doi: 10.1186/1746-4269-4-24.
- Zhu, L., Tang, SY., Chen, DP. et al. Synthesis and antibacterial activity of indole 3-substituted-[1,2,4]triazole derivatives. Chem. Pap. 77, 895–907 (2023). https://doi.org/10.1007/s11696-022-02393-9
- Ayati A., Emami S., Foroumadi A. The importance of triazole scaffold in the development of anticonvulsant agents. Eur J Med Chem. 2016;109:380–392
- Briguglio I., Piras S., Corona P., et al. Benzotriazole: an overview on its versatile biological behavior. Eur J Med Chem. 2015;97:612–648
- Abdellatif K.R.A., Bakr R.B. New advances in synthesis and clinical aspects of pyrazolo[3,4-d]pyrimidine scaffolds. Bioorg Chem. 2018;78:341–357
- Herr R.J. 5-Substituted-1H-tetrazoles as carboxylic acid isosteres: medicinal chemistry and synthetic methods. Bioorg Med Chem. 2002;10:3379–3393.
- Al?Akayleh, F., Khalid, R.M., Hawash, D., Al?Kaissi, E., Al?Adham, I.S.I., Al?Muhtaseb, N., Jaber, N., Al?Remawi, M. and Collier, P.J., 2022. Antimicrobial potential of natural deep eutectic solvents. Letters in Applied Microbiology, 75(3), pp.607-615.
- Reddy GKK, Padmavathi AR, Nancharaiah YV. Fungal infections: Pathogenesis, antifungals and alternate treatment approaches. Curr Res Microb Sci. 2022 Apr 27;3:100137. doi: 10.1016/j.crmicr.2022.100137. PMID: 35909631; PMCID: PMC9325902.
- Ruiz-Baca, E., Arredondo-Sánchez, R.I., Corral-Pérez, K., López-Rodríguez, A., Meneses-Morales, I., Ayala-García, V.M. and Martínez-Rocha, A.L., 2021. Molecular mechanisms of resistance to antifungals in Candida albicans. Adv. Candida Albicans, 39, p.5772.
- Colin J Jackson, David C Lamb, Diane E Kelly, Steven L Kelly, Bactericidal and inhibitory effects of azole antifungal compounds on Mycobacterium smegmatis, FEMS Microbiology Letters, Volume 192, Issue 2, November 2000, Pages 159–162, https://doi.org/10.1111/j.1574-6968.2000.tb09375.x
- Hasim S, Coleman JJ. 2019. Targeting the fungal cell wall: current therapies and implications for development of alternative antifungal agents. Future Med Chem 11:869–883. doi: 10.4155/fmc-2018-0465.
- El-Gendy, M., Shaaban, M., Shaaban, K.A., El-Bondkly, A.M. and Laatsch, H., 2008. Essramycin: a first triazolopyrimidine antibiotic isolated from nature. The Journal of antibiotics, 61(3), pp.149-157.
- Sahu, P.K., Sahu, P.K., Sharma, Y. and Agarwal, D.D., 2014. Synthesis and Mechanistic Study of Triheterocyclic 4H?Pyrimido [2, 1?b] benzothiazole derivatives, One?Pot Three?component Reaction under Solvent?Free Conditions. Journal of heterocyclic Chemistry, 51(4), pp.1193-1198.
- Rostovtsev, V.V., Green, L.G., Fokin, V.V. and Sharpless, K.B., 2002. A stepwise huisgen cycloaddition process: copper (I)-catalyzed regioselective “ligation” of azides and terminal alkynes. Angewandte Chemie International Edition, 41(14), pp.2596-2599.
- Liu, X., Zhang, S.B., Zhu, H. and Dong, Z.B., 2018. Copper (I)-catalyzed tandem one-pot synthesis of 2-arylthiobenzothiazoles and 2-arylthiobenzoxazoles in water. The Journal of Organic Chemistry, 83(19), pp.11703-11711.
- Reinus, B. and Kerwin, S.M., 2019. Preparation and utility of N-alkynyl azoles in synthesis. Molecules, 24(3), p.422.
- Rahman, A.M., Ali, R., Jahng, Y. and Kadi, A.A., 2012. A Facile Solvent Free Claisen-Schmidt Reaction: Synthesis of ?, ??-bis-(Substituted-benzylidene) cycloalkanones and ?, ??-bis-(Substituted-alkylidene) cycloalkanones. Molecules, 17(1), pp.571-583.
- Almalki ASA, Nazreen S, Malebari AM, Ali NM, Elhenawy AA, Alghamdi AAA, Ahmad A, Alfaifi SYM, Alsharif MA, Alam MM. Synthesis and Biological Evaluation of 1,2,3-Triazole Tethered Thymol-1,3,4-Oxadiazole Derivatives as Anticancer and Antimicrobial Agents. Pharmaceuticals (Basel). 2021 Aug 28;14(9):866. doi: 10.3390/ph14090866. PMID: 34577567; PMCID: PMC8468421.
- B?benek E, Jastrz?bska M, Kadela-Tomanek M, Chrobak E, Orzechowska B, Zwoli?ska K, Latocha M, Mertas A, Czuba Z, Boryczka S. Novel Triazole Hybrids of Betulin: Synthesis and Biological Activity Profile. Molecules. 2017 Nov 1;22(11):1876. doi: 10.3390/molecules22111876. PMID: 29104263; PMCID: PMC6150379.
- Bellahouel, Salima & Nadia, Kambouche. (2021). Theoretical affinities study of some hypothetical proposed triazoles derivatives with main enzymes responsible on inflammations and fungal infections. Journal of Pharmacy and Pharmacology. 8. 009-024. 10.15413/ajpp.2020.0110.
- Matta, R., Pochampally, J., Dhoddi, B.N. et al. Synthesis, antimicrobial and antioxidant activity of triazole, pyrazole containing thiazole derivatives and molecular docking studies on COVID-19. BMC Chemistry 17, 61 (2023). https://doi.org/10.1186/s13065-023-00965-8
- Fichtali, I., Chraibi, M., Aroussi, F.E., Ben-Tama, A., Hadrami, E.M.E., Benbrahim, K.F. and Stiriba, S.E., 2016. Synthesis of some 1, 2, 3-triazoles derivatives and evaluation of their antimicrobial activity. Der Pharma Chem, 8(5), pp.236-242.
- Alkhaldi, A. A.M., Abdelgawad, M. A., Youssif, B. G.M., El-Gendy, A. O. and De Koning, H. (2019) Synthesis, antimicrobial activities and GAPDH docking of new 1,2,3-triazole derivatives. Tropical Journal of Pharmaceutical Research, 18(5), 1108. (doi: 10.4314/tjpr.v18i5.27)
- Matta R, Pochampally J, Dhoddi BN, Bhookya S, Bitla S, Akkiraju AG. Synthesis, antimicrobial and antioxidant activity of triazole, pyrazole containing thiazole derivatives and molecular docking studies on COVID-19. BMC Chem. 2023 Jun 17;17(1):61. doi: 10.1186/s13065-023-00965-8. PMID: 37330518; PMCID: PMC10276907.
- Pokhodylo N, Shyyka O, Matiychuk V. Synthesis of 1,2,3-Triazole Derivatives and Evaluation of their Anticancer Activity. Sci Pharm. 2013 Apr 14;81(3):663-76. doi: 10.3797/scipharm.1302-04. PMID: 24106665; PMCID: PMC3791931.
- Eswaran S, Adhikari AV, Shetty NS. Synthesis and antimicrobial activities of novel quinoline derivatives carrying 1,2,4-triazole moiety. Eur J Med Chem. 2009 Nov;44(11):4637-47. doi: 10.1016/j.ejmech.2009.06.031. Epub 2009 Jul 3. PMID: 19647905.
- Ellouz M, Sebbar NK, Fichtali I, Ouzidan Y, Mennane Z, Charof R, Mague JT, Urrutigoïty M, Essassi EM. Synthesis and antibacterial activity of new 1,2,3-triazolylmethyl-2H-1,4-benzothiazin-3(4H)-one derivatives. Chem Cent J. 2018 Nov 29;12(1):123. doi: 10.1186/s13065-018-0494-2. PMID: 30499014; PMCID: PMC6768024.
- Patil, Bhimagouda S., Ganganaika Krishnamurthy, Nellisara D. Shashikumar, M. R. Lokesh and Halehatti S. Bhojya Naik. “Synthesis and Antimicrobial Activity of Some [1,2,4]-Triazole Derivatives.” Journal of Chemistry 2013 (2013): 1-7.
- Al-Abdullah, Ebtehal S et al. “Synthesis, antimicrobial, and anti-inflammatory activity, of novel S-substituted and N-substituted 5-(1-adamantyl)-1,2,4-triazole-3-thiols.” Drug design, development and therapy vol. 8 505-18. 12 May. 2014, doi:10.2147/DDDT.S62465
- Saxena, V. and Sharma, C. S. (2021) “Synthesis, antimicrobial activity, drug likeness and in silico toxicity study of some novel Triazole derivatives”, International Journal of Pharmaceutical Sciences and Drug Research, 13(1), pp. 93-98. doi: 10.25004/IJPSDR.2021.130114.
- Anwar, Rebaz & RASH?D, Rzgar & Othman, Khdir. (2023). Exploring The Synthesis of 1,2,4-Triazole Derivatives: A Comprehensive Review. Journal of Physical Chemistry and Functional Materials. 6. 10.54565/jphcfum.1263834.
- Sangshetti, J.N., Lokwani, D.K., Sarkate, A.P. and Shinde, D.B., 2011. Synthesis, Antifungal Activity, and Docking Study of Some New 1, 2, 4?triazole Analogs. Chemical biology & drug design, 78(5), pp.800-809.


 Abhiman S. Jadhav*
Abhiman S. Jadhav*
 Vinayak S. Marulkar
Vinayak S. Marulkar















 10.5281/zenodo.13292951
10.5281/zenodo.13292951