Abstract
The heterocyclic compounds have great importance in medicinal chemistry. One of the most important heterocycles in medicinal chemistry are thiophenes possessing wide spectrum of biological properties like anti-cancer, anti-inflammatory, anti-bacterial, anti-fungal, anti-tumor, anti-malarial, anti-microbial, anti-anxiety and many more activities. This skeleton is an important pharmacophore considered as a privileged structure. This review highlights the recent advances in the synthesis of thiophene derivatives with wide variety of pharmacological activities.
Keywords
Heterocycle, Thiophene, Antimicrobial, Pharmacological activities
Introduction
As the world's population grows at an alarming rate, health issues are becoming a major clinical concern. Because of this it is imperative that scientists develop and find novel medicinal molecules, since they may hold the key to some of the most promising futures. However, pharmacologically active heterocyclic compounds continue to be used in clinical settings on a large scale [1]. Heterocyclic compounds are widely distributed in nature, and have diverse synthetic applications, and biological activity. Heterocyclic compounds have aided medicinal chemists in organizing, planning and implementing creative approaches to the development of new medications [2].
Heterocyclic Compounds
A heterocyclic compound is also known as ring structure is a cyclic compound whose ring contains atoms from at least two different elements [3]. Heterocyclic chemistry is the branch of organic chemistry that deals with the synthesis, properties, and applications of these heterocycles. The most prevalent heteroatoms are nitrogen, oxygen, and sulfur, but heterocyclic rings with additional heteroatoms are also frequently employed [4]. There are huge growing number of heterocyclic compounds currently recognized. All nucleic acids, bulk of pharmaceuticals, biomass (cellulose and related materials), and several synthetic and natural dyes are examples of heterocyclic compounds. More than half of compounds are heterocycles [5].
Classification Of Heterocyclic Compound
The electrical configuration of heterocyclic compounds allows them to divide them into two categories:
1. Aliphatic Heterocyclic Compounds
2. Aromatic Heterocyclic Compounds
Aliphatic Heterocyclic Compounds: Cyclic heterocycles without a double bond are known as aliphatic heterocyclic compounds.vRing strain predominantly affects the characteristics of aliphatic heterocyclic compounds. Tetrahydrofuran(THF), dioxane, Pyrrolidine, Piperidine, Aziridine, Ethylene Oxide, Thiirane, Oxetane, Azetidine, Thietane, etc. are a few examples of aliphatic heterocyclic compounds [6].
Aromatic Heterocyclic Compounds: Aromatic heterocyclic compounds, as the name suggests, are cyclic aromatic compounds. Aromatic Heterocyclic compounds obey Huckell’s Rule, i.e. It should be cyclic. It should be planar. It should not contain any sp3 hybridized atoms. It must have (4n+2) ? electrons. n=number of degenerate orbitals. Aromatic Heterocyclic compounds are analogous to Benzene. Examples: Furan, Pyrrole, Thiophene, Indole, Benzofuran, Carbazole, Quinoline, Iso quinoline, Imidazole, Oxazole, Pyrazole, Pyridazine, Pyrimidine, Purine, etc [7].
Introduction To Thiophene
THIOPHENE was discovered as a contaminant in benzene [8]. When Isatin (1H-indole-2, 3-Dione) is combined with sulfuric acid and crude benzene, it has been found to generate a blue dye. Victor Meyer succeeded in identifying the material that was causing this reaction. The compound was found to be a heterocyclic compound – thiophene [9]. Derivatives of thiophene have long been recognized in medicinal chemistry for their potential therapeutic uses. Simple thiophenes are stable liquids that have a boiling point and even a smell that is quite similar to the comparable benzene compounds [10]. They occur in coal tar distillates. One of the most well-known tales in organic chemistry is the finding of thiophene in coal tar benzene [11].

Figure-1: Structure of Thiophene
Thiophene derivatives provide useful intermediaries in various areas of science & industry, with a wide range of applications, and therapeutic properties [12]. Thiophene derivatives attract both great academic interest, and interest from the agrochemical, pharmaceutical and dye industry, as well. Heterocyclic compounds have historically played an important role in the search for bioactive products [13]. Thiophene has a structure that is analogous to the structure of pyrrole, and due to pie electron cloud, it behaves like an extremely reactive benzene derivative [14].
Properties Of Thiophene
- At room temperature, thiophene is a toxic, flammable and colorless liquid with a mildly pleasant odor reminiscent of benzene.
- The molecular mass of thiophene is 84.1416 g/mole, density is 1.051 g/ml and melting point is -38°C.
- It is insoluble in water but soluble in most of the organic solvents including alcohol and ether [15].
Aromaticity Of Thiophene
- Thiophene have 4C and 1S, all are sp?2; hybrid3ized.
Sp2 hybridization forms a planar thiophene ring structure in a planar manner
- Each ring atom also contains un hybridized p orbital that is perpendicular to the plane of sigma bonds (plane of ring).
- Here p orbitals are parallel to each other, so overlapping between p orbitals is possible.
- The resonance of 6 electrons follows HUCKEL’S rule.
- So, the THIOPHENE is aromatic [16].
Biological Activity Of Thiophene
As far as biological activity is concerned. The thiophene derivatives are known to be associated with broad spectrum of biological activity like antifungal, antibacterial activity. Thiophene can be fused with various heterocyclic system give rise to new heterocyclic system with enhanced biological activity [17]. Many thiophene derivatives have been developed as chemo therapeutic agents are widely used. One of the most significant heterocycles with notable pharmacological properties is the thiophene nucleus. It is also used in the creation of pesticides and metal complexing agents. There are further applications for the higher alkylated thiophenes. For example, 2-hexylthiophene is widely employed as a raw material in patents pertaining to liquid crystals.
Fused hetero-aromatic systems are frequently more interesting than monocyclic molecules in terms of biological activity [18].
The Thiophene containing compounds shows a various therapeutic activity such as:
- Antimicrobial
- Anti-inflammatory
- Anti-cancer
- Anti-psychotic
- Anti-anxiety
- Anti-fungal
- Anti-tumor
- Antimalarial
- Anthelmintic
- Antitubercular
- Anti-oxidant
- Anti-viral
- Anti-HIV
- Anti-arrhythmic
- Anti-bacterial
- Cardiovascular
- Anti-convulsant
- Analgesic
Table-1: List Of Drugs Containing Thiophene Nucleus In Their Structure And Their Uses
|
S.no
|
Drug name
|
structure
|
Brand name
|
Uses
|
|
1.
|
Temocillin
|

|
Negaban
|
Used in investigated infection, liver dysfunction, and UTI
|
|
2.
|
Lipoic acid
|

|
ALA max protects
|
Nutritional supplement,
Used to treat dietary shortage or imbalance
|
|
3.
|
Ketotifen
|

|
Zaditor,
Alaway
|
Used to treat mild atopic asthma, and allergic conjunctivitis
|
|
4.
|
Duloxetine
|

|
Cymbalta
|
Used to treat generalized anxiety disorder, and stress
|
|
5.
|
Clopidogrel
|

|
Pavix
|
Used to prevent cerebrovascular disease
|
|
6.
|
Canagliflozin
|

|
Invokana
|
Used in Type-2 diabetes mellitus
|
|
7.
|
Ticlopidine
|

|
Ticlid
|
Used in the prevention of conditions associated with thrombi, such as stroke and Transient Ischemic Attacks (TIA)
|
|
8.
|
Pizotifen
|

|
Sandonigran
|
A serotonin and tryptamine antagonist indicated for migraine.
|
|
9.
|
Prasugrel
|

|
Effient,
Efient
|
A P2Y12 platelet inhibitor used to reduce risk of thrombotic cardiovascular events in instable angina.
|
|
10.
|
Rivaroxaban
|

|
Xarelto
|
Used to treat deep vein thrombosis (DVT) and pulmonary embolism (PE)
Also used as thrombosis prophylaxis in specific situations
|
|
11.
|
Pyrantel
|

|
Pin-X, Pin-Rid
|
Used to treat helminth infections
|
|
12.
|
Tinoridine
|

|
Clinivex
|
Tinoridine is under investigation in clinical trial NCT01224756 (Efficacy of tinoridine in treating pain and inflammation in adults)
|
|
13.
|
Tienilic acid
|

|
Diflurex
|
Used for the treatment of hypertension
|
|
14.
|
Vaborbactam
|

|
Vabomere
|
Used in trails investigating the treatment of bacterial infections in subjects with varying degree of renal insufficiency.
|
|
15.
|
Raloxifene
|

|
Evista
|
Used to treat post-menopausal osteoporosis and the risk of reduction of invasive breast cancer in post-menopausal women
|
|
16.
|
Sitaxentan
|

|
Thelin
|
Used in pulmonary hypertension, connective tissue diseases, congestive heart failure
|
|
17.
|
Olanzapine
|

|
Oleanz, Olan
|
Used to treat the symptoms of schizophrenia and bipolar disorder
|
|
18.
|
Articaine
|

|
ketocaine
|
Local anesthetic
|
Some Of The Therapeutic Activities Of Thiophene
Anti Inflammatory Activity:
The mechanism of action of anti-inflammatory drugs containing thiophene is to inhibit the activity of cyclooxygenase (COX) and lipoxygenase (LOX) enzymes:
- Cyclooxygenase (COX): An enzyme that the body uses to produce prostaglandins, which are involved in inflammatory events like increased blood flow and vascular permeability.
- Lipoxygenase (LOX): An enzyme that produces leukotrienes, which promote allergic inflammation [19]
Example: Tenidap
Figure-2: Anti-inflammatory Activities
Anti-Cancer Activity:
Anti-cancer drugs containing thiophene can work by binding to cancer-specific proteins, inhibiting signalling pathways, or disrupting microtubules:
- Binding to cancer-specific proteins
Thiophene analogues can bind to oncogenic kinases like FLT3 tyrosine kinase, checkpoint kinase, PI3K, IKK-2.
- Inhibiting signalling pathways
Thiophene analogues can inhibit signalling pathways involved in cancer.
Some thiophene-containing drugs, like ELR510444, can disrupt microtubules to inhibit cell proliferation.
Nocodazole, a thiophene-containing drug, can suppress mitosis by binding to ?-tubulin.
- Inhibiting tubulin polymerization
Benzothiophene derivatives can inhibit tubulin polymerization by binding to the colchicine binding site [20].
Example: Raltitrexed
Figure-3: Anti-cancer activity
ANTI TUMOUR ACTIVITY:
Thiophene-based anti-tumour drugs work in a variety of ways, including:
- Binding to protein targets: Thiophene analogues can bind to cancer-specific proteins.
- Inhibiting signalling pathways: Thiophene analogues can inhibit signalling pathways involved in cancer.
- Targeting oncogenic kinases: Some thiophene-based drugs target oncogenic kinases like FLT3 tyrosine kinase, PI3K, and IKK-2.
- Suppressing mitosis: Some thiophene-based drugs, like Nocodazole, bind to ?-tubulin to suppress mitosis.
- Disrupting microtubules: Some thiophene-based drugs, like ELR510444, disrupt microtubules to inhibit cell proliferation.
- Inhibiting tubulin polymerization: Some thiophene-based drugs, like Benzothiophene derivatives, bind to the colchicine binding site to inhibit tubulin polymerization.
- Inhibiting ribonucleotide reductase: Some thiophene-based drugs, like triapine, inhibit the enzyme ribonucleotide reductase [21].
Example: Thiophen Furin
Figure-4: Anti tumour activity
Local Anesthetic Activity
The mechanism of action of local anaesthetic drugs containing thiophene, like articaine, is to block nerve conduction by binding to sodium channels in the nerve:
Articaine binds to the voltage-gated sodium channels in the nerve, reducing the amount of sodium that can enter the cell. This prevents the threshold potential from being reached, stopping the conduction of impulses.
Articaine's thiophene ring makes it more lipid soluble, which helps it diffuse across the nerve membrane to reach its target receptors.
Ester group
Articaine also contains an ester group, which causes it to be metabolized and excreted in the kidneys after hydrolysis in the plasma [22].
Example: Articaine
Figure-5: Local anaesthetic Activity
Anti Tubercular Activity:
Anti-tubercular drugs containing thiophene may have a few different mechanisms of action, including:
Some thiophene compounds kill Mycobacterium tuberculosis (Mtb) by inhibiting Pks13, which is required for mycolic acid biosynthesis. This mechanism of action is bactericidal and similar to the first-line drug isoniazid.
- Allosterically targeting DNA gyrase
Some thiophene-based compounds bind to a pocket on DNA gyrase that is remote from Domain A. This binding site is potentially druggable and may reduce the likelihood of cross-resistance to fluoroquinolones.
Some thiophene compounds may inhibit DprE1 to exert antimycobacterial activity [23].
Example: TCA1
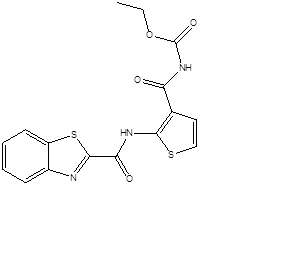
Figure-6: Anti tubercular activity
Anti-Malarial Activity:
The mechanism of action of anti-malarial drugs containing thiophene is
- Inhibition of lipid synthesis: MMV-0206 disrupts parasite membrane function.
- Inhibition of pyrimidine synthesis: DSM-1 inhibits pDHODH, disrupting DNA synthesis.
- Mitochondrial electron transport inhibition: Thiostrepton disrupts energy production.
- Cysteine protease inhibition: K77 disrupts haemoglobin degradation.
- Phospholipid synthesis inhibition: SJ000030570 disrupts membrane function.
Anti-malarial Drugs
1. MMV-0206: Inhibits plasmodial lipid synthesis, disrupting parasite membrane function.
2. DSM-1: Inhibits plasmodial dihydroorotate dehydrogenase (pDHODH), disrupting pyrimidine synthesis.
3. Thiostrepton: Inhibits plasmodial mitochondrial electron transport, disrupting energy production.
4. K77: Inhibits plasmodial cysteine proteases, disrupting haemoglobin degradation.
5. SJ000030570: Inhibits plasmodial phospholipid synthesis, disrupting membrane function [24].
Example: Benzothiophene
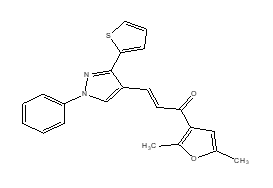
Figure-7: Anti-malarial activity
ANTI PSYCHOTIC ACTIVITY:
The mechanism of anti-psychotic drugs containing thiophene shows mechanism such as inhibiting the enzymes cyclooxygenase (COX) and lipoxygenase (LOX).
- Thiothixene: Blocks dopamine, serotonin, histamine, alpha1/alpha2, and muscarinic receptors
- First-generation antipsychotics: Blocks dopamine, serotonin, histamine, alpha1/alpha2, and muscarinic receptors
- Second-generation antipsychotics: Blocks D2 dopamine receptors and acts as a serotonin receptor antagonist
- Atypical antipsychotics: Enhances 5-HT, norepinephrine, and/or dopamine transmission [25].
Example: Olanzapine

Figure-8: Anti-psychotic activity
CONCLUSION
In this review I have described the recent advances in pharmacological diversification of thiophene derivatives. Some of the pharmacological activities that has described in this review are anti-cancer, anti-malarial, anti-inflammatory, anti-psychotic, local anesthetic, anti-tumor and anti-tubercular activities.
REFERENCES
- Nenajdenko VG, Tkachenko SS, Balenkova ES. Pharmacologically active heterocyclic compounds: Synthesis and biological evaluation. J Pharm Pharmacol 2020; 72(8): 1081-1095.
- Nenajdenko VG, Tkachenko SS, Balenkova ES. Synthetic applications of heterocyclic compounds. J Heterocycle Chem 2020; 57(5): 1315-1325.
- Katritzky AR, Ramsden CA. Heterocyclic chemistry: An overview. J Heterocycle Chem 2022; 59(3): 531-542.
- Nenajdenko VG, Tkachenko SS, Balenkova ES. Heterocyclic compounds: Synthesis, properties, and applications. Eur J Org Chem 2018; 2018(25): 3331-3345.
- Grimmett MR. Heterocyclic chemistry: Recent advances. J Chem Soc, Perkin Trans 1 2020; 46: 5123-5135.
- Eicher T, Hauptmann S. The Chemistry of Heterocycles: Aliphatic and Aromatic Compounds. Adv Heterocycle Chem 2017; 113: 1-15.
- Albini A, Pietra S. Aromatic Heterocyclic Compounds: Properties and Applications. J Heterocycle Chem 2015; 52(5): 1231-1245.
- Nenajdenko VG, et al. Thiophene-based materials: Synthesis, properties, and applications. Eur J Org Chem 2017; 2017(25): 3331-3345.
- Aly A, El-Sayed E, Abd-El-Aziz A. Thiophene-containing polymers: A review. J Polym Sci 2022; 60(10): 1511-1533.
- Joule JA, Mills K. Thiophene and its derivatives: Synthesis and applications. Chem Rev 2018; 118(15): 7211-7235.
- Grimmett MR. Thiophene and its derivatives: Recent advances. J Chem Soc, Perkin Trans 1 2019; 53(10): 2411-2422.
- Nenajdenko VG, et al. Thiophene-based materials: Synthesis, properties, and applications. Eur J Org Chem 2017; 2017(25): 3331-3345.
- Katritzky AR, et al. Heterocyclic compounds: Thiophene and its derivatives. J Heterocycle Chem 2020; 57(5): 1315-1325.
- El-Sayed E, et al. Thiophene derivatives: Synthesis, applications, and therapeutic properties. Eur J Med Chem 2020; 200: 112456.
- Aly A, El-Sayed E, Abd-El-Aziz A. Thiophene-containing polymers: A review. J Polym Sci 2022; 60(10): 1511-1533.
- Deepa S, et al. Aromaticity in heterocyclic compounds: A review. J Heterocycle Chem 2020; 57(3): 631-645.
- Joule JA, Mills K. Thiophene and its derivatives: Synthesis, properties, and biological applications. Chem Rev 2018; 118(15): 7211-7235.
- Nenajdenko VG, et al. Thiophene-based heterocycles: Synthesis, properties, and biological applications. Chem Rev 2018; 118(15): 7236-7264.
- Wang Y, Zhang W, Lv X, et al. Design, synthesis, and biological evaluation of thiophene-based COX-2 inhibitors. Eur J Med Chem 2020; 187: 112036. doi: 10.1016/j.ejmech.2020.112036. [PMID: 32065943].
- Kumar et al. Thiophene-containing PI3K inhibitors: design, synthesis, and anti-cancer activity. Bioorg Med Chem Lett 2021; 31: 127947. doi: 10.1016/j.bmcl.2021.127947. [PMID: 33706212]
- Lee et al. Benzothiophene derivatives inhibit tubulin polymerization and exhibit anti-tumor activity. Biochem Pharmocol 2018; 151: 279-289. doi: 10.1016/j.bcp.2018.02.005. [PMID: 29454247]
- Santos et al. Pharmacological and pharmacokinetic profile of Articaine: a review. J Clin Pharm Ther 2022; 47(3): 539-548. doi: 10.1111/jcpt.13585. [PMID: 35076141]
- Chakraborty et al. Structure-based design of thiophene-based inhibitors of DprE1: a potential target for anti-tubercular therapy. Bioorg Med Chem Lett 2019; 29(14): 1658-1665. doi: 10.1016/j.bmcl.2019.04.046. [PMID: 31054555]
- Kumar et al. Design, synthesis, and biological evaluation of Benzothiophene-based anti-malarial agents. Eur J Med Chem 2022; 231: 114294. doi: 10.1016/j.ejmech.2022.114294. [PMID: 35030585]
- Liu et al. Thiophene-based second-generation antipsychotics: design, synthesis, and biological evaluation. Bioorg Med Chem Lett 2020; 30(15): 127095. doi: 10.1016/j.bmcl.2020.127095. [PMID: 32563855]


 Syed Afreen*
Syed Afreen*
 K. Chandra Sekhar
K. Chandra Sekhar




 10.5281/zenodo.14253213
10.5281/zenodo.14253213