Abstract
Controlled and sustained drug delivery has become a novelty in recent years Modern drug design standards and in-depth research Steps have been taken to achieve significantly better drug efficacy. Reliability and security. In situ gel systems have become one of the most innovative systems in recent years. New drug delivery systems have Advantages such as prolonged drug effect and patient improvement Comparing compliance and reducing dosing frequency Traditional drug delivery systems (DDS). In this system This preparation is in the form of a solution and after contact with It condenses into a gel in body fluids. Gel formation Depends on factors such as temperature changes, pH changes, and presence of ions and ultraviolet radiation. Various biodegradable polymers, such as gellan gum Use gum, pectin, alginic acid, chitosan, xyloglucan, etc. In situ gel Administered in various ways such as B. Oral, ocular, nasal, rectal, Vaginally, parenterally, and intraperitoneally. The production of such equipment is It is also less complex, thus reducing investment and manufacturing costs.
Keywords
NDDS, Gelling systems, Gelation, in-situ gel, HPMC, PLGA, TDDS.
Introduction
The primary goal of any drug delivery system is to modulate pharmacokinetics and/or tissue Distribute medications in a favourable manner. Growing demand for more Patient friendly dosage form. Therefore, the demand for its technology has increased. Pharmaceutical companies suffer as development costs for new chemical devices are very high Focus is on developing new drug delivery systems for existing drugs to improve efficacy Efficacy and bioavailability and reduced dosing frequency to minimize side effects. (1) The development of controlled and managed systems has received increasing attention in recent decades Extensively researched sustained drug delivery system Development of polymeric drug delivery systems. Development of in situ gel systems Has received great attention in recent years. In situ is a Latin word meaning “in him.” “Original position” or “in place.” In situ gel drug delivery system capable of releasing drugs in a sustainable manner while maintaining a relatively constant plasma distribution. Last few years, More and more in situ gel systems are being studied and a large number of patents have been filed. Registered for use in various biomedical applications including drug delivery Reported. This interest was prompted by the potential benefits of in situ Forming a polymer delivery system such as a simple manufacturing process and easy operation Management, reduce the frequency of management, improve patient compliance and Convenient compared to traditional dosage forms. It also facilitates the delivery of accurate doses It also increases the time the drug remains at the site of administration. In situ gel formation Occurs due to one or a combination of different stimuli, such as changes in pH, temperature, or solvents Exchange, ion cross-linking, ionization, UV irradiation. Research has been conducted by various routes such as oral, ocular, nasal, rectal, vaginal, injection, parenteral and Intra-abdominal. (2) Intelligent polymeric systems demonstrate effective drug delivery capabilities. These polymers undergo a sol-gel transition upon administration. Research on natural and synthetic polymers for controlled release formulations began in the early 1970s. The benefits of utilizing biodegradable polymers in clinical settings are evident. A variety of natural and synthetic polymers are employed in the development of in situ gel-forming drug delivery systems. (3) The gelation process of gellan gum takes place in situ as a result of temperature variations or the presence of certain cations. The combination of temperature and the ionic condition, specifically the presence of Ca2+ ions, in tear fluid triggers the transformation of the gellan gum solution from a liquid state to a gel state (4). Carbopol, also known as poly acrylic acid, is a widely recognized polymer that exhibits pH dependence. It remains in a soluble state under acidic conditions but transforms into a low viscosity gel when exposed to alkaline pH. In order to develop an in-situ gel for delivering ofloxacin to the eyes, a combination of carbopol and hydroxypropyl methylcellulose (HPMC) was formulated. HPMC was utilized to enhance the viscosity of the Carbopol solution while simultaneously reducing its acidity. (5) However, a plasmid DNA delivery system was created and formulated using an aqueous solution of Carbopol-H PMC (6). Pluronic F-127, a nonionic triblock copolymer, forms a gel in situ upon temperature change. It was combined with Carbopol 934 and HPMC to create a vaginal in situ gel containing clotrimazole-?-cyclodextrin complex, aiming to prolong the drug’s presence at the site of application. (7) Chitosan solution in water creates a gel when the pH is above 6.2. By introducing polyol salts with a single anionic head, such as glucose phosphate salts, into the chitosan solution, the cationic polysaccharides solution can be converted into a gel that is sensitive to both temperature and pH. The liquid form of this mixture solidifies into gel implants when injected into a living organism. This method has been effectively utilized in tissue engineering projects. (8)
RECENT PROGRESS IN SITU GEL POLYMER IN DRUG DELIVERY SYSTEMS.
The Pharmaceutical industry is currently working on developing a range of effective treatments, but the formulation of drug delivery systems poses a significant challenge. Among the most complex systems is the one designed for delivering medication to the eyes. These systems are utilized to enhance treatment outcomes and improve the release of medication from the formulation. One particularly challenging ocular medication delivery system is the In-Situ gel formulation. Recent advancements have led to the creation of labile macromolecular therapeutic medicines, which require intricate preparation for effective administration. By combining the hydrophilic solvent N-stearoyl L-alanine (m) ethyl ester, an injectable, in situ forming organogel can be produced. When loaded with Leuprolide, the organogel gradually decomposes and releases the medication for a period of 14 to 25 days after subcutaneous injection. In situ gelling drug delivery systems can be developed using various mechanisms and techniques that are already established at the application site. These systems allow for the creation of gels based on the triggers involved in the phase transitions from Sol-to-Gel phase.
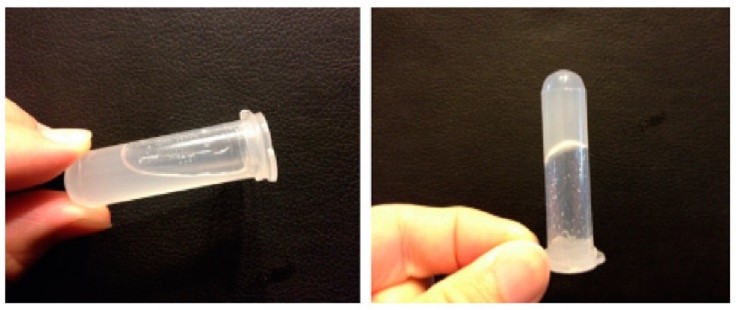
Fig 1. The formation of in situ gel under both room temperature and body temperature conditions.
Importance of in situ gelling system
- The “Sol-Gel” transition of an in-situ gel facilitates the controlled and sustained release of drugs.
- Minimal drug doses are necessary, leading to decreased frequency of drug intake and minimizing the risk of drug accumulation and adverse reactions.
- Enhances the absorption of drugs, resulting in improved bioavailability.
- Gel formation prolongs the presence of the drug in the body, extending its residence time(9)
Advantages
- Ensure a controlled and prolonged release of the medication
- Simplify the process of administering the medication
- Suitable for use on patients who are unconscious
- Enhance patient adherence and comfort
- Reduce the frequency of dosing and the risk of drug toxicity
- Improve the absorption of the drug
- Offer compatibility with the body and breakdown naturally due to the inclusion of natural polymers
- Natural polymers possess unique qualities such as compatibility with the body, natural breakdown, and components that support cellular functions
- Synthetic polymers typically have precise structures that can be adjusted to achieve acceptable breakdown and functionality
- In situ gels can be designed to adhere to biological tissues, aiding in drug delivery, particularly through mucous membranes, without the need for invasive procedures
- In situ gels possess a valuable stealth property in the body, thanks to their water-loving nature, which extends the time the drug delivery system remains in circulation by avoiding immune responses and reducing the activity of phagocytes. (10)
Disadvantages of in situ gel system
- A significant amount of fluids is necessary.
- The drug in sol form is more prone to degradation.
- There is a possibility of stability issues caused by chemical degradation.
- Consumption of food and beverages may be limited for a few hours after taking the medication.
- The amount and uniformity of drug loading into hydrogels may be restricted, especially for hydrophobic drugs.
- Only drugs with low dosage requirements can be administered.
- Reduced mechanical strength may lead to early dissolution or displacement of the hydrogel from the intended local area. (11
Ideal characteristics of polymers for preparation of in situ gel
-
- The polymer must possess the ability to adhere to the mucous membrane.
- It should exhibit excellent compatibility and should not cause any toxic effects.
- The polymer should demonstrate pseudo plastic behavior.
- It should be able to reduce viscosity as the shear rate increases.
- The desired characteristic of the polymer is pseudo plastic behavior.
- A higher preference is given to the polymer that exhibits good tolerance and optical clarity. (12)
Approaches
There are 4 mechanisms for triggering the in-situ Gelling formation of biomaterials. These Include:
- In situ gel formation is triggered by physiological stimuli.
- Temperature triggered in situ gel systems
- pH triggered in situ gelling systems
- The formation of a gel in situ is a result of the ion activated system
- In situ gel formation due to physical Mechanism
- Swelling
- Diffusion
4. Chemical reactions lead to the formation of in situ gel.
- Ionic cross-linking
- Enzymatically cross linking
- Photo-polymerization
- In situ gel formation is triggered by physiological stimuli:
Certain polymers exhibit significant and unforeseen alterations in both physical and chemical properties when exposed to minor changes in their surrounding environmental factors. These polymers are referred to as stimuli-responsive polymers, also known as stimuli-sensitive, intelligent, smart, or environmentally sensitive polymers. They have the ability to perceive a stimulus as a cue, assess its intensity, and subsequently modify their chain conformation accordingly. (13)
A. Temperature induced in-situ gelling system:
Temperature-induced systems are extensively utilized in in-situ gelling formulations. These systems do not necessitate any external heat apart from the body temperature to induce gelation. There exist three distinct types of temperature-induced systems, a few of which are mentioned here.
- Negatively thermo sensitive type Eg: poly(N-isopropylacrylamide)
- Positively thermo sensitive type Eg: Polyacrylic acid
- Thermally reversible type8 Eg: Poloxamer, Pluronics, Tetronics
Temperature responsive polymers, also known as thermo responsive polymers, are utilized in temperature induced gelling systems. These polymers undergo a significant and abrupt alteration in their physical properties when exposed to temperature changes. They fall under the category of stimuli responsive materials, which means that their properties continuously adapt to environmental conditions. At high or low temperatures, these polymers exhibit a miscibility gap and possess an upper or lower critical solution temperature. The upper critical solution temperature range for these polymers is 0-100?C. In this particular approach, the solution remains in a liquid state at room temperature. However, when it comes into contact with body fluid and is exposed to body temperature, it undergoes a transformation into a gel. Since the body cannot maintain the upper critical solution temperature, polymers with a lower critical solution temperature are employed. These polymers undergo polymer-polymer interactions, resulting in a sudden change in polymer solubility. As the solution is in liquid form, the hydrogen bonding between the polymer and water causes an abrupt change, ultimately leading to the formation of a gel. (14)
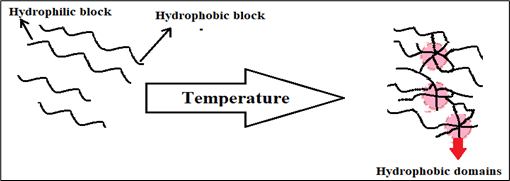
Fig 2: Mechanism involved in temperature triggered system.
B. pH triggered systems:
Gel formation is induced by a change in pH within this system. pH responsive or pH sensitive polymers are utilized in this approach. These polymers contain ionisable functional groups, either acidic or alkaline, known as polyelectrolytes. The presence of polyelectrolytes in the formulation causes an increase in the external pH, resulting in the swelling of the hydrogel and the subsequent formation of an in-situ gel. Polymers with anionic groups are particularly suitable for pH triggered systems. Examples of such polymers include cellulose acetate phthalate (CAP), Carbomer and its derivatives, Polyethylene glycol (PEG), Pseudo latexes, and poly methacrylic acid (PMC), among others. (15)

Fig 3: Mechanism of pH triggered in situ gel system.
- In situ gel formation due to ion-activated System
The induction of gelation in the instilled solution is caused by a change in ionic strength. It is widely believed that the rate of Gel formation is dictated by the osmotic gradient across. the surface of the gel. In the presence of mono and divalent cations commonly found in tear fluids, the aqueous polymer solution forms a transparent gel. The electrolytes present in tear fluid, particularly Na+, Ca2+, and Mg2+ cations, play a crucial role in initiating gelation when the solution is applied to the conjunctival cul-de-sac. Polymers such as gelrite or Gellan gum, hyaluronic acid, and alginates are examples of substances that undergo osmotically induced gelation. (16)
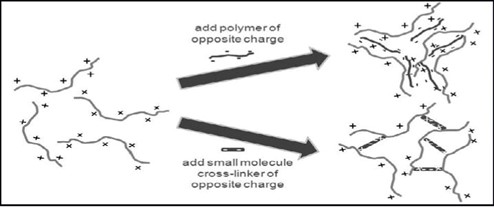
Fig 4: Ionic cross-linking system
3. In situ gel formation due to physical Mechanism
-
- Diffusion
- swelling
- Diffusion
is a physical method employed in in-situ gel formulations. This technique entails the gradual release and dispersion of solvent from a polymer solution into the surrounding tissue, leading to the precipitation or coagulation of the polymer matrix. (17)
- Swelling
In-situ development may also transpire when a substance assimilates moisture from its encompassing surroundings and enlarges to occupy the intended area. Through this technique, the polymer assimilates external fluids found in the outer environment and expands to gradually release the medication (18)
- In situ gel formation due to chemical reactions
Chemical reactions that lead to in-situ gelation can involve enzymatic mechanisms, the formation of inorganic solids from highly concentrated ionic solutions, and the initiation of the process through light exposure.
Ionic crosslinking
The phenomenon where polymers undergo a phase transition upon exposure to various ions, including Na+, K+, Ca+, and Mg+. Certain polysaccharides, such as k-carrageenan and iota-carrageenan, exhibit ion sensitivity. When k-carrageenan comes into contact with small amounts of K+, it forms a rigid and fragile gel. On the other hand, iota-carrageenan primarily forms flexible gels in the presence of Ca2+. Gellan gum, an anionic polysaccharide, undergoes in-situ gelation when exposed1. Monovalent and divalent cations such as calcium (Ca2+), magnesium (Mg2+), potassium (K+), and sodium (Na+). to m. The gelation of low methoxy pectin is induced by divalent cations, particularly Ca2+. Similarly, alginic acid gels when it interacts with divalent/polyvalent cations such as Ca2+, due to the interaction between the alginate chain and the glucuronic acid block. (19)
Enzymatic cross-linking
The process involves the formation of a gel using enzymes present in bodily fluids. Although the in-situ formation catalyzed by natural enzymes has not been extensively studied, it seems to have several advantages compared to chemical and photochemical methods. One significant advantage is that enzymatic processes can effectively function under physiological conditions without the use of potentially harmful chemicals like monomers or initiators. Currently, researchers are exploring the development of intelligent stimulus-responsive delivery systems that utilize hydrogels capable of releasing insulin. These systems employ cationic pH-sensitive polymers containing immobilized insulin and glucose oxidase. As blood sugar levels fluctuate, these polymers can expand and release insulin in a pulsatile manner. Moreover, the amount of enzyme can be adjusted to regulate the rate of gel formation, enabling the mixture to be injected before the gel solidifies. (20)
POLYMERS USED AS IN SITU GELLING AGENTS
Gellan gum
Gellan gum, an anionic polysaccharide, is synthesized by the microorganism Sphingomonas elodea. It is comprised of glucose, rhamnose, and glucuronic acid, which combine to create a tetrasaccharide unit. Gelrite is a deacetylated variant of gellan gum, obtained by subjecting it to alkaline treatment to eliminate the acetyl group from the molecule. The presence of calcium ions in gelrite gives it a gel-like consistency. Gelation occurs through the formation of double-helical crossings, followed by the aggregation of double helical segments into three-dimensional networks through complexation with cations and hydrogen bonding water. In the food industry, gellan gum is employed as a suspension and stabilizing agent.
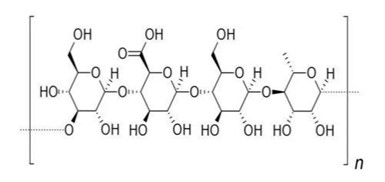
Fig 5: Structure of Gellan gum
Alginic acid
Alginate is a linear block copolymer polysaccharide that consists of ?-D-mannuronic acid and ?-L-glucuronic acid residues connected by 1,4-glycosidic bonds. The arrangement of these blocks on each chain varies depending on the source of algae. When divalent and trivalent metal ions are introduced through a suitable method, the aqueous alginate solutions undergo crosslinking, resulting in the formation of strong gels. Alginic acid, which is commonly used as an ophthalmic flat vehicle, possesses excellent properties such as biodegradability and non-toxicity
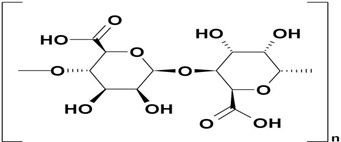
Fig 6: Structure of Alginic Acid
Pectins
Pectins are a type of polysaccharide characterized by a polymer that is predominantly composed of galacturonic ?— (1-4) --- D- corrosive accumulation. When exposed to free carbon particles, gels can easily form in aqueous solutions with low methoxy gelatins (esterification level < 50>
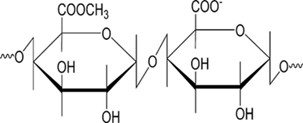
Fig 7: Structure of Pectins
Xyloglucan
Xyloglucan is derived from tamarind seeds and is composed of a (1?4)-?-D-glucan backbone with (1?6)-?-D-xylose branches that are partially substituted by (1?2)-?-D-galactoxylose. When partially degraded by ?-galactosidase, xyloglucan exhibits thermally reversible gelation through the lateral stacking of rod-like chains. These gels form at body temperature and have demonstrated potential uses in oral, intraperitoneal, ocular, and rectal drug delivery (22)
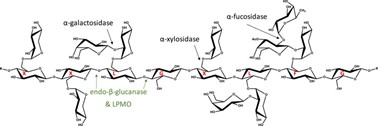
Fig 8: Structure of Xyloglucan
Chitosan
Chitosan, a polycationic polymer, is derived from chitin found in the shells of shrimp and crabs through alkaline deacetylation. It is biodegradable and thermosensitive. In aqueous solutions, chitosan remains dissolved until the pH reaches 6.2 However, when the pH is raised above 6.2, a gel-like precipitate is formed. Incorporating polyol salts containing a sole anionic head, like glycerol, sorbitol, fructose, or glucose phosphate salts, into a chitosan aqueous solution, the pH gelling cationic polysaccharides solution The text can undergo a conversion process to become thermally responsive, pH-responsive gel-forming aqueous solutions, without the need for any chemical alteration or cross-linking.
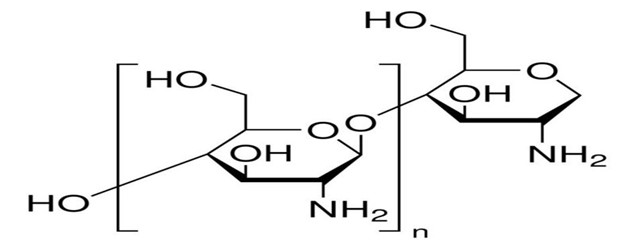
Fig 9: Structure Of Chitosan
Carbopol:
Carbopol is a widely recognized polymer that is pH dependent. It remains in a solution state when the pH is acidic, but transforms into a low viscosity gel when the pH becomes alkaline. To enhance the viscosity of the Carbopol solution and reduce its acidity, HPMC is used in conjunction with Carbopol. These polymeric systems fall under the category of pH-induced in-situ precipitating systems. Building upon this concept, the formulation and assessment of an ophthalmic delivery system for indomethacin, aimed at treating Uveitis, was conducted. Ismail et al. Designed and developed a pH-induced in-situ precipitating polymeric system, specifically an aqueous solution of Carbopol-HPMC, for the delivery of plasmid DNA. (23)
Fig 10: Structure Of Carbopol
Methods Of preparation
-
-
- Solution polymerization or cross linking
In this approach, multifunctional crosslinking agents are combined with ionic or neutral monomers. Polymerization can be initiated through thermal means, UV light, or a redox initiator system. The presence of a solvent helps in minimizing temperature control issues and acts as a heat sink. The resulting hydrogels need to be washed with distilled water to eliminate any unreacted materials, crosslinking agents, and initiators. An excellent illustration of this technique is the production of poly (2-hydroxyethyl methacrylate) hydrogels from hydroxyethyl methacrylate, utilizing ethylene glycol dimethacrylate as the crosslinking agent.
-
-
- Suspension polymerization
This technique is commonly employed to produce spherical hydrogel microparticles ranging in size from 1µm to 1mm. The monomer solution is dispersed in a non-solvent to create fine droplets, which are then stabilized by the addition of a stabilizer. Polymerization is initiated through the thermal decomposition of free radicals, resulting in the formation of the microparticles. Additional washing is necessary to eliminate any unreacted monomers, cross-linking agents, and initiators. Poly (vinyl alcohol) and hydroxyethyl methacrylate hydrogel microparticles were synthesized using the suspension polymerization method.
-
-
- Polymerization by irradiation
High-energy radiations, such as gamma and electron beams, are utilized in the preparation of hydrogels made from unsaturated compounds. When an aqueous polymer solution is irradiated, radicals are formed on the polymer chains, leading to the creation of microradicals. The recombination of these microradicals on different chains results in the formation of covalent bonds, ultimately leading to the development of a cross-linked structure. To prevent interaction with oxygen during radiation, the process is carried out in an inert atmosphere using nitrogen or argon gas. Examples of polymers that can be synthesized using this method include poly (vinyl alcohol), poly (ethylene glycol), and poly (acrylic acid).
D. Chemically crossed linked hydrogels
Polymers that possess functional groups such as -OH, -COOH, and -NH2 exhibit solubility in water. The presence of these functional groups on the polymer chain allows for the formation of hydrogels through the establishment of covalent linkages between polymer chains and complementary reactive groups. Examples of such reactive groups include amine-carboxylic acid, isocyanate -OH or -NH2, and Schiff’s base formation. To prepare hydrogels, glutaraldehyde can be employed as a cross-linking agent for polymers containing -OH groups like poly(vinyl alcohol) and polymers containing amine groups such as albumin, gelatin, and polysaccharides. This cross-linking agent interacts with the functional groups present on the polymer through addition reactions. However, it is crucial to extract any unreacted cross-linking agents due to their high toxicity. Additionally, the reaction must be conducted in organic solvents as water can react with the cross-linking agents. (24)
APPLICATION OF IN SITU POLYMERIC
DRUG DELIVERY SYSTEM
Ocular- Delivery
Natural polymers like gellan gum, alginic acid, and xyloglucan are frequently utilized in in situ gels for ocular delivery. Local ophthalmic drug delivery is employed for a variety of compounds including antimicrobial agents, anti-inflammatory agents, and autonomic drugs. Drugs utilized to alleviate intraocular tension in glaucoma. Traditional delivery methods frequently lead to low bioavailability and therapeutic efficacy due to the rapid elimination of the drug from the eye caused by high tear fluids and dynamics. In order to address bioavailability issues, ophthalmic in situ gels were created. Much attention in the pharmaceutical field has been focused on the use of gellan gum for ophthalmic drug delivery. The drug release from these in situ gels is extended because of the longer precorneal contact times of the viscous gels compared to conventional eye drops. Miyazaki et al. Experimented with formulating in situ gels for ocular delivery using Xyloglucan (1.5% w/w) as the natural polymer. These in situ forming polymeric systems were found to induce a significant mitotic response for a period of 4 hours when administered into the lower cul-de-sac of a rabbit’s eye. The development and assessment of an ophthalmic delivery system for indomethacin for the treatment of uveitis were conducted. A sustained release of indomethacin was observed for a period of 8 hours in-vitro, indicating that this system, in combination with the water-soluble Carbopol system, is a promising candidate.
An in-situ gel system for nasal delivery
Mometasone furoate was developed and assessed for its efficacy in treating allergic rhinitis. Gellan gum and xanthan gum were utilized as polymers to form an in-situ gel. Animal studies were conducted using an allergic rhinitis model to observe the effect of the in-situ gel on antigen-induced nasal symptoms in sensitized rats. The in-situ gel was found to effectively inhibit the increase in nasal symptoms compared to the marketed formulation, Nasonex (mometasone furoate suspension 0.05%). Histopathology of the rat nasal cavity revealed intact ciliated respiratory epithelium and normal goblet cell appearance, indicating the safety of these formulations for nasal administration. Wu et al. Developed a new thermosensitive hydrogel by simply mixing N-[(2-hydroxy-3-Methyltrimethylammonium) propyl] chitosan chloride and poly (ethylene glycol) with a small amount of ?-?-glycerophosphate for nasal delivery of insulin. The formulation was in solution form at room temperature and transformed into a gel form when kept at 37°C. Animal experiments demonstrated the effectiveness of the formulation. Hydrogel formulation is designed to reduce the blood-glucose concentration by 40-50% of the initial values for a duration of 4-5 hours after administration, without causing any observable harm to cells. Consequently, these particular systems prove to be highly appropriate for delivering protein and peptide drugs via the nasal route. (25)
Rectal and vaginal drug delivery system
Various drugs can be administered through the rectal route in different forms such as liquid, semisolid (ointments, creams, and foams), and solid dosage forms (suppositories). Acetaminophen, an anti-inflammatory drug, can be formulated as a rectal in situ gel using synthetic polymers like Polycarbophil, poloxamer F188, and poloxamer 407. This in situ gelling liquid suppository, which is considered an effective method, demonstrates enhanced bioavailability.
Injectable drug delivery system
The drug delivery system includes in situ gels formulated over the past decade due to their benefits, such as not requiring surgical procedures and ensuring patient compliance. Injectable in situ gels are primarily composed of synthetic polymers and block copolymers. An example of an anti-inflammatory drug, Bupivacaine, is formulated as an injectable in situ gel using poly(D, L-lactide), poly(D,L-lactide coglycolide), and PLGA as polymers, demonstrating prolonged drug action under gel conditions.(26)
Oral drug delivery system
Pectin, Xyloglucan, and gellan gum natural polymers are utilized for the oral in situ gel delivery system. A pectin formulation has been reported for the sustained delivery of paracetamol. One advantage of pectin is its water solubility, eliminating the need for organic solvents. Cross-linked dextran hydrogels exhibit faster swelling under high pH conditions, similar to other polysaccharides like amide pectins, guar gum, and insulin. These were investigated to develop a potential colon-specific drug delivery system. W. Kubo et al. developed formulations of gellan and sodium alginate, both containing complexed calcium ions that undergo gelation upon release of these ions in the acidic environment of the stomach. The oral delivery of paracetamol was studied. Hydrogels made of varying proportions of PAA derivatives and crosslinked PEG allowed for the preparation of silicone microspheres. These microspheres released prednisolone in the gastric medium or exhibited gastroprotective properties. (27)
EVALUATION AND CHARACTERIZATION:
Sol to gel Transition Temperature and Gelling Time:
The determination of the sol to gel transition temperature and pH is essential for in situ gel forming systems. The gelling time refers to the duration required for the initial detection of gelation in the in-situ gelling system. It is crucial to assess the thermosensitive in situ gel for its ability to undergo in situ gelling at body temperature.
Clarity:
The clarity of formulated solutions can be determined by visual inspection against black and White background.
Texture Analysis:
The texture analyzer is utilized to evaluate the firmness, consistency, and cohesiveness of the formulation. This assessment primarily determines the syringeability of the solution, ensuring that the formulation can be administered effortlessly in-vivo. In order to establish a close and lasting connection with surfaces such as tissues, gels require higher levels of adhesiveness. (28)
Spreading coefficient
The apparatus is composed of a ground glass slide affixed to the wooden block. Each sample, weighing approximately 2 grams, was positioned and examined on this ground slide. Subsequently, gel preparation was inserted between this slide and a second slide of identical dimensions to the fixed glass slide. The second slide was equipped with a 1-gram hook weight placed on top of the two slides for 5 minutes to eliminate air and establish a consistent gel film between them. A calibrated weight was placed on a pan connected to the pulley using a hook. The time taken for the upper slide to detach from the ground slide was recorded. A shorter duration indicates a better spreading coefficient (S)
Gelling capacity
A rheometer is utilized to assess the gel strength, which relies on the gel-forming capacity of the specific polymer. The standard process includes creating a designated quantity of gel in a container. The container with the gel is then lifted at a set speed, and a probe is carefully inserted through the gel. The force on the probe is determined by the penetration depth of the gel surface.
Viscosity and Rheology
The Brookfield viscometer is used to assess the viscosity of the in-situ gel, both at room temperature and body temperature. Once the gel is formed, the formulated system should exhibit both pseudo-plastic and Newtonian flow. The viscosity value range during the solution stage should fall between 5-1000 m Pas, and after gelation, it should remain within the same range of 5-1000 m Pas. (29)
Fourier transform infra-red spectroscopy and Thermal analysis
This technique allows for the evaluation of the nature of interacting forces during the gelation process. The potassium bromide pellet method is employed to achieve this. In order to quantitate the percentage of water in hydrogel, thermo-gravimetric analysis can be conducted for in situ forming polymeric systems. Additionally, differential scanning calorimetry is utilized to observe any changes in thermograms, indicating the interactions when compared to the pure ingredients used.
In-vitro drug release studies
In order to administer in situ gel formulations orally, ocularly, or rectally, drug release studies are conducted using a plastic dialysis cell24. This cell consists of two half cells: a donor compartment and a receptor compartment, separated by a cellulose membrane. The sol form of the formulation is placed in the donor compartment. The assembled cell is then horizontally shaken in an incubator. The total volume the receptor solution can be periodically replaced with fresh media. This solution is then analyzed using analytical technique28 to determine the drug release. In the case of injectable in situ gels, the formulation is placed in vials containing receptor media and placed on a shaker water bath at the desired temperature and oscillation rate. Samples are withdrawn at regular intervals and analyzed. (30)
RECENT ADVANCES
The pharmaceutical industry today faces a significant challenge in developing effective treatment options that are widely accepted by both physicians and patients. In order to offer viable alternatives to current pharmaceutical delivery methods, delivery systems must also play a role in improving therapeutic outcomes. In situ gel formulations present a complex drug delivery system that requires careful consideration. While various biodegradable polymers are utilized in the formulation of in situ gels, issues such as fabrication difficulties, process complexity, the use of organic solvents (particularly in synthetic polymer-based systems), burst effects, and inconsistent drug release kinetics are common. Although natural polymers possess ideal characteristics, ensuring batch-to-batch reproducibility is challenging, leading to the utilization of synthetic polymers. Recent advancements in biotechnologies have enabled the development of labile macromolecular therapeutic agents that necessitate intricate formulations for effective administration. The combination of N-stearoyl L-alanine (m) ethyl esters with a vegetable oil and a biocompatible hydrophilic solvent has resulted in the creation of an injectable, in situ forming organ gel. Upon subcutaneous injection, the leuprolide-loaded organ gel degrades gradually, releasing leuprolide over a period of 14 to 25 days. (31)
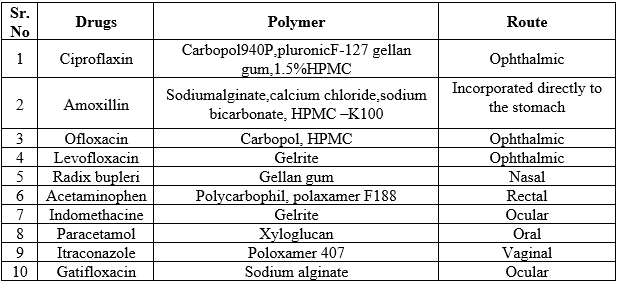
Table No. 1 List of a drug developed as an in situ gel drug delivery system (32)
CONCLUSION
This review article provides a comprehensive overview of the 'in situ gel' method, which has emerged as one of the most effective drug delivery systems currently available. It offers long-term and controDlled drug release, improved patient compliance, and enhanced comfort. The in situ gel system utilizes synthetic polymers that form a gel at the site of application, making it suitable for oral, ocular, transdermal, buccal, intraperitoneal, parenteral, injectable, rectal, and vaginal routes. Extensive research has been conducted on this system, enabling the development of advanced technology in various drug delivery systems. Patient compliance is the key factor in ensuring the success of controlled release products, which can be achieved through the use of in situ gels. In situ gels offer the flexibility to administer drugs with different therapeutic effects through various routes. The utilization of polymeric in situ gels for controlled release of drugs presents numerous advantages over traditional dosage forms, including sustained and prolonged drug release, stability, and biocompatibility. Incorporating biodegradable and water-soluble polymers in the formulation of in situ gels enhances their acceptability and makes them an excellent drug delivery system.
REFERENCES
- Kulkarni V, Kumbhar R, Butte K, Rathod S. Development and Evaluation of curcumin Liposomal gel, IJAPR. 2013;4.
- Nerkar Tushar S, Gujarathi Nayan A, Rane Bhushan R, Bakliwal Sunil R, Pawar SP. Insitu Gel: Novel Approaches in sustained and controlled drug delivery system. 2013;4(4).
- Kant A, Reddy S, Shankraiah MM, Venkatesh JS, Nagesh C. In situ gelling system – an Overview, Pharmacologyonline. 2011; 2:28-44.
- Al-Shamklani A, Bhakoo M, Tuboku MA, Duncan R editors. Evaluation of the biological properties of alginates and gellan and xanthan gum. Proc Int Symp Control Release Bioact Mater. 1991 pp. 213–4.
- Srividya B, Cardoza RM, Amin PD. Sustained ophthalmic delivery of ofloxacin from a pH Triggered in situ gelling system. J Control Release. 2001;73(2-3):205–11.
- Ismail FA, Napaporn J, Hughes JA, Brazeau GA. In situ gel formulations for gene delivery: Release and myotoxicity studies. Pharm Dev Technol. 2000;5(3):391–7.
- Bilensoy E, Rouf MA, Vural I, Sen M, Hincal AA. Mucoadhesive, thermosensitive, Prolonged-release vaginal gel for clotrimazole: beta -cyclodextrin complex. AAPS PharmSciTech. 2006;7(2): E38.
- Chenite A, Chaput C, Wang D, Combes C, Buschmann MD, Hoemann CD, et al. Novel Injectable neutral solutions of chitosan form biodegradable gels in situ. Biomaterials. 2000;21(21):2155–61
- Vidhi Upadhaya, Neha Tiwari A Review: in-situ Gel Drug Delivery System, JETIR2302293 Journal of Emerging Technologies and Innovative Research (JETIR) www.jetir.org c715
- S. Cohen, E. Lobel, A. Trevgoda, Y. Peled. A novel in situ-forming ophthalmic drug delivery system from alginates undergoing gelation in the eye. J. Control. Release. 44, 1997, 201–208.
- Sirish vodithala, Sadhna Khatry, Nalini Shastri, M. Sadanandam, Formulation and evaluation of ion activated ocular gels of ketorolac tromethamine International Journal of Current Pharmaceutical Research, 2(3), 2010.
- Rajas NJ, Kavitha K, Gounder T, Mani T, In-Situ ophthalmic gels a developing trend, Int J Pharm Sci Rev and Res, 7, 2011, 8-14.
- Kant A, Reddy S, Shankraiah MM, Venkatesh JS, Nagesh In situ gelling System – an Overview, Pharmacology online. 2011; 2:28-44.
- Dongare PS, Darekar AB, Gondkar SB, Saudagar RB Floating Drug Delivery System: A Better Approach. IJPBS, (2013); 3(4):72-85.
- Motto F, Gailloud P, et al., In-vitro assessment of new embolic liquids prepared from Preformed polymers and water miscible solvents aneurysm treatment. Biomaterials 2000,21:803-11
- Soniya R. Devasani, Asish Dev, S. R etal. An overview of in situ gelling system PHARMACEUTICAL AND BIOLOGICAL EVALUATIONS February 2016; vol. 3 (Issue 1):60-69.
- Motto F, Gailloud P, et al., In-vitro assessment of new embolic liquids prepared from Preformed polymers and water miscible solvents aneurysm treatment. Biomaterials, 21; 2000:803-11
- Esposito E, Carratto V et al. Comparative analysis of tetracycline containing dental gels; Poloxomers and Mono-glycerides based formulation. Int.J.Pharm.1996;142:9-23
- Guo J-H, Skinner GW, Harcum WW, Barnum PE. Pharmaceutical applications of naturally Occurring watersoluble polymers. Pharm Sci & Technol Today 1998; 1:254-61
- Podual K, Doyle III FJ, Peppas NA. Dynamic behavior of glucose oxidase-containing Microparticles of Poly(ethylene)- grafted cationic hydrogels in an environment of changing pH. Biomaterials 2000; 21:1439-50.
- Swarnim Srivastava Role of In Situ Gel in Drug Delivery System International Journal of Pharmaceutical Research and Applications Volume 5, Issue 2, pp: 449-461
- Nirmal HB, Bakliwal SR, Pawar SP. In situ Gel: New trends in controlled and sustained Nirmal HB, Bakliwal SR, Pawar SP. In situ gel: New trends in controlled and sustained drug Delivery system. International Journal of PharmTech Research. 2010;2(2):1398-408
- Kalia Neha*, Nirmala, Harikumar SL. INSITU GELLING SYSTEM: A REVIEW Journal of Drug Delivery & Therapeutics; 2014, 4(4), 93-103
- Soniya R. Devasani*, Asish Dev, S. Rathod, Ganesh Deshmukh An overview of in situ gelling systems PHARMACEUTICAL AND BIOLOGICAL EVALUATIONS February 2016; vol. 3 (Issue 1): 60-69.
- Miyazaki S, Hirotatsu A, Kawasaki N, Wataru K, Attwood D. In situ gelling gellan Formulations as Vehicles for oral drug delivery. J Control Rel 1999; 60:287-95
- Ramesh CR, Zentner GM, Jeong B. Macro med, Inc. Biodegradable low molecular weight Triblock poly (lactide-Co- glycolide) polyethylene glycolcopolymers having reverse Thermal Gelation properties. US patent 6201072. 2001
- Sonjoy KMJ, Thimmasetty GL, Prabhu S, Geetha MS. Formulation and evaluation of an In-situ gel forming ophthalmic Formulation of Moxiflaxin hydrochloride. Int J Pharma Investig 2012; 2: 2
- P.R. Patil*, S.S. Shaikh, K.J. Shivsharan, S.R. Shahi IN SITU GEL: A NOVEL DRUG DELIVERY SYSTEM Indo American Journal of Pharmaceutical Research, 2014 ISSN NO: 2231-6876
- Allamsetti Geethanjali, Praveen Sivadasu* and Padmalatha K AN OVERVIEW ON IN-SITU GELLING SYSTEM INDO AMERICAN JOURNAL OF Pharmaceutical SCIENCES SJIF Impact Factor: 7.187 http://doi.org/10.5281/zenodo.4940113
- Nirmal H.B.*, Bakliwal S.R., Pawar S.P In-Situ gel: New trends in Controlled and Sustained Drug Delivery System International Journal of PharmTech Research CODEN (USA): IJPRIF ISSN: 0974-4304 Vol.2, No.2, pp 1398-1408, April-June 2010
- Harnish Patel1, Priyanka Patel2, Tushar Brahmbhatt1 and Mayur Suthar1 In-Situ Gelling System: A Review J. Chem. Pharm. Res., 2011, 3(6):217
- Li B, Gao S, Qiao X. The preparation and analysis of low molecular weight chitosan. Zhongguo Shenghua Yaowu Zazhi ,1999; 20: 292–294.


 Akash Shendge *
Akash Shendge *
 Vikas B. Wamane
Vikas B. Wamane
 Jayshree R. Shejul
Jayshree R. Shejul










 10.5281/zenodo.12679670
10.5281/zenodo.12679670