Abstract
The most frequent cause of end-stage renal disease (ESRD) is diabetic kidney disease (DKD). The incidence of DKD persists despite rigorous therapy that includes blood pressure management, hyperglycemic control, and renin-angiotensin system blockades. According to recent studies, there has been significant advancement in the development of new DKD treatments, and the spectrum of DKD may have shifted.
Keywords
Microalbuminuria, Macroalbuminuria End stage renal disease, Diabetic Nephropathy, SGLT2 inhibitors
Introduction
Diabetes mellitus is a long-term metabolic disease characterized by elevated blood sugar levels and altered protein, lipid, and carbohydrate metabolism. This ailment hinders the body's capacity to metabolize blood sugar as. This elevated blood glucose level has the following effect. It results in diabetes mellitus in our bodies. [1] About 30% of people worldwide suffer from diabetic kidney disease (DKD), which is one of the most prevalent microvascular consequences. It is also linked to greater mortality and higher medical care expenditures. In many communities, it is the primary cause of end-stage renal disease. Renal fibrosis is the primary pathophysiology of diabetic kidney disease (DKD), and it is caused by inflammatory processes, an overactive renin-angiotensin-aldosterone system (RAAS), ischemia, and excessively reactive oxidative stress, at least in part. Novel domains of DKD pathogenesis, including mitochondria malfunction podocyte autophagy and genetic and epigenetic control have been investigated by recent molecular and cellular studies. [2] One of the most common illnesses that contributes to morbidity and mortality globally is diabetes mellitus (DM). The organs most frequently impacted are the kidney and liver, leading to non-alcoholic fatty liver disease (NAFLD) and diabetic kidney disease (DKD). But pathophysiological processes that might be Despite sharing an underlying etiology, the common features of both DKD and NAFLD have not been thoroughly explored. In addition to investigating possible medication treatments for DKD and NAFLD, this study sought to discover the hub genes shared by both conditions. [3] The primary cause of kidney failure globally is diabetic renal disease, which affects more than 50% of patients starting dialysis or kidney transplant programs in the United States. In contrast to other diabetes problems, the past 30 years have seen no drop in the prevalence of diabetic kidney disease. Diabetic kidney disease is primarily caused by hyperglycemia as the aetiological component. Once hyperglycemia is established, a number of pathophysiological abnormalities lead to the progressive glomerular sclerosis and the decline in glomerular filtration rate. These abnormalities include hypertension, altered tubuloglomerular feedback, renal hypoxia, lipotoxicity, podocyte injury, inflammation, mitochondrial dysfunction, impaired autophagy, and increased activity of the sodium-hydrogen exchanger. It is yet unknown how much each of these anomalies contributes quantitatively to the development of diabetic kidney damage and how they affect people with type 1 and type 2 diabetes. [4] Since the 1995 release of the previous edition of Diabetes in America, significant advancements have been accomplished.in treating diabetic kidney disease and comprehending its causes and progression . Based on the evaluation of albuminuria and estimated glomerular filtration rate (eGFR) , widely accepted criteria for the staging of chronic kidney disease (CKD) have been devised; in 1995, estimations of GFR and CKD staging criteria had not yet been developed. However, among those with kidney disease, it continues to be a leading cause of morbidity and death. diabetics, as seen by the sharp rise in the number of patients undergoing renal replacement treatment in the 1980s. [5] The most common cause of kidney impairment in patients starting kidney transplantation is diabetic renal failure, which affects about 30% of those with type 1 and type 2 diabetes. The course and pathogenesis of the disease are examined in this article. Special structural and functional alterations in the kidneys are brought on by diabetic nephropathy. Mesangial expansion with extracellular matrix protein accumulation (collagen, fibronectin, laminin), glomerular hyperfiltration, glomerular and kidney enlargement, and enhanced basement layer thicknesses are among the earliest kidney alterations associated with diabetes mellitus. glomerulosclerosis, interstitial fibrosis, reduced creatinine elimination, glomerular filtration rate decline, and macroalbuminuria (>300 mg/day) are the hallmarks of diabetic kidney failure. Even though inadequate glycemic management is a risk factor, end-stage kidney disease in diabetes only affects a subset of the population. Numerous mechanisms, including systemic and glomerular hypertension, advanced glycation end products, and the aldose reductase system, have been linked to diabetic kidney failure through extensive research.[6] Diabetic kidney disease is is becoming more common as a result of public ageing and rising obesity rates. Reducing the heart rate, controlling blood sugar, and inhibiting the renin-angiotensin-aldosterone axis are the three primary methods for stopping or postponing its progression. Additionally, new treatments have been tried.[7] Diabetic kidney disease (DKD) is the most frequent cause of renal deterioration worldwide and the best indicator of death for those with diabetes. DKD is the classic gene-environment interaction disorder. The incidence of diabetics renal failure (DKD) is significantly reduced by intensive control of sugar, indicating that metabolic dysfunctions caused by hyperglycemia such as altered energy metabolism and mitochondrial dysfunction play a significant role in the illness's genesis. Controlling blood sugar levels, especially with the use of medications that block the angiotensin system, is the only effective way to stop the problem from getting worse. Despite the fact that kidney disease is believed to be a microvascular aftereffect of diabetes, an increasing amount of evidence indicates that podocyte loss and epithelial dysfunction may play a major role. Inflammation, cell hypertrophy, and dedifferentiation brought on by the activation of conventional regenerative mechanisms all contribute to the illness's progression.[8]
Literature review

Epidemiology of diabetic kidney disease
Throughout their lives, one-third of people with T1DM and nearly half of those with T2DM develop diabetic kidney damage. It is among the most common, costly, and time-consuming long-term consequences of diabetes. Approximately 20% of T2DM adults will achieve an eGFR of less than 60 mL/min/1.73 m2, and between 30 and 50% will have higher excretion of albumin in their urine. According to the UK Prospective Diabetes Study, 28% of participants had albuminuria and 28% had an eGFR < 60> Regardless of the type of diabetes, low eGFR incidence ranges from 2% to 4% annually.Since people with diabetes may have various causes of CKD in addition to diabetes, and since kidney biopsies are rarely done to verify the accurate diagnosis, it is unknown with precision what proportion of patients have CKD caused by diabetes. Other causes of chronic kidney disease (CKD), such as hypertension, dyslipidemia, obesity, intra-renal vascular disease, acute kidney injury (AKI), glomerular atherosclerosis, or age-related kidney loss, are frequently prevalent, especially in those with type 2 diabetes. [9] Diabetes is currently the major cause of end-stage renal disease (ESRD) worldwide. Type 2 diabetes was the major cause of end-stage renal disease (ESRD) in Malaysia, Mexico, and Singapore in 2009–2011. Israel, Korea, Hong Kong, Taiwan, Philippines, Japan, the United States, and New Zealand, all have End Stage Renal Disease incidences of 40% to 50%. type 2 Diabetes-related ESRD is more prevalent in older persons. In 2011, the incidence rates of ESRD caused by diabetes in the United States were 44, 266, and 584 per million for ages groups 20-44, 45-64, and 65-74 years. [10] A Changing Concept: From Diabetic Nephropathy to Diabetic KidneyDisease Until recently, diabetic nephropathy was defined as the existence of a kidney abnormality in an individual with diabetes, as shown by elevated protein levels equal to or greater than 300 mg/day. Typically, this clinical scenario was accompanied by diabetic retinopathy and hypertension, resulting in a steady decline in renal function. Nonetheless, the absence of diabetic retinopathy does not rule out the diagnosis of renal failure. The typical progression of diabetic renal disease varies among type 1 and type 2. The 5 classical phases outlined in type 1 DM may not occur in type 2 DM since type 2 diabetes is occasionally diagnosed after other linked illnesses such as high blood pressure, proteinuria, or kidney disease.The most prevalent clinical signs are the development of microalbuminuria and subsequent progressing to overt proteinuria. Yet, contrary to the conventional strategy estimate for chronic kidney participation, a significant proportion of patients with diabetes and impaired renal filtration do not have significantly increased urine protein excretion rates. All of the research on this subject are observing, with the majority lacking biopsy results. To completely understand this entity, an effective biopsy investigation and a series of intervention experiments are required. In reference to this point, Tervaert et al. published a novel pathology classification of diabetics renal infections in 2010. The researchers stressed that there are various types of kidney damage that are distinct from the traditional nodular or global glomerulosclerosis and that primarily affect the tubules, interstitium, or blood vessels. Due to these recent developments, the term "diabetics Nephropathy is " (formerly known as "diabetic nephropathy") has been replaced with "diabetic chronic renal diseases". [11]
Structure of Diabetic Kidney Disease
Fig: 1
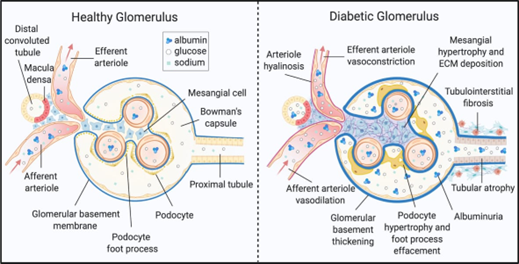
The kidney is a metabolically active organ that contains several highly developed cell types. A thorough understanding of DKD onset and development in different cell types is a considerable challenge. DKD causes diverse metabolic or morphological alterations, some of which are well documented ( Figure 1). kidney disease is distinguished by the early thickening of the glomerular basement membrane. The glomerulus's mesangial cells are significantly hypertrophied and fibrotic. Podocytes, which control the the glomerulus' size-restriction barrier, show foot process effacement, hypertrophy, and death. Diabetes renal show border brushing weakening, tubular epithelial cell destruction, and separation. The first studies on chronic kidney disease found lipid-rich lesions in tubular and glomerular cells. Pathological changes may take place in the kidney interstitium, which consists of the extracellular matrix, fluid, and cells that surrounds kidney cells and capillaries. Immune cells and mesenchymal TECs activate and move in the renal system of chronic kidney disease patients, causing swelling and ECM accumulation. DKD development results in interstitial and tubular fibrosis, as well as glomerulosclerosis. [12]
Molecular Mechanisms of Diabetic Kidney Disease
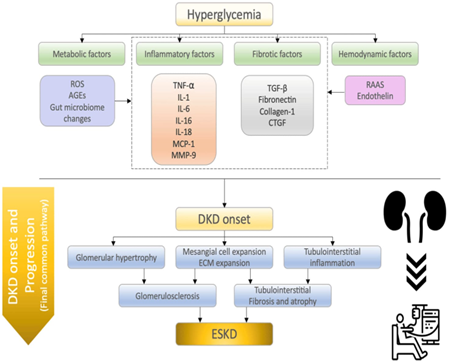
Fig: 2.
Overview about the cellular mechanisms behind the start and development of diabetic kidney disease (DKD). Several pathways influence DKD development, including metabolic, inflammatory, fibrotic, and hemodynamic variables. These elements all cause albuminuria and impair renal function. DKD patients eventually develop ESKD by a final common pathway (glomerulosclerosis, tubulointerstitial fibrosis, and atrophy). ROS refers to reactive oxygen species; Advanced glycation end products (AGEs) TNF-? (tumor necrosis factor-?), IL-1 (interleukin-1), IL-6 (interleukin-6), IL-16 (interleukin-16), IL-18 (interleukin-18), MCP-1 (monocyte chemoattractant protein-1), MMP-9 (matrix metalloproteinase-9), TGF-? (transforming growth factor), CTGF (connective tissue growth factor), RAAS (renin-angiotensin-aldosterone system), DKD (diabetic kidney disease), and ESKD (end-stage kidney disease). Inflammatory factors:. Continuous inflammation is a key factor in the development of DKD. Kidney biopsy samples from DKD patients contained high levels of inflammatory cytokines, chemokines, and growth components. Diabetes alters many immune system factors, especially circulating leukocytes, which cause fluctuations in the amounts of certain cytokine and chemokines in Pathological modifications in DKD include mesangial enlargement, swelling of the glomerular and tubular basement membranes, or podocyte reduction as a result of continued glomerular hypertension and hyperfiltration, followed by gradual progression to glomerular sclerosis and tubular atrophy, and finally a decrease in kidney function. A high blood sugar level promotes mesangial enlargement by cytokines and growth factors such TGF-?, VEGF, and fibronectin. Because mesangial enlargement is directly associated to kidney function damage, these cytokines and growth hormones have significant pathophysiological implications. This part summarizes recent results on inflammatory variables related to DKD etiology, including TNF-?, IL-1, IL-6, IL-16, IL-18, MCP-1, and MMP-9.
TNF-?:
Produced by activated macrophages and causes cytokines, chemokines, apoptosis, and cytotoxic effects.
IL-1:
Hyperglycemia and macrophage infiltration are significantly associated with IL-1?.
IL-6:
Causes tubulointerstitial neutrophil infiltration and is linked to GBM thickness and podocyte hypertrophic invasion.
IL-16:
Immunomodulatory cytokine that correlates with the severity of DKD, but precise processes have yet to be defined.
IL-18:
Links most significantly with DKD severity among cytokines and is activated by the inflammation (NLRP3).
MCP-1:
Increases the production of various inflammatory factors, including inflammatory cytokines and inflammatory cells like monocytes/macrophages.
MMP-9:
controls extracellular matrix breakdown during fibrosis in the proximal renal tubular epithelial cell and inflammatory cells such as monocytes/macrophages
Fibrotic Factors:
Fibrosis can be defined by the formation of myofibroblasts, which are collagen-depositing cells. In addition to bone marrow-derived myofibroblasts, local fibroblasts serve a significant role in the progression of kidney fibrosis. Tubulointerstitial fibrosis is the final common mechanism for all renal disorders, includes DKD, and is linked to tubular shrinkage and extracellular matrix accumulation. This part covers new results on typical fibrotic factors related to DKD, including transforming growth factor-, fibronectin, collagen-1, and connective tissue growth factor.
TGF-?:
The primary regulator of inflammation and fibrosis. TGF-?1 and Smad3 (downstream of TGF-?) are highly harmful.
Fibronectin:
Accumulation in glomerular mesangial infections is related with worsening renal function.
Hemodynamic Factors:
Glomerular hyperfiltration is a common disease in the early phases of DKD, while glomerular expansion is a histological feature in both DKD and obesity-related nephropathy. The initial stage of conventional and typical diabetic nephropathy is glomerular hyperfiltration, which leads to increasing albuminuria, decreased GFR, and, eventually, end-stage kidney disorder. Lifestyle factors including nutrition and body weight can also have an impact on glomerular hypertension. A high blood sugar level combines with higher amounts of circulating amino acids induced by a high-protein diet, triggering glomerular hyperfiltration. SGLT2 is one of the proposed mechanisms for glomerular hyperfiltration. SGLT2 promotes reabsorption of glucose in the proximal tubules, lowering sodium chloride supply to the macula densa. In this part, we focused on the renin-angiotensin system (angiotensin II and aldosterone) and endothelin as hemodynamic variables.
RAAS:
Promotes formation of ROS and podocyte damage via angiotensin-II-mediated calcium entry into podocytes during DKD development
Endothelin:
ET-1 promotes proinflammatory and profibrotic pathways, and it causes endothelial dysfunction and oxidative stress. [13]
Metabollic factors:
The metabolic pathways of diabetic nephropathy. A high blood sugar level causes the buildup of AGEs and other glucose-metabolizing products.
Stimulation of any of these mechanisms can harm the kidney's function. AGEs can cause cell damage through both receptor and non-receptor routes. Out of the cells, they can induce damage to tissues by glycating proteins like collagen, which reduces tissue compliance through crosslinking. High glucose influx can lead to the activation of pathways such polyol, hexosamine, and PKC, which can cause cellular damage and kidney failure.
ROS: DKD is facilitated by a high number of podocytes. Causes epithelial-mesenchymal transition and the death of cells.
AGEs: Changes the extracellular matrix structure. RAGE-mediated regulation of cellular activities
Gut microbiome changes: Dietary AGEs interact with colonic bacteria, causing local pain and the production of pro-inflammatory cytokines. [14]
Classification of Clinical Stages of Diabetic Nephropathy:
First stage : hyperfiltration
Early in the course of type I diabetes, the glomerular filtration rate may increase to well above normal.This stage has been studied less in type II diabetes, the prevalence of microalbuminuria (which occurs in later stages, see below) ranged from 20% to 40%. The duration of stage 1 varies,lasting up to 15 years.What causes hyperfiltration is not completely understood; however, glomerular filtration increases with even mild hyperglycemia, and improved glycemic control leads to a marked reduction in hyperfiltration Usually absent in this stage are albuminuria and, presumably, any histologic changes of diabetes mellitus in the kidneys. [15]
Second Stage: Microalbuminuria
UAE becomes more prevalent as kidney involvement progresses. It is also known as hidden or subclinical nephropathy. In this period, the traditional strip test is frequently negative. At this period, the risk of cardiovascular disease increases. Changes in albuminuria rate over time are linked to decreasing kidney function. Microalbuminuric patients with rising, steady, or decreased albumin exertion had a high, moderate, or low risk of GFR. In type 1 diabetes, higher levels of albuminuria increase the risk of microvascular sequelae such retinopathy and neuropathy.Our study found that 35.2% of patients with type 2 diabetes have microalbuminuria. Hyperlipidemia, age, and diabetes duration all increase the incidence of microalbuminuria. Early diagnosis can prevent clinical nephropathy or return to normal albuminuria. Without therapy, type 1 diabetic patients face a high chance of developing clinical nephropathy after 10-15 years (more than 75%) and type 2 diabetic patients after 15-20 years (20-40%).
Third stage: Macroalbuminuria
This stage, also known as clinical nephropathy, occurs approximately 10-20 years after the onset of diabetes (5-10 years after the onset of microalbuminuria). This stage sees an increase in coronary artery disease and cerebrovascular incidents compared to prior stages. Approximately 75% of patients in this stage have elevated blood pressure. Controlling blood pressure in type 2 diabetes patients with past hypertension is more challenging. At this point, a traditional dipstick test indicates proteinuria and a urine albumin excretion rate of over 300 mg per 24 hours. Following this, GFR decreases by 10-12 ml yearly. Without treatment, clinical nephropathy leads to a gradual loss in kidney function and increased proteinuria, maybe reaching nephrotic levels. Diabetic retinopathy helps corroborate the diagnosis of diabetic nephropathy. Hypertension and proteinuria cause decreased GFR and progression to ESRD. [16].
Fourth Stage: ESRD
In stage 5, renal function progressively declines, ultimately leading to end-stage renal disease and the need for dialysis or transplan-tation. Patients with end-stage renal disease of any cause may have hyperkalemia, uremic symptoms, and abnormalities of calcium, phosphorus, vitamin D, and parathyroid hormone. In general, management of this stage is similar to that for nondiabetic end-stage renal disease, and the indications for dialysis and transplantation are the same. Because specialists rather than primary-care physicians usually undertake the care of such patients, we will not discuss it in detail here
Stages Diabetic Kidney Disease
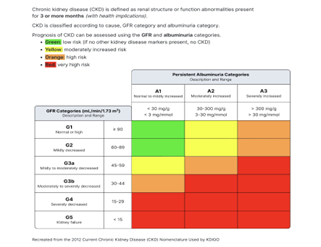
Fig3
Sign and Symptoms:
In the early stages of the disease, there are often no symptoms at all. Yet, once the glomeruli and arteries are injured, the following typical signs and symptoms of diabetic nephropathy occur.
- Weaknesses
- Fatigue
- High blood pressure
- frequently peeing with protein
- Feeling sick
- Sleepiness
- Breathlessness.
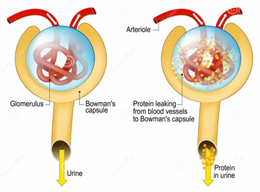
Fig 4
- Anaemia (low blood count)
- High Blood Pressure,
- Nausea
- Having trouble sleeping or concentrating
- Trouble understanding properly
- Losing weight
- Very dry skin with itching cramps in the muscles (especially in the legs).
- Loss of appetite
- Retention of fluids as a result of swelling toes and knees.
- Swelling behind the eyes
- Having to pass urine more regularly.[19]
Causes of Diabetic Nepropathy
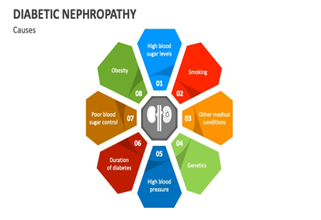
Fig 5
Risk Factors for Development of Diabetic Nephropathy
Non modifiable
- Increase age
- Early start of diabetes
- Prolonged duration of diabetes
- Gene-related variables
- Cultural background
- Family history of diabetic kidney disease, type 2 diabetes, non-diabetic chronic kidney disease, high blood pressure, and resistance to insulin.
- development slowing within the uterus
- Maternal gestational diabetes or developing hyperglycemia intolerance.
Modifiable
- inadequate management of glucose.
- High blood pressure.
- Dyslipidemia.
- Poverty due to economic status.
- A sedentary lifestyle or low intensity exercises.
- Obesity.
- Consuming cigarettes.
Complications of diabetic kidney disease

Diagnosis of Diabetic kidney disease
DKD can be diagnosed in T1DM with prolonged moderate (A2) or severe (A3) albuminuria or a persistent decline in eGFR to <60>
Routine screening tests may include:
Urinary albumin test:
The method used detects a blood protein called albumin in urine. Usually, the kidneys fail to remove albumin from the blood. Too much albumin in your urine can indicate that your kidneys are not performing properly.
Albumin/creatinine ratio:
Creatinine is a chemical waste product that healthy kidneys remove from the blood. The albumin/creatinine ratio compares the amount of albumin to creatinine in a urine sample. It reveals how well the kidneys perform.
Glomerular filtration rate (GFR):
The concentration of creatinine in a blood sample can be used to determine how rapidly the kidneys filter blood. This is the glomerular filtration rate. A low level indicates that the kidneys are not functioning effectively. [23]
Prevention Of Diabetic Kidney Disease

Fig 7
Diabetic Kidney Disease : Potential Treatment
Glycemic controlThe most efficient strategy of preventing diabetic kidney disease is extreme glycemic control. The term of hyperglycemic poisoning ("glucotoxicity") is widely regarded as a major mechanism of DKD. Studies in both type 1 and type 2 diabetes have shown that strict management of glucose levels has a significant benefit in lowering the likelihood of developing microalbuminuria and progressing to kidney failure. The DCCTEDIC study found that stringent glucose management had a long-term protective effect on DN (metabolic memory). The level of glycemic management to enhance safety is still under contention, according to the updated recommendation. Following 3 significant clinical trials, a goal HbA1c of 7% is now advised. It revealed no benefit to more intense glucose management but demonstrated a higher risk of severe hypoglycemia, when the objective is strict glucose management for older people.
Blood Pressure Control:
According to the most recent Joint National Committee (JNC) 8 and KDIGO guidelines, blood pressure in diabetic should be less than 140/90 mmHg [121 - 123]. Hypertension is one of the most prevalent concurrent illnesses in DKD. Hypertension of the arteries occurs early and frequently with every Glomerular Filtration Rate decline. Diabetics people with CKD have increased blood pressure, which is more prevalent than in non-diabetic CKD patients. Approximately two-thirds of all diabetic individuals have hypertension. Hypertension control, independent of the antihypertensive medication used, can help reduce the development of DN. ACEIs and ARBs are still suggested as the first choice in DKD patients. On average, people with DN require three medications to correct their hypertension, including diuretics and calcium blockers. Strict blood sugar control, defined as a blood pressure of less than 140/90 mmHg, lowered the risk of death and diabetes-related complications.
Weight Loss :
Overweight is a growing problem in both the general population and the diabetic community. Several studies show that severe obesity (BMI > 40 kg/m 2) accelerates the progression of renal failure. Losing weight enhances control of glycemic levels. While weight loss surgery has been linked to reduced albuminuria and improved glycemic management, its impact on the course of diabetic kidney failure is unknown.
Protein Restriction
The DKD guidelines currently propose a daily protein intake of 0.8-1 g/kg body weight. While animals investigations have revealed strong renoprotective effects of a reduced protein diet, human clinical studies examining the effects of protein restriction on diabetic nephropathy did not show apparent benefits of a low protein-restricted diet
Lipid Lowering Drugs:
Lipid-lowering medications are recommended for primary and secondary prevention of CVD in diabetes, hypertension, and kidney disease, all of which enhance CVD morbidity and mortality. Pathophysiology of Diabetic Nephropathy MichalH6@clalit.org.il 54 The Study of Heart and Renal Protection (SHARP) randomised trial found that individuals with initial CKD exhibit lipid abnormalities such as elevated low-density lipoprotein (LDL) cholesterol levels, increased lipoprotein levels, and reduced high-density lipoprotein (HDL) cholesterol levels. Total and LDL cholesterol levels in patients with type 1 diabetes mellitus have been identified as dependent risk factors for kidney disease development.
Prevention of Acute Kidney Injury:
Individuals with DKD are more vulnerable than those without DKD to acute kidney damage (AKI). Patients with chronic kidney disease repair their kidneys far less after an acute kidney injury event, and such an occurrence may result in irreparable kidney harm. Regardless of other acute kidney injury risk factors, the use of RAS antagonists may raise the risk of acute kidney injury. In a recent investigation, any type of acute kidney injury was associated with an increased risk of development to stage 4 CKD in 3679 diabetic individuals observed from January of 1999 to December of 2008.
Angiotensin Converting Enzyme Inhibitors and Angiotensin Receptor Blockers
For the last twenty-five years, treating diabetic kidney disease with angiotensin-converting enzyme (ACE) inhibitors or angiotensin II receptor blockers (ARBs) has been the first line therapy due to their ability to diminish proteinuria. Kidney outcomes are less common in nephrotic patients with and without diabetes mellitus when the RAS is blocked. However, for many individuals, RAS blockage is inadequate to stop the course of diabetic renal disease. Long-term protection against the development of diabetic glomerulopathy is demonstrated by ACE inhibitors in diabetic animal models, but extensive human clinical trials have not demonstrated the same degree of protective effect on human kidney function.[25]
Dietary protein restriction.
Patients with ESKD or on the verge of it may see a reduction in uremic signs by diet restrictions. Its usefulness in treating DN is questionable, nevertheless. A low-protein diet (0.8g/kg/d) has been demonstrated in small studies to significantly decrease proteinuria with increasing albumin in plasma in patients with Type 2 diabetes who have moderately elevated albuminuria. Standard recommendations should be followed for the management of any additional chronic kidney disease problems. [26]
Novel Therapies for Diabetic Kidney Disease


Fig 8
Recent therapeutic advancements and putative pathways for decreasing diabetic kidney disease development. DKD should be treated with an integrated strategy that includes lifestyle modifications, RAS blockers, SGLT2 inhibitors, Ns-MRAs, and GLP-1RA. Meanwhile, clinical trials of PTF, selonsertib, and baricitinib have shown promise for the management of DKD. Yet, further RCTs are needed to assess their effectiveness. RAS: Renin-angiotensin/aldosterone system; SGLT2: sodium-glucose cotransporter 2; Ns-MRAs: nonsteroidal mineralocorticoid receptor antagonists; GLP-1RAs: glucagon-like peptide 1 receptor agonists; ASK1: apoptosis signal-regulating kinase 1; JAK: Janus kinase.
CONCLUSION
DKD is involved in a variety of hemodynamic, inflammatory, and metabolic processes, which eventually lead to the fibrotic pathway. Even if glycemia is stabilized, DKD might spread due to persistent production of proinflammatory and profibrotic mediators. Lifestyle and epigenetic factors are also linked to the development of DKD. Although the development of RAS blockades, SGLT2 inhibitors, and NS-MRAs, existing therapy choices are limited in their capacity to successfully slow renal disease development and reduce the risk of morbidity and mortality in DKD patients. RAS blockers, SGLT2 inhibitors, and NS-MRAs have shown significant success in lowering the risk of kidney disease
REFERENCE
- By Dr.M.Senthil Kumar and Dr.S. Valarmathi et. al, Dr.R.Sathish, E.Naveena, D.Pavithra, P.Nivash, G.Praveen, N.Naveenkumar. Formulation and Evaluation of Polyherbal Syrup with Anti-diabetic activity. International journal of pharmaceutical research and applications. Volume 7 , pp-867-872, Published by Jan-feb 2022. DOI: 10.35629/7781-0701867872. www.ijprajournal.com.
- Yumin Zhang and Wei Li et. al,Yunting Zhou. Identification of Hub genes in Diabetic Kidney Disease. National Library of Medicine. Volume 8, Published on: July 29th 2020. DOI:10.21037/atm-20-5171.
- Kaberi Datta. Common Hub Genes and Drug Candidates for Diabetic Kidney Disease and Non-Alcoholic Fatty Liver Disease. International Journal of Pharmaceuticals Science and Research. Published on: 30 January 2024. DOI :10.25004/IJPSDR.2024.160114.
- DeFronzo, R.A., Reeves, W.B. & Awad, A.S. Pathophysiology of diabetic kidney disease impact of SGLT2 inhibitors. Nat Rev Nephrol 17, 319–334 (2021). https://doi.org/10.1038/s41581-021-00393-8. Published on: 5 February 2021.
- Meda E. Pavkov, and MD et. al. PhD, Allan J. Collins, MD, Josef Coresh, MD, PhD, and Robert G. Nelson, MD, PhD. Kidney Disease in Diabetes . Published on :July 2017.
- Deepak N Parchwani and Amit A Upadhyah. Diabetic Nephropathy: Progression and Pathophysiology. International Journal of Medical Science and Public Health. Published on 24-7-2012. Voume1. Issues 2. DOI: 10.5455/ijmsph.2012.1.59-70.
- Gerald apple and MD. Detecting and controlling diabetic nephropathy: What do we know? . Cleveland Clinic Journal Of Medicine. Volume 80, Number 4. Published on :April 5, 2024.DOI: https://doi.org/10.3949/ccjm.80gr.12006 . www.ccjm.org.
- Kimberly Reidy and Hyun Mi Kang et. al. Thomas Hostetter, Katalin Susztak. Molecular mechanisms of diabetic kidney disease. The Journal of Clinical Investigation. Volume 124, Number 6. Published on: June 2014. doi:10.1172/JCI72271.
- Ellen K. Hoogeveen. The Epidemiology of Diabetic Kidney Disease. Kidney Dial. 2022, 2, 433–442. Published: 1 August 2022. https://doi.org/10.3390/kidneydial2030038 epidemiology of dkd
- Andy KH Lim. Diabetic nephropathy – complications and treatment. nternational Journal of Nephrology and Renovascular Disease. Published on 12 August 2014. Volume 2014:7 Pages 361—381. DOI https://doi.org/10.2147/IJNRD.S40172.
- Alberto Martinez-Castelao and Juan F. Navarro-Gonzalez et.al Jose Luis Gorriz and Fernando de Alvaro 4. The Concept and the Epidemiology of Diabetic Nephropathy Have Changed in Recent Years. Journal of clinical Medicine. Published on : 28 May 2015. doi:10.3390/jcm4061207 the cocept
- Rebekah J. Nicholson and Marcus G. Pezzolesi et. al. Scott A. Summers. Rotten to the Cortex: Ceramide-Mediated Lipotoxicity in Diabetic Kidney Disease. Frontiers in Endocrinology. Volume 11.Published: 28 January 2021. doi:10.3389/fendo.2020.622692 structure of dkd
- Kimio Watanabe and Emiko Sato et.al. Eikan Mishima, Mariko Miyazaki Tetsuhiro Tanaka What’s New in the Molecular Mechanisms of Diabetic Kidney Disease: Recent Advances International Journal of Molecular Sciences Published: 29 December 2022 https://doi.org/10.3390/ijms24010570 molecular mechanism of dkd
- Rajiv Agarwal, MD, MS . Pathogenesis of diabetic nephropathy.
- James K Salem MD And Byron J . Hoogwerf, MD. Diabetic nephropathy strategies for preventing renal failure CLEVELAND CLINIC JOURNAL OF MEDICINE VOLUME 63 • NUMBER 6 OCTOBER 1996. www.ccjm.org
- Heshmatollah Shahbazian and Isa Rezaii. Diabetic kidney disease; review of the current knowledge. Journal of Renal Injury Prevention. Published on: 1 June 2013. Volume 2, Number 2. DOI: 10.12861/jrip.2013.24. http://journalrip.com. stages
- Stages of diabetic kidney disease
- file:///storage/emulated/0/Download/F9h6o8pWcAAM1uS (1).jpeg
- Diabetic Kidney Nephropathy. https://medtour.help/disease/diabetic-nephropathy/
- Diabetic nephropathy. https://my.clevelandclinic.org/health/diseases/24183-diabetic-nephropathy#symptoms-and-causes
- Causes of diabetic nephropathy. https://www.collidu.com/presentation-diabetic-nephropathy
- Merlin C. Thomas and Michael Brownlee et. al. Katalin Susztak, Kumar Sharma, Karin A. M. Jandeleit-Dahm, Sophia Zoungas, Peter Rossing, Per-Henrik Groop, Mark E. Cooper. Diabetic kidney disease. Published on: 30 July 2015. doi:10.1038/nrdp.2015.18. www.nature.com/nrdp. Risk factor and complications.
- Nicholas M Selby and Maarten W Taal. An updated overview of diabetic nephropathy: Diagnosis, prognosis, treatment goals and latest guidelines. Article in Diabetes Obesity and Metabolism. Published on: 13 February 2020. DOI: 10.1111/dom.14007. https://www.researchgate.net/publication/340509679.
- Diabetic nepropathy kidney disease . https://www.mayoclinic.org/diseases-conditions/diabetic-nephropathy/diagnosis-treatment/drc-20354562.
- https://www.shutterstock.com/image-vector/prevention-kidney-failure-infographic-useful-advices-1978161260 Prevention
- MichalHerman-Edelstein and Sonia Q. Doi, Pathophysiology of Diabetic Nephropathy.Proteinuria: Basic Mechanisms, Pathophysiology and Clinical Relevance.Proteinuria: Basic Mechanisms, Published on 04 February 2017 DOI 10.1007/978-3-319-43359-2_4. https://www.researchgate.net/publication/309304847. Treatment
- Devada Sindhu, MD , Gaurav Shekhar Sharma, MD, DM, Damodar Kumbala, MD, DM, FASS, FASDIN Management of diabetic kidney disease: where do we stand?A narrative review. Medicine (2023) 102:13 Accepted:6 March 2023. DOI: 10.1097/MD.0000000000033366
- Carmen Muntean, Iuliana Magdalena Starcea, Claudia Banescu.Diabetic kidney disease in pediatric patients: A current review.World Journal of W J D Diabetes. Published on : August 15,2022. Volume 13, Issue 8. DOI: 10.4239/wjd.v13.i8.587. Novel drug
- Na Wang and Chun Zhang et.al. Recent Advances in the Management of Diabetic Kidney Disease: Slowing Progression. International Journal of Molecular Sciences. Published: 7 March 2024. https://doi.org/10.3390/ijms25063086.


 Bhangare Suvarna Balu *
Bhangare Suvarna Balu *
 Ravindra S Lad
Ravindra S Lad










 10.5281/zenodo.12602883
10.5281/zenodo.12602883