Abstract
The OECD Guidelines for the Testing of Chemicals (TGs) are regularly reviewed in light of evolving assessment techniques, scientific advancements, and animal welfare issues. The TG 451 update has conducted concurrently with the Test Guidelines 452, Chronic Toxicity Studies (TG 452), and 453, Studies of combined chronic toxicity/carcinogenicity, with the aim of collecting more data from the study's animal subjects and supplying more information on the choice of dosage. The research assists in determining accumulating dangers, target organs and safety standards for exposure to humans.
Keywords
OECD, Carcinogenicity, Animal subjects, Safety standards.
Introduction
The OECD Guidelines for the Testing of Chemicals (TGs) are regularly reviewed in light of evolving assessment techniques, scientific advancements, and animal welfare issues. Adopted in 1981, Test Guideline 451 on Carcinogenicity Studies was initially developed. Creation of It was decided that TG 451 needed to be updated to reflect recent advancements in the sector of legal standards and animal welfare[1],[2],[3],[4],[5]. The TG 451 update has conducted concurrently with the Test Guidelines 452, Chronic Toxicity Studies, and 453, Studies of Combined Chronic Toxicity/Carcinogenicity, with the aim of collecting more data from the study's animal subjects and supplying more information on the choice of dosage. This test guideline is intended to be applied to a wide variety of chemical tests, such as those involving industrial and pesticide chemicals. However, it should be mentioned that specifics and specifications may vary for medications (see International Conference on Harmonization). (ICH) Guidance S1B on Pharmaceutical Carcinogenicity Testing). Most of the Studies on carcinogenicity are conducted on rodent species, and the purpose of this test guideline is to mostly apply to research done on these animals as a result. Are these studies supposed to be necessary in non-rodent animals, the guidelines and practices described here along Repeated Dose 90-day Oral Toxicity Study in Non-Rodents[6] should be used, with the necessary adjustments, in conjunction with those described in OECD TG 409.

The OECD Guidance Document No. 116[7] on the Design and Conduct of Chronic Toxicity and Carcinogenicity Studies[7] has more recommendations. In carcinogenicity investigations, oral, cutaneous, and inhalation are the three primary modes of delivery. The selection of the physical and chemical properties of the test determine the delivery method. Chemical and the main way that people are exposed to it. More details regarding the option Guidance Document No. 116[7] provides an outline of the exposure route. This recommendation is centered around oral exposure, which is the method most frequently employed in investigations on carcinogenicity. Although research on the carcinogenicity of exposure through the skin or respiratory pathways may also essential for evaluating the danger to human health and/or might be needed in accordance with specific regulatory regimes, there is a significant amount of technical complexity in both exposure pathways. These investigations will must be created on an individual basis, even though the Guidelines provided here for the Oral administration's examination and evaluation of carcinogenicity could serve as the foundation for a procedure for inhalation[7] and/or skin research, considering therapy suggestions time frames, pathology and clinical characteristics, etc. The OECD Guidance can be found on the application of test substances via the skin and respiratory systems. The OECD Guidance Document on acute inhalation testing[7],[8] and TG 412[9] and 413[10] should be specifically examined when designing longer-term studies incorporating exposure by inhaling. If testing is conducted, TG 410[11] should be consulted by the dermal[7] pathway. The carcinogenicity research offers details on potential health risks. Risks that could result from continuous exposure for a length of time that could extend up to the lifetime of the animal employed.
Information about the substance's hazardous effects, including its potential for cancer, will be provided by the study. It may also identify target organs and the probability of buildup. For hazardous effects, it can give an approximation of the no-observed-adverse-effect level; additionally, in the event of non-genotoxic carcinogens, which can be utilized to determine safety, for tumor responses standards for exposure to humans. The necessity of closely observing the animals' clinical conditions in order to It is also emphasized to get as much information as you can.
OBJECTIVES OF CARCINOGENICITY STUDIES:
The objectives of carcinogenicity studies covered by this test guideline include:
- The discovery of a chemical's carcinogenic characteristics, which leads to a rise in the frequency of neoplasms, a rise in the percentage of malignant neoplasms, or a decrease in the amount of time before neoplasms occur in comparison to concurrent control groups,
- Determining the target organ or organs that are cancerous;
- Determining the duration before neoplasms manifest;
- Determination of the dose-response relationship for tumors;
- Establishing a starting point or no-observed-adverse-effect level (NOAEL) for creation of a BMD, or benchmark dose;
- Applying carcinogenic effects to human exposure levels at low doses;
- Data sharing to test theories about the manner of action[2],[7],[12],[13],[14],[15].
INITIAL CONSIDERATIONS:
Before beginning a research to assess a chemical's potential carcinogenicity, the testing laboratory should take into account all available information about the test chemical. This will help to focus the study's design and enable more effective testing for carcinogenic potential and to reduce the use of animals. Details about and thought on the It's crucial to understand how a suspected carcinogen[2],[7],[12],[13],[14],[15] works. Given that the ideal design could vary based on whether the material is a known or a possible genotoxic carcinogen. Additional direction regarding mode of action considerations can be included in Document No. 116[7] of the Guidance.
The identity, chemical structure, and physical-chemical characteristics of the test chemical; the findings of any in vitro or in vivo toxicity tests, including genotoxicity tests; the planned use(s) and potential for human exposure; and the information that is currently available will all help with the research design. (Q)SAR data, carcinogenicity, mutagenicity/genotoxicity, and further toxicological data on structurally similar compounds; accessible toxicokinetic information (for both single and repeated doses) kinetics, if available), and information obtained from other experiments with repeated exposure. Evaluation of carcinogenicity should be conducted following the acquisition of preliminary data regarding toxicity.
from multiple dose toxicity experiments conducted over 28 or 90 days. Progression and commencement of short-term cancer Tests might potentially yield insightful data. When evaluating a chemical's possible negative health consequences, a phased testing strategy to carcinogenicity testing should be taken into account[16],[17],[18],[19]. Before the study is started, the statistical techniques best suited for the analysis of the data should be determined, taking into account the goals and design of the experiment. Among the things to think about are if the statistics need to account for survival and cumulative tumor hazards in relation to the length of survival, examination of the tumor's onset time, and analysis in the incident where one or more groups end prematurely. Advice on the suitable statistical The analysis and important sources of globally recognized statistical techniques are provided in 116[7] of the Guidance Document, as well as in Guidance Document No. 35 regarding the examination and assessment of research on carcinogenicity[20] and longterm toxicity.The guidelines and factors provided in the OECD Guidance Document No. 19 on the identification, evaluation, and application of clinical symptoms as humane endpoints for carcinogenicity research should be followed when performing a study. Particularly when it comes to safety evaluations[21], experimental animals should always be subsequently. The text reads as follows: "In experiments with repeated dosage, when an animal exhibits escalating clinical indications that cause the illness to worsen even worse, an The choice of whether or not to kill the animal humanely should be well-informed. The decision should take into account the importance of the knowledge that will be obtained from the ongoing care for the research animal in terms of its general health. In the event that it is decided to continue testing the animal, more frequent observations should be performed as needed. It might also be able to temporarily halt the test without having a negative impact on its goal. Dosage if it may lessen discomfort or agony, or cut back on the test dosage.” Both guidelines Document No.116[7] and two publications from the International Life Sciences Institute provide comprehensive guidelines[22],[23] and a discussion of the fundamentals of dosage selection for research pertaining to chronic toxicity and carcinogenicity. The primary method for choosing a dose is depending on the main goal or goals of the research. When choosing the right dosage levels, it's important to strike a balance between, on the one hand, danger screening and On the other hand, the relevance of low-dose reactions and their characterisation. In particular, relevant when a combined chronic toxicity and carcinogenicity research is conducted (TG 453) is to be executed. Rather than conducting separate studies for chronic toxicity (TG 452) and carcinogenicity (TG 451), consideration should be made to conducting a combined chronic toxicity and carcinogenicity research (TG 453). Greater efficiency is provided by the combination test. without sacrificing quality, in terms of time and expense when compared to carrying out two independent studies the level of data quality in the phases related to carcinogenicity or chronicity. Exercise caution But while doing a dose selection, thought should be given to the guidelines. study (TG 453) on the combined chronic toxicity and carcinogenicity, and it is acknowledged that Some regulatory frameworks may necessitate independent study. Terms utilized in the Guidance Document No. 116[7] contains the context for this test guideline.
PRINCIPLE OF THE TEST:
For the majority of the test animals' lives, the test chemical is given orally to multiple groups of animals at progressive doses every day. It may also be suitable to conduct testing via the skin or respiratory routes. The animals are continuously watched for any indications of toxicity and for the growth of cancerous lesions. Animals who pass away or are killed while being tested are animals are killed and necropsied when the test is over, and any animals that survive are also examined.
DESCRIPTION OF METHOD:
Selection of animal species:-

Fig. No. 3
The assessment and evaluation of rodent carcinogenicity is the main focus of this guideline. When information indicates that non-rodent species are more pertinent for predicting the effects on human health, then using them may be taken into consideration. The species selection needs to make sense. Although other rodent species, such as the mouse, may be preferred, the rat is the favored species utilized. Despite the potential limitations[24],[25],[26] of using mice in carcinogenicity testing usefulness, carcinogenicity testing in the present regulatory programs A mouse is still necessary. Mice and rats have traditionally been used as experimental models because of their extensive usage in toxicological and pharmacological research, their rather short lifespan research, how easily they can develop tumors, and the availability of enough strains that were characteristic. Because of these traits, a wealth of knowledge regarding their physiology and disease is accessible. Guidance Document No. 116[7] contains more details on the selection of species and strain. wholesome mature animals that are young It is best to use commonly used laboratory strains. The study on carcinogenicity ought to should ideally be conducted on animals that are of the same strain and origin as those utilized in preliminary, shorter-term toxicity study(ies), however, if animals from this strain and sources are recognized to pose challenges to meeting the commonly acknowledged survival standards. Regarding long-term research (see to Guidance Document No. 116)[7], thought should be given to choosing an animal strain suitable for the long-term research in terms of survival. The females ought to be devoid of children and nulliparous.
Housing and feeding:-

Fig. No. 4
Animals can be kept in small groups of the same sex or individually; individual housing should only be taken into consideration when it is supported by science[24],[25],[26]. It is important to arrange cages so as to minimize any potential impacts from their placement. The ideal temperature for the experimental animal chamber is 22°C (± 3°C). Even so, the relative Except in the room, humidity should be at least 30% and ideally not more than 70%. cleaning, 50–60% should be the goal. There should be artificial lighting with a 12-hour cycle.
12 hours of darkness, 12 hours of brightness. Traditional laboratory meals can be fed indefinitely. access to potable water. In addition to meeting the nutritional needs of the tested species, the food should be free of any dietary contaminants, such as pesticide residues, persistent organic pollutants, phytoestrogens, heavy metals, and mycotoxins. impact the test's result, should be kept to a minimum. analytical data regarding the levels of dietary contaminants and nutrients should be generated on a regular basis, preferably at the when the batch utilized changes at the start of the trial and should be included in the completed report. Analytical data about the study's drinking water should likewise be supplied. When a test chemical is given to animals by the food route, the need to guarantee an appropriate mixing of the chemical and to fulfill the animals' nutritional needs may impact the diet selection.
Preparation of animals:-
Fig. No. 5
Use of healthy animals that have not been exposed to prior experimental treatments and have been acclimated to laboratory conditions for at least seven days is recommended. When it comes to rats, the animals should start receiving doses as soon as they are weaned and acclimation, and ideally before to the pups' eight-week age. The test subjects ought to be described in terms of age, weight, sex, species, strain, and source. When the first of the The weight fluctuation of each sex of animal utilized in the study should be kept to a minimum, not to exceed ± 20. percentage of the average weight of every animal in the study, broken down by sex. The control and treatment groups should be randomly assigned to the animals. Following randomization, the mean body weights of the groups within each sex should not differ significantly from one another. If statistically significant differences exist, the randomization process must to be carried out once more. feasible. Every animal should have a special identifying number that is assigned to them permanently. tagged with this number through a microchip implantation, tattoo, or other appropriate technique.
PROCEDURE:
Number and sex of animals:-
It is best to use both sexes. It is important to employ a sufficient number of animals to provide a comprehensive biological and statistical analysis. For this reason, a minimum of 50 animals per sex should be present in each dosing group and concurrent control group. Depending on the study's objectives, It could be feasible to enhance the key estimates' statistical power by differential allocation animals disproportionately to the different treatment groups, with over 50 animals in the low dose group groupings, for example, to calculate the low-dose carcinogenic potential. It is important to acknowledge that even a slight increase in the group size will not significantly boost the study's statistical power. More details on the study's statistical design and selection In Guidance Document No. 116[7], dosage levels to maximize statistical power are given.
Provision for interim kills and satellite (sentinel) groups:-
If scientifically justifiable, the study may allow for interim kills, such as those at 12 months, to offer information on the evolution of neoplastic alterations and mechanistic details. When such data is already accessible due to earlier repeat dosage toxicity investigations on the in actuality, temporary killings could not be supported by science. If temporary killings are part of the study design, the quantity of animals slated for an intermediate death in each treatment group will typically consist of ten animals each sex, and the overall number of animals used in the research plan should be raised in accordance with the quantity of animals slated for slaughter prior to the the research. If necessary, a second group of sentinel animals—typically five animals per sex—may be added to the study[30] to monitor the status of the disease. Guidance Document No. 116[7] has more instructions.
Dose groups and dosage:-
Guidance Document No. 116[7] offers advice on every facet of dose selection and dose level spacing. It is recommended to use a contemporaneous control and a minimum of three dosing levels. Usually, the outcomes of shorter-term repeated dose or range finding will determine the dosage levels. research and ought to consider any relevant toxicological and toxicokinetic data. for the relevant ingredients or the test chemical. When choosing a dosage, the researcher ought to additionally Take into account and guarantee that the data produced is sufficient to meet the regulatory requirements throughout OECD nations as necessary (e.g., risk and hazard assessment, categorization and labeling, ED evaluation, etc.). When determining the primary target organs and toxic effects, the greatest dose level should be selected to prevent suffering, severe toxicity, morbidity, or death, unless restricted by the physical-chemical nature or biological effects of the test chemical. Considering the criteria listed below, it is usually best to use the greatest dosage level in order to elicit evidence of toxicity, as demonstrated, for instance, by a 10% increase in body weight that is depressed. However, depending on the study's goals, a maximum dose that is less than the dose that offers proof of toxicity, such as when a dosage causes an unfavorable consequence that raises concerns, may be selected. nonetheless, has no effect on body weight or lifetime. To determine a dose-response and, based on the test chemical's mode of action, a NOAEL or another desired study outcome, such as a BMD at the lowest dose, dose levels and dose level spacing may be chosen degree. When placing smaller doses, some factors to take into account include the anticipated the levels at which significant changes in the slope of the dose-response curve may occur in where a threshold is anticipated, where a point of hazardous action or metabolism deviation is anticipated for the low-dose extrapolation. The features of the test chemical will determine the dosage level spacing, which is not specified in this guideline. However, two to four fold intervals often yield satisfactory test performance for setting the falling dose levels, and adding a fourth test group is frequently better than using extremely wide intervals (such as more than a factor of around 6-10) between amounts. Generally speaking, using factors bigger than 10 should only be justified and avoided. if applied.
As elaborated upon in Guidance Document No. 116[7], factors must be taken into account in dosage selection consist of:
- Toxicokinetics, and dose ranges where metabolic induction, saturation, or nonlinearity between external and internal doses does or does not occur;
- Known or suspected nonlinearities or inflection points in the dose-response;
- Precursor lesions, effect markers, or clues regarding the functionality of important underlying biological mechanisms;
- Important (or suspected) elements of the method of action, like the starting doses for cytotoxicity to appear, hormonal imbalances occur, overburdening homeostatic processes, etc.;
- The dose-response curve regions where extra-rugged estimate is required, such as in the predicted BMD's range or a potential threshold;
- Taking expected human exposure levels into account.
If a vehicle is employed to administer the test chemical, the control group must be either the untreated group or the vehicle-control group. Animals in the control group should be treated in the same way as those in the test groups, with the exception of being given the test chemical. If a car When applied, the vehicle in the dose group with the largest volume used will be given to the control group. If a test chemical is added to the diet and results in a considerable decrease in dietary consumption because of the diet's decreased palatability, an extra pair-fed control group might be beneficial, acting as a better fit control.
Preparation of doses and administration of test chemical:
Usually, the test chemical is given orally, by gavage, through the food or drink. Guidance Document No. 116[7] contains more details on administration routes and techniques. The administration's path and technique are determined by the goal of the investigation, the test substance's physical and molecular characteristics, its bioavailability, and the principal pathway and means of human exposure. There should be an explanation given for the selected delivery route and administrative style. For the sake of the welfare of the animals, oral gavage Typically, only those agents who qualify for this route and technique of administration (such as medications) logically depict possible human exposure. Chemicals found in food or the environment, such as pesticides, are usually administered by drinking water or diet. But in certain cases, such as those involving occupational exposure, administration through alternate ways might be more suitable. The test chemical is suspended or dissolved in an appropriate vehicle as needed. In addition to other ingredients, if necessary, the following features of the car should be taken into account: impacts on the distribution, metabolism, absorption, or retention of the test chemical; impacts on the test chemical's chemical characteristics that could change its harmful features; as well as impacts on the amount of food or water consumed or the nutritional state of the creatures. It is advised to utilize an aqueous solution or suspension whenever feasible. be taken into consideration initially, then an oil-based solution or emulsion (such as corn oil), and then by a potential fix in different cars. If a vehicle is not a watercraft, the hazardous It is important to understand the vehicle's features. Data on the homogeneity of dosage solutions or diets (as appropriate) under the circumstances of administration (e.g., diet) and the stability of the test chemical should be accessible. For drugs consumed or consumed through diet water Making sure the amounts of the test chemical involved don't interact is crucial. with a regular diet or water equilibrium. In long-term dietary toxicity studies administration, the chemical's concentration in the meal shouldn't typically be higher than an 5% of the entire diet as a maximum, to prevent nutritional imbalances. When the test chemical is fed to the animal, either a fixed dose level expressed in terms of the animal's body weight (mg/kg body weight), or a constant dietary concentration (mg/kg diet or ppm), should be determined. could be employed once a week. A description of the substitute should be provided. When the test chemical is administered orally, the animals receive a daily dose (seven days per week), often for a 24-month duration in the case of rodents. Any additional dose schedule, such as five days a week, must be supported. When it comes to topical application, animals are typically exposed to the test chemical for the required minimum of six hours per day, seven days a week. for a duration of 24 months in TG 410[11]. The inhalation route of exposure lasts for six hours a day, seven days a week; however, exposure for five days a week may also be utilized, provided appropriate. See also TG 412[9]. Typically, there will be an exposure period of 24 months. Using a stomach tube or an appropriate intubation cannula, the test chemical should be given to the animals by gavage at regular intervals throughout the day. Typically, a single dose is given once a day; however, in cases when a substance, for instance, is a local irritant, it may be It is feasible to sustain the daily dosage rate by giving it in two separate doses each day. The largest amount of liquid that can be given at once depends on the test's size creature. The loudness ought to be maintained as low as is reasonably possible, typically not going above 1 ml/100g[31] of rodent body weight. In order to maintain a constant volume at all dosage levels, the concentration should be adjusted to minimize test volume variation. The exception is materials that may be caustic or irritating; they should be diluted to prevent adverse local effects. Assessing at levels that could potentially cause harm or irritation to the gastrointestinal system ought to be stayed away from.
Duration of study:-
For rodents, who make up the majority of the animals to be utilized in the study, the study will typically last 24 months. Depending on how long an animal strain in the study lives, several study lengths may be employed, but they must be justified. For a period of 18 months, certain mouse strains (such as AKR/J, C3H/J, or C57BL/6J strains) might be more suitable. The following offers some direction regarding the length and end of the investigation and survival; further advice, such as taking into account the acceptability of a The OECD Guidance provides negative carcinogenicity in relation to study survival. The Design and Conduct of Chronic Toxicity and Carcinogenicity Document No. 116[7] Research.
- When the percentage of survival in the control group or lower dose groups drops below 25%, the trial should be terminated.
- If toxicity causes only the high dose group to pass away too soon, this shouldn't cause the study to end.
- Each sex's survival should be taken into account independently.
- The investigation shouldn't be continued past the time when the study's data are no longer available. are no longer adequate to allow for the creation of a statistically sound assessment.
OBSERVATIONS:
Every animal should have its morbidity and mortality assessed, usually at the start and end of each day, including on weekends and public holidays. Additionally, animals should be examined once daily for particular indicators of toxicological significance, keeping in mind the peak time of expected consequences following gavage delivery in terms of dosage. Special consideration ought to be given to the growth of tumors; and the timing, location, and size of the tumors Every tumor that is palpably or grossly evident should have its appearance and progression documented.
Body weight, food/water consumption and food efficiency:-
Weighing should be done on every animal at the beginning of treatment, then every week for the first 13 weeks, and then every month after that. For the first 13 weeks, food consumption and food efficiency should be measured at least weekly; after that, measurements should be conducted at least monthly. Water For the first 13 weeks, consumption should be measured at least weekly, and then at least monthly. subsequently, when the drug is added to drinking water. usage of water Measurements should be taken into account for research where drinking behavior is changed.
Haematology, clinical biochemistry and other measurements:-
To optimize the data gathered from the investigation, particularly with mode of action considerations, blood samples may be extracted for haematology and clinical biochemistry, subject to the research director's judgment. A urinalysis can be necessary as well. Additional direction Guidance provides information on the need of collecting such samples as part of a carcinogenicity investigation. Referencing Document No. 116[7]: Blood sample for haematological and Urinalysis and clinical chemistry assessments may be carried out as part of an interim kill and on a minimum of 10 animals per sex per group at the end of the trial. Blood samples should be drawn from a designated location, such as the retro-orbital sinus while under anesthesia or by heart puncture, and kept, if relevant, in the right way. Smears of blood could additionally be ready for inspection, especially if the target organ seems to be the bone marrow. While the usefulness of such testing in determining a substance's propensity to cause cancer or other diseases has been called into doubt[32].
PATHOLOGY:-
Gross necropsy:-
A thorough, complete gross necropsy should be performed on every animal involved in the study, with the exception of sentinel animals and other satellite animals. This necropsy should involve a meticulous inspection of the body's exterior, all orifices, and the cranial, thoracic, and abdominal cavities, as well as their contents. On an individual basis, the study director may decide that necropsy is necessary for satellite animals and sentinel animals. Organ weights are typically not included in carcinogenesis studies because the relevance of organ weight data is muddled by aging changes and the later development of tumors. However, they might be essential for carrying out an assessment of the weight of the evidence, particularly when considering a course of action. Should they be a component of a satellite research, they ought to be gathered no later than a year following the study's start. The following tissues need to be kept in the best fixing media possible for both the kind of tissue and the planned histopathological analysis[33] that will follow (tissues in Square brackets are not required.):
Table No. 1
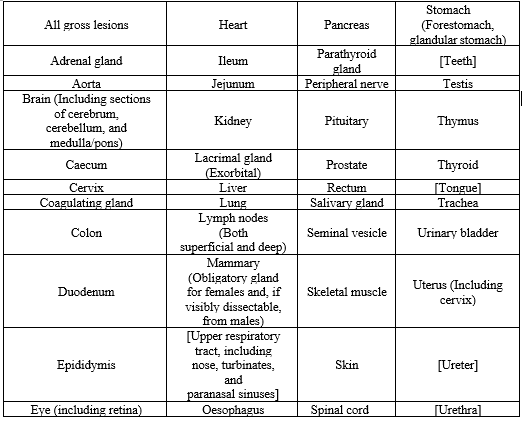
When two organs are coupled, such as the kidney and adrenal, both should be kept intact. Additional tissues may need to be examined, as suggested by the clinical and other results. Additionally, any organs that are thought to be target organs according to the test chemical's known qualities must to be maintained. The list of organs specified for the oral route should be maintained in studies using the dermal route of administration. It is also crucial to preserve and carefully sample the skin at the application site. The list of maintained and analyzed respiratory tract tissues for inhalation investigations should adhere to TG 412[9] and TG 413[10] guidelines.
Histopathology:-
There are guidelines available for doing toxicological pathology[33] investigations in the best possible way.
- All tissues from the high dose and control groups;
- All tissues from animals killed or dying during the study;
- All tissues exhibiting macroscopic abnormalities, including tumors;
- All animals in all other dose groups whose tissues exhibit treatment-related histopathological changes;
- All paired organs, such as the kidney and adrenal, should have both organs examined. These are the minimal tissues that should be examined.
DATA AND REPORTING:
Data :-
Data specific to each animal should be supplied for each parameter under consideration. All information should also be compiled into a table that lists the number of animals in each test group at the beginning of the experiment, the number of animals discovered deceased throughout the experiment, and the number of animals killed for humane the cause and timing of any fatalities or compassionate killings, the quantity exhibiting toxicological symptoms, a description of the toxicity indicators seen, including the intensity, duration, and time of any harmful effects, the quantity, kind, and percentage of animals exhibiting lesions of animals with every kind of lesion. In addition to the grading of lesions, summary data tables should provide the means and standard deviations (for continuous test data) of animals exhibiting harmful effects or lesions. In certain situations, such as when there are signs that the data from the concurrent controls are significantly out of line when compared to more recent data from control animals from the same test, historical control data may be helpful in interpreting the study's findings. establishment/colony. If analyzed, historical control data ought to be provided from the same
laboratory and connect to creatures of the same age and strain produced over the course of five years. before the relevant study. When appropriate, numerical outcomes ought to be assessed by an suitable and widely acknowledged statistical technique. The data and statistical techniques to be examined ought to be chosen throughout the study's design. Selection ought to include provisions. for any necessary adaptations for survival.
Test report :-
The following details must to be included in the test report:
Test chemical:
- Physical nature, purity, and physicochemical properties;
- Identification data;
- Source of substance;
- Batch number;
- Certificate of chemical analysis;
Vehicle (if appropriate):
- An explanation of the vehicle selection (if not a water vehicle);
Test animals:
- The animals' number, age, and sex at the beginning of the test;
- The species or strain employed and the rationale behind the selection;
- The source, the housing circumstances, the food, etc.;
- The weights of the animals individually before the test begins;
Test conditions:
- Justification for dosage and route of administration; - Data analysis techniques, if appropriate; - Specifics of test chemical formulation and diet planning.
- analytical information regarding the preparation's homogeneity, stability, and attained concentration;
- Directions for administering the test chemical, including specifics;
- For inhalation experiments, using the entire body or just the nose;
- Real dosages (mg/kg body weight/day), as well as the conversion factor derived from the drinking water/diet test chemical concentration, if appropriate, in milligrams per kilogram or parts per million;
- Information about the quality of food and water;
Results (summary tabulated data and individual animal data should be presented).
General:
- Data on survival;
- Body weight and weight fluctuations;
- Food intake, including any food efficiency calculations;
- Water consumption, if any relevant;
- If accessible, toxicokinetic information;
- Opthalmoscopy, assuming it is accessible;
- Haematology, assuming it is accessible;
- Clinical chemistry, if it's accessible;
Clinical findings:
- Toxicological symptoms;
- The frequency and, if scored, the degree of any anomaly;
- The kind, intensity, and length of clinical observations (whether temporary or long-term);
Necropsy data:
- Body weight at death;
- Organ weights and ratios, if relevant;
- Results of necropsy;
- The frequency and gravity of anomalies;
Histopathology:
- Correlation between macroscopic and microscopic findings;
- Non-neoplastic histopathological findings;
- Neoplastic histopathological findings;
- A thorough explanation of every treatment-related histopathology result, taking into account its severity ratings
- A report on any slide peer review;
Statistical treatment of results, as appropriate.
- Discussion of results including:
- A discussion of modeling methodologies;
- Relationships between dose and response;
- Historical control data;
- Taking into account information on any mode of action, such as BMD, NOAEL, or LOAEL determination; Human relevance;
CONCLUSION
To sum up, the goal of the updated OECD Test Guideline 451 and associated guidelines is to improve the evaluation of studies on chronic toxicity and carcinogenicity by including more specific details on dosage selection and possible health risks from repeated exposure. The research assists in determining accumulating dangers, target organs, and safety standards for
contact with non-genotoxic carcinogens in humans.
REFERENCES
- OECD (1995), Report of the Consultation Meeting on Sub-chronic and Chronic Toxicity/Carcinogenicity Testing (Rome, 1995), internal working document, Environment Directorate, OECD, Paris.
- EPA (2005). Guidelines for Carcinogen Risk Assessment Risk Assessment Forum U.S. Environmental Protection Agency Washington, DC http://cfpub.epa.gov/ncea/cfm/recordis pl ay.cfm?deid=116283&CFID=1267360&CFTOKEN=65052793&jsessionid=9830b2c4116e3 d8fbbf0174 14e1 a782e7f79TR
- Combes RD, Gaunt, I, Balls M (2004). A Scientific and Animal Welfare Assessment of the OECD Health Effects Test Guidelines for the Safety Testing of Chemicals under the European Union REACH System. ATLA 32, 163-208.
- Barlow SM, Greig JB, Bridges JW et al (2002). Hazard identification by methods of animal based toxicology. Food. Chem. Toxicol. 40, 145-191.
- Chhabra RS, Bucher JR, Wolfe M, Portier C (2003). Toxicity characterization of environmental chemicals by the US National Toxicology Programme: an overview. Int. J. Hyg. Environ. Health 206, 437-445.
- OECD (1998), Repeat Dose 90-day Oral Toxicity Study in Non-Rodents, Test Guideline No. 409, OECD Guidelines for the Testing of Chemicals, OECD, Paris.
- OECD (2009), Draft Guidance Document on the Design and Conduct of Chronic Toxicity and Carcinogenicity Studies, Series on Testing and Assessment No. 116, available on the OECD public website for Test Guideline at www.oecd.org/env/testguidelines.
- OECD (2009), Guidance Document on Acute Inhalation Toxicity Testing, Series on Testing and Assessment No. 39, ENV/JM/MONO(2009)28, OECD, Paris.
- OECD (2009), Subacute Inhalation Toxicity: 28-Day Study, Test Guideline No. 412, OECD Guidelines for the Testing of Chemicals, OECD, Paris.
- OECD (2009), Subchronic Inhalation Toxicity: 90-Day Study, Test Guideline No. 413, OECD Guidelines for the Testing of Chemicals, OECD, Paris.
- OECD (1981), Repeated Dose Dermal Toxicity: 21/28-day Study, Test Guideline No. 410, OECD Guidelines for the Testing of Chemicals, OECD, Paris.
- Boobis, A.R., Cohen, S.M., Dellarco, V., McGregor, D., Meek, M.E., Vickers, C., Willcocks, D. & Farland, W. IPCS Framework for analyzing the Relevance of a Cancer Mode of Action for Humans. Crit. Rev. in Toxicol, (2006) 36:793-801.
- Cohen, S.M., Meek, M.E., Klaunig, J.E., Patton, D.E., and Fenner-Crisp, P.A. (2003) The human relevance of information on carcinogenic Modes of Action: An Overview. Crit. Rev. Toxicol. 33:581-589
- Holsapple, M.P., Pitot, H.C., Cohen, S.N., Boobis, A.R., Klaunig, J.E., Pastoor, T., Dellarco, V.L. & Dragan, Y.P. (2006) Mode of Action in Relevance of Rodent Liver Tumors to Human Cancer Risk. Toxicol. Sci. 89:51-56.
- Meek, E.M., Bucher, J.R., Cohen, S.M., Dellarco, V., Hill, R.N., Lehman-McKemmon, L.D., Longfellow, D.G., Pastoor, T., Seed, J. & Patton, D.E. A Framework for Human Relevance analysis of Information on Carcinogenic Modes of Action. Crit. Rev. Toxicol. (2003). 33:591-653.
- Carmichael NG, Barton HA, Boobis AR et al (2006). Agricultural Chemical Safety Assessment: A Multisector Approach to the Modernization of Human Safety Requirements. Critical Reviews in Toxicology 36, 1-7.
- Barton HA, Pastoor TP, Baetcke T et al (2006). The Acquisition and Application of Absorption, Distribution, Metabolism, and Excretion (ADME) Data in Agricultural Chemical Safety Assessments. Critical Reviews in Toxicology 36, 9-35.
- Doe JE, Boobis AR, Blacker A et al (2006). A Tiered Approach to Systemic Toxicity Testing for Agricultural Chemical Safety Assessment. Critical Reviews in Toxicology 36, 37- 68.
- Cooper RL, Lamb JS, Barlow SM et al (2006). A Tiered Approach to Life Stages Testing for Agricultural Chemical Safety Assessment. Critical Reviews in Toxicology 36, 69-98.
- OECD (2002), Guidance Notes for Analysis and Evaluation of Chronic Toxicity and Carcinogenicity Studies, Series on Testing and Assessment No. 35 and Series on Pesticides No. 14, ENV/JM/MONO(2002)19, OECD, Paris.
- OECD (2000), Guidance Document on the recognition, assessment, and use of clinical signs as humane endpoints for experimental animals used in safety evaluation, Series on Testing and Assessment No. 19, ENV/JM/MONO(2000)7, OECD, Paris.
- Rhomberg, LR, Baetcke, K, Blancato, J, Bus, J, Cohen, S, Conolly, R, Dixit R, Doe, J, Ekelman, K, Fenner-Crisp, P, Harvey, P, Hattis, D, Jacobs, A, Jacobson-Kram, D, Lewandowski, T, Liteplo, R, Pelkonen, O, Rice, J, Somers, D, Turturro, A, West, W, Olin, S. Issues in the Design and Interpretation of Chronic Toxicity and Carcinogenicity Studies in Rodents: Approaches to Dose Selection Crit Rev. Toxicol. 37 (9) 729 - 837 (2007).
- ILSI (International Life Sciences Institute) (1997). Principles for the Selection of Doses in Chronic Rodent Bioassays. Foran JA (Ed.). ILSI Press, Washington, DC.
- Griffiths SA, Parkinson C, McAuslane JAN and Lumley CE (1994) The utility of the second rodent species in the carcinogenicity testing of pharmaceuticals. The Toxicologist 14(1):214.
- Usui T, Griffiths SA and Lumley CE (1996). The utility of the mouse for the assessment of the carcinogenic potential of pharmaceuticals. In D'Arcy POF & Harron DWG (eds). Proceedings of the Third International Conference on Harmonisation. Queen's University Press, Belfast. pp 279-284.
- Carmichael NG, Enzmann H, Pate I, Waechter, F (1997). The Significance of Mouse Liver Tumor Formation for Carcinogenic Risk Assessment: Results and Conclusions from a Survey of Ten Years of Testing by the Agrochemical Industry. Environ Health Perspect 105:1196- 1203
- EEC Council Directive 86/609/EEC on the approximation of laws, regulations, and administrative provisions of the Member States regarding the protection of animals used for experimental and other scientific purposes. Official Journal 29, L358, 18 December 1986.
- National Research Council, 1985. Guide for the care and use of laboratory animals. NIH Publication No. 86-23. Washington, D.C., US Dept. of Health and Human Services.
- GV-SOLAS (Society for Laboratory Animal Science, Gesellschaft für Versuchstierkunde, 1988). Publication on the Planning and Structure of Animal Facilities for Institutes Performing Animal Experiments. ISBN 3-906255-04-2. http://www.gv-solas.de/publ/heft1_1988.pdf
- GV-SOLAS (Society for Laboratory Animal Science, Gesellschaft für Versuchstierkunde, 2006). Microbiological monitoring of laboratory animals in various housing systems. http://www.gv solas.de/auss/hyg/hyg-p7_e.html
- Diehl K-H, Hull R, Morton D, Pfister R, Rabemampianina Y, Smith D, Vidal J-M, van de Vorstenbosch C. 2001. A good practice guide to the administration of substances and removal of blood, including routes and volumes. Journal of Applied Toxicology, 21:15-23. Available at: http://www.ff.up.pt/farmacologia/pdf/good_practice_lab_animals.pdf
- Weingand, K., et al. 1996. Harmonization of Animal Clinical Pathology Testing in Toxicity and Safety Studies. Fund. Appl. Toxicol. 29: 198-201.
- Crissman, J., Goodman D., Hildebrandt P., et al. (2004). Best Practices Guideline: Toxicological Histopathology. Toxicologic Pathology 32, 126-131


 Aniket Dattatraya Takale*
Aniket Dattatraya Takale*
 Dr. Ajay Y. Kale
Dr. Ajay Y. Kale
 Dr. Kishor V. Otari
Dr. Kishor V. Otari





 10.5281/zenodo.13737920
10.5281/zenodo.13737920