Abstract
A key component of contemporary business is packaging, which includes the art and science of encasing goods for consumer appeal, protection, and communication. This succinct description offers a quick review of packaging's crucial position in the current business environment, highlighting its variety of uses, material advancements, and environmental issues. Packaging has various critical responsibilities and acts as a vital link between producers and consumers. It offers product protection, making sure that goods are delivered to customers in the best possible condition. Additionally, packaging conveys important information like product specifics, usage guidelines, and safety cautions. It also helps with marketing and branding, influencing consumer decisions and promoting brand identification. Modern society has many different uses for packaging, which is frequently referred to as a product's silent ambassador. Packaging has various critical responsibilities and acts as a vital link between producers and consumers. It offers product protection, making sure that goods are delivered to customers in the best possible condition. This abstract explores the crucial nexus between sustainability and packaging. This abstract scrutinizes the environmental consequences of packaging choices and underscores the importance of adopting circular economy practices to mitigate the environmental footprint of packaging materials, including recycling and waste reduction. This abstract examines the contributions of cutting-edge packaging technologies to increased sustainability and efficiency, including eco-friendly materials, intelligent packaging, and automation.
Keywords
Packing, Container, pharmaceutical products, delivery, storage.
Introduction
A pharmaceutical packaging container is a device designed to contain pharmaceutical products, and its contact with the product may vary. It is imperative that the container exhibits stability when intended for pharmaceutical applications1. Packaging encompasses the expertise, scientific principles, and technological practices involved in encapsulating and safeguarding products intended for distribution, storage, sale, and utilization. Because it facilitates the movement of products from production facilities to consumers and clients, packaging holds a crucial role in the business sphere2. Pharmaceutical packaging plays a vital role in preserving the quality of pharmaceutical products throughout their storage, transportation, delivery, sale, and usage3. It serves the purpose of enclosing or safeguarding pharmaceutical items, ensuring their safe delivery to patients. To guarantee the presence of a secure and efficient dosage form until the products' expiration date, it is crucial to highlight that packaging maintains the integrity and excellence of pharmaceutical items while safeguarding them from any form of deterioration or tampering4. Furthermore, packaging must provide safeguards against physical harm, material or ingredient loss, and the infiltration of undesirable environmental factors, such as oxygen, water vapor, and light5.
Despite decades of government engagement and industry efforts to promote eco-friendly packaging alternatives, there is still a rising trend in packaging waste volumes in various regions across the globe6,7. While effective waste management systems for packaging can mitigate the adverse environmental impacts of packaging waste, challenges such as the proliferation of ocean garbage patches on a global scale continue to persist8, 9. Considering that approximately one-third of the food produced globally is estimated to go to waste10, Packaging that reduces food waste has significant social and environmental implications11. Food packaging developers now have to take into account a number of, sometimes conflicting, Environmental packaging demands add to an already intricate mix of marketing, logistics, and production prerequisites. Reducing packaging and creating recyclable packaging solutions have been longstanding focal points for the packaging development sector, there are now additional environmental packaging requirements that must be taken into consideration11.There are research projects to broaden our understanding of the function of packaging in preventing food waste12 For the environmental optimisation of packaging, a number of tools and models have been proposed.13,14 yet it's uncommon to do an integrative analysis of product-packaging pairings15 Even though the food industry has actively engaged in various high-level initiatives aimed at fostering the development of more sustainable packaging 16,17 research has also shown that environmental factors are only partially taken into account while developing packaging on a daily basis18. When a novel chemical or biologic entity is discovered and new packaging is created to help the product get through regulatory approval and eventually reach the market, now is the perfect time to upgrade and improve the materials used in pharmaceutical packaging19. Depending on its purpose and the type of substance utilised, a particular type of pharmaceutical packaging may be employed. Last but not least, all packing materials must be examined through testing of chosen materials, sterilisation, storage, and stability studies20, 21.
Packaging:
Any substance utilized to encompass or enclose a product from the moment of its production until its ultimate utilization is termed packaging material 22, 23. The fundamental roles of packaging materials include the presentation and safeguarding of products. Packaging serves to protect the product throughout storage, preventing the loss of color, flavor, or odor, both during transit and use. Furthermore, packaging enhances ease of handling and product adherence 23, 24. Packaging is made up of a variety of distinct parts that are connected to a pharmaceutical product from the time it is produced until it is used25. Additionally, packaging makes contributions to emerging science, which includes design engineering26. It shouldn't interact with any excipients used in the preparation's formulation or the active ingredient27.
FUNCTION OF PACKAGING:
- Barrier Protection: Its purpose is to shield the product from detrimental external elements that could alter its attributes, encompassing moisture, light, oxygen, and temperature variations. The use of blister packaging can give this kind of protection.
- Physical Protection: It is intended to guard against physical injury to pharmaceutical dosage forms.
- Identification: It is intended to identify the product28.
- Protection against Light: To stop product photo-degradation, containers that are opaque or amber-colored might be utilised.
- Protection Against Compression: Product protection against compression is aided by secondary packing. To create additional packs, cardboard is utilised.
- Protection Against Impact: Impact action causes the product to drop. The primary pack can be cushioned and protected from impact damage by being placed within the secondary pack29-33.
- Desirable Characteristics of a Pharmaceutical
Packaging:
- Alkali shouldn't bleed into the contents.
- The container shouldn't permit the growth of mould.
- The container must withstand the heat when being sterilised.
- The container shouldn't absorb the contents of the container.
- The container should be constructed from neutral or inert materials.
- There must be no interaction between any element of the container or closure.
- It should provide the desired level of environmental protection 34, 35.
Types of Packaging Materials:
The primary and secondary packaging of pharmaceutical products represents a critical stage in the value-added process and is intricately linked to their manufacturing. The quality control department has been responsible for overseeing the regulation of pharmaceutical packaging materials, this entails the receipt, sampling, and testing of these materials to assess their acceptability or non-acceptability as containers and closures for drug products 36.
Primary Packaging:
The component of the packaging that comes into direct contact with the dosage form is termed a primary packaging component37.
Secondary Packaging:
This packaging's main purpose is to group all of the primary packages together. This includes items such as boxes, cartons, injection trays, and shipping containers38. To ensure efficient wrapping, the use of multiple attachments and heat tunnels is necessary39.
Tertiary Packaging:
This kind of packaging makes it easier to ship and handle the product in quantity. Some examples are containers, barrels, and edge guards 40.
Table No. 1. Table illustrating various primary and secondary packaging materials:
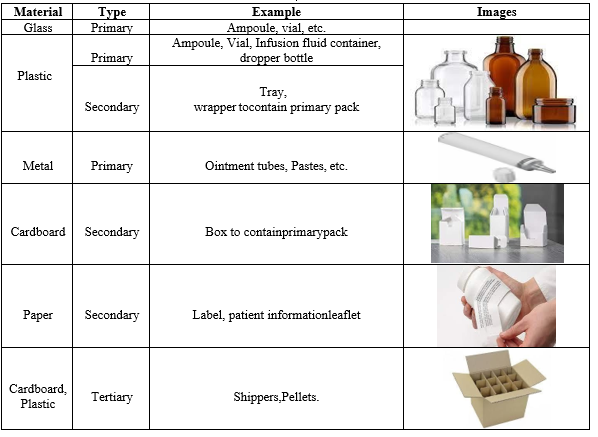
Materials used For Packaging:
Primary Package:
- Plastic
- Glass
- Metal
- Plastic:
Polycondensation or addition polymerization of monomer units is the process employed in the production of plastics41. Plastic constitutes 20% of the total packaging weight. As plastic coatings and packaging materials are often identified as major environmental concerns, there is a current phase of technological advancement aimed at exploring innovative approaches to decrease the environmental footprint of packaging42-46. Plastic stands out as one of the most versatile material categories utilized in packaging due to its ability to fulfill a wide range of requirements47. Polyolefin, polyester, polyvinyl chloride, polyvinylidene chloride, polystyrene, polyamide, and ethylene vinyl alcohol are among the plastics employed for food packaging. While more than 30 distinct plastic types have been utilized for packaging purposes, polyolefins and polyesters are the most commonly employed41.
In the market, two primary categories of biodegradable plastics exist: hydro-biodegradable plastics (HBP) and oxo- biodegradable plastics (OBP)48.
Materials employed in the production of plastic containers:
- Polyethylene:
One particularly favored polymer is polyethylene, available in various grades, including high density, medium density, and low density. In pharmaceutical applications, high-density polyethylene is predominantly used. It provides effective moisture protection but offers limited defense against oxygen and other environmental gases. Containers crafted from polyethylene cannot undergo sterilization through heat, however autoclaving them is a simple process. The melting point range for polyethylene is 120 to 150 oC. It has exceptional chemical resistance, which means it can withstand powerful acids and alkalis without harm. It is used to prepare plastic bottles, films, bags, and other items.
- Polypropylene:
It is a highly common material for pharmaceutical containers. It has many qualities of polyethylene. It has a greater melting point than polyethylene at 170 oC, making it acceptable for sterilisation at high temperatures. It provides good resistance to almost all chemical types. It cannot be compared to low density polyethylene.
- Polyvinylchloride (PVC):
The oxygen barrier is effective, and the clarity is superb. Immediately following polyethylene and polypropylene, it is the third most common polymer produced. The heat resistance of PVC is notably low, with a melting point of 160°C. While it is less heat-resistant than polyethylene and polypropylene, it offers good insulating qualities. PVC can be used to create catheters, bypass sets, hemodialysis sets, blood and blood component containers, and other items. The key benefits of PVC are its biocompatibility, low weight, softness, appropriateness for sterilisation, and transparency 49-53.
Advantages of Plastic Packaging Material:
- The ease of manufacture, the availability of a wide range of quality, the freedom of design, and the exceptional breaking resistance.
- The shape, size, and style of plastic packaging may be customised by manufacturers to meet the needs of their customers because it is a very flexible and adaptable type of packaging54.
Disadvantages of Plastic Packaging Material:
- The primary drawback is penetration. Environmental gases, vapours, or liquids from the immediate vicinity can easily travel into plastic containers.
- Another issue is sorption, which is the process by which the packaging material removes one or more elements from the product. This may reduce the product's ability to treat conditions 55,56,57.
- Glass
- Glass constitutes 20% of the total packaging weight. It is considered environmentally friendly due to the abundance of its raw materials in nature. It can be easily reused and recycled to produce new containers, thereby reducing its negative environmental footprint. The United States Food & Drug Administration has classified only glass packaging as "GRAS," which stands for generally recognized as safe58, 59, 60, 61. Glass is commonly employed as a means of environmentally responsible packaging for pharmaceuticals. Pharmacopoeias classify different kinds of glasses according to their use and chemical properties62. Tinplate: Administration of low carbon steel is used to make tinplate.63,64.
Manufacturing Process of glass :
It primarily consists of four steps.
- Blowing:
The molten glass is formed in a mould cavity using compressed air.
- Drawing:
Soft glass is shaped in this by having molten glass dragged through dies. Ampules and vials are among the containers created using this procedure.
- Pressing:
In this, molten glass is pressed or forced against a mold's ride using mechanical force.
- Casting:
To start the creation of molten glass in the hollow, for example, one could employ gravity or centrifugal force.
Types of Glass:
Type I Glass: Borosilicate Glass (Pyrex):
This type of glass is composed of boric oxide, aluminum oxide, and alkali or alkaline earth oxides. Glass containers for pharmaceutical preparations, except for type I glass containers, should not be reused. It is very resistant to hydrolysis and thermal shock. It is primarily used to prepare laboratory glass equipment, injection containers, and injection water.
Type II Glass: De-alkalized Soda-Lime Glass:
It contains higher levels of sodium and calcium oxide. Compared to type I glass, it has a lower level of leaching resistance, but type III glass has a higher level of resistance. It is used to keep plasma, alkali-sensitive goods, and infusion fluids in storage.
Type III: Standard Soda-Lime Glass:
This type of glass consists of earth oxides, primarily calcium oxides, sodium oxides, aluminum, and metal oxides. It is utilised to hold liquid compositions that are resistant to alkalis. Additionally, it is employed to keep all forms of solid dosage forms.
Advantages of Glass Packaging Material:
- It is simple to label them.
- They are readily sealable, allowing for hermetic sealing.
Disadvantages of Glass packaging Material:
- Due of their considerable weight, their transportation costs are higher.
- They can be broken55, 65, 66.
- Metal:
The most adaptable pharmaceutical packaging material is metal. "These materials offer excellent physical protection, malleability, long-lasting performance, decorative possibilities, appeal to consumers, and are environmentally friendly". They also have great barrier qualities67,68. Metals are frequently employed in pharmaceutical container packing. The primary metals frequently employed for this purpose encompass aluminum, lead, tin, and comparable metallic elements.
Benefits of using metals:
- They are less heavy than glass.
- They are powerful.
- They are gas, light, and moisture resistant.
Drawbacks of metals:
- They could produce harmful products if certain medications or chemicals react with them.
- They tend to come at a higher cost compared to plastic.
Material used for making metal containers
- Aluminum:
Due of its light weight and sophisticated appearance, aluminum is very frequently employed. Compared to tin, they are less expensive. Aluminum is protected from further oxidation by the transparent oxide coating that forms on its surface when oxygen reacts with it, but some Corrosion can occur when complexing agents or substances with either high or low pH levels interact with the oxide coating. Aluminum finds application in the production of items such as ointment containers, pill and capsule packaging, and screw closures.
- Tin:
It comes at a higher cost compared to other metals utilized in the manufacturing of pharmaceutical containers. It is resistant to chemical corrosion. It possesses an attractive appearance and remains chemically inert. It is used in the production of food containers.
- Lead:
It is more affordable than all other metals. Its nature is kind. To make lead harder, antimony is typically added at a rate of 3%. The primary drawback of lead is the potential for lead poisoning if consumed internally.
Varieties of metal containers:
- Collapsible Tubes:
They are crafted from materials like lead, tin, or aluminum. They're utilised to package preparation that's semisolid. Creams and dental paste are dispensed through aluminium tubes. Creams and dental paste are dispensed through aluminium tubes.
- Metal containers for solid dosage forms:
This mostly involves the usage of aluminium. Consequently, the capsule and tablet containers are sufficiently sturdy and light in weight.
- Metal Foil:
These foils serve the purpose of packaging individual pessaries or suppositories. For this, primarily aluminium foil is utilised. Strip and blister packaging of pills and capsules both employ metal foil69, 70.
Secondary Package:
Primary packages containing pharmaceutical preparations are kept in secondary packages. Primary package and secondary package are in direct contact, however pharmaceutical preparations are not. Shippers or cartons that provide the primary package with physical protection are typically seen in the secondary package. These are made from wood-derived cellulose fibres.
- Paper and paperboards.
- Rubber.
- Closures.
1. Paper and paperboards:
Paperboards, which have more weight and thickness compared to paper, are also employed as secondary packaging. White board, solid board, chipboard, and fiberboard are some instances of paperboards. Polyethylene or waxes are primarily used to laminate these boards. Recycled paper is used to make chipboard. Solid boards that are laminated with polyethylene include bleached sulphate boards.71, 72. A network of cellulose fibres derived from wood are woven together to form paper and paperboard. When used as primary packaging, paper is always treated with waxes, resins, laminates, or lacquers to enhance its protective and functional qualities.73, 74, 75, 76. Paperboard is thicker than paper and consists of multiple layers, resulting in a high weight per square inch due to the amalgamation of these characteristics. Paperboards can be classified as:
- Solid Board:
Bleached sulphate board is present in several layers. It is used to produce liquid cartons, commonly referred to as milk board, through the process of lamination with polyethylene.
- White Board:
It is composed of numerous layers of chemically bleached pulp. When wax is applied to the surface or when it is laminated with polyethylene for heat sealing, it serves as the primary packaging material. Furthermore, the inner layer of a carton can also consist of white board paper.
- Chipboard:
The outer layers of the cartons are made from recycled paper and are reinforced with white board lining to enhance both aesthetics and durability.
- Fiberboard:
There are two variants: solid and corrugated. Solid board features an outer layer of kraft and an inner layer of white board. In contrast, corrugated type comprises two layers of kraft paper bonded together with a central corrugating (or fluting) material. It is well-suited for product packaging during shipping due to its remarkable resistance to impact and abrasion77. Paper is the naturally existing material that can be recycled the most, thus it makes sense to use it for environmentally friendly pharmaceutical packaging78.Paperboard materials are frequently employed in the manufacture of corrugated boxes for secondary pharmaceutical packaging63, 79, 80.
2. Rubber:
Latex, which is found in some plant secretions, is used to make rubber. It serves as closures in drug packing.81, 82. It is employed in the creation of vial closures, fluid transfusion bottle closures, etc. Butyl rubber is widely used because it has a low absorption rate and because it is less expensive than other synthetic rubber, although it decomposes at temperatures exceeding 130oC. Water permeability & absorption are extremely low in silicon rubbers, which are also quite stable. But the price is exorbitant. A base polymer and additives are combined to create rubber. The rubber being manufactured is in a non- vulcanized state.
Ingredients employed in the preparation of rubber:
- Fillers:
There exist two distinct categories of filler material. For example, reinforce Other materials, such as carbon black, are not reinforced. calcareous carbonate. Due to the widespread use of carbon black, Most rubber products tend to be of a black color.
- Plasticizers:
These substances are utilized to improve the flow characteristics of rubber throughout its processing, including the incorporation of oils and paraffins.
- Vulcanizing agent:
Crosslinking is introduced using vulcanizing chemicals. such vulcanizing agents include: sulphate, etc.
- Accelerators:
Reducing the sulfur content, these compounds are employed to accelerate the crosslinking process and enhance the properties of the vulcanized product. Thiazoles serve as a notable illustration.
- Activators:
These substances initiate the vulcanization process. Notable examples include zinc oxide and stearic acid83.
3. Closures:
An effective sealing mechanism helps to safeguard the product from environmental contamination, minimize material loss from the container, and prevent the intrusion of microorganisms.
Desirable characteristics of a closure:
- It should exhibit non-reactive properties.
- It should be economically efficient.
Design of closures:
There are five primary designs of closures available. These are the specifics.
- Threaded Screw Type:
They are made from materials such as plastic, tin, or aluminum. These kinds of closures offer the product an efficient seal that safeguards it from physical and chemical reaction. Due to their resistance to corrosion, plastic caps are more common than metal ones.
- Crown Caps:
These metal caps are frequently used on beverage bottles. These also offer a strong seal and are impossible to open with hands. They cannot be sealed once more.
- Roll on closures:
The roll-on closure includes an aluminum cap that can be easily sealed, opened, and reclosed. These come in forms that are resealable, non-sealable, and pilfer proof 65, 84, 85.
PHARMACEUTICAL PACKAGES :
Various drug-holding containers are included in pharmaceutical packaging. Containers can be grouped based on factors such as shape and closure method 86. Containers are categorized based on their closure and usage into various groups, including tightly sealed, airtight, hermetically sealed, light-resistant, single- dose, multidose, and aerosol containers 87. The containers and other pharmaceutical packaging materials will be covered in the section after this one.
- Well-Closed Container:
They stop unwanted foreign particles from getting inside and contaminating the product. The loss of contents during use is also prevented by tightly-closed containers88. In standard storage, handling, and transportation environments, a tightly closed container safeguards its contents against contamination by external liquids and solids while also preventing content loss.
- Airtight Container:
Containers with an airtight seal guarantee that the container will remain airtight after being reclosed. Airtight containers maintain its ability to keep out solids, liquids, and gases during routine usage and storage. Under standard conditions of storage, handling, and transportation, an airtight container is impermeable to liquids, solids, and gases, effectively preventing the passage of these substances89.
- Single-Dose Container:
Single-dose containers, such as ampoules or vials, are utilized to package products intended for one-time use. A container specifically designed to hold a drug quantity intended for single-dose administration is termed a unit dose container, as it cannot be reused. After opening the container, the medication must be used right away90.
- Multidose Container:
A multidose container refers to a container designed for the sterile delivery of parenteral drugs through infusion or injection. In a multiple-dose container, more than one dose of a drug product is expected to be included91.
- Ampoules:
Ampoules are thin-walled glass containers. These closures can be sealed either by employing the pull method or the tip approach and are intended for single-dose usage. A variety of ampoules in the 0.50–50 mL range can hold various volumes of formulation92.
EVALUATION OF PACKAGING MATERIALS:
- Plastic Container.
- Metal Container for Eye Ointments.
1. Plastic Containers:
- Leakage Test:
Ten plastic bottles are to be equipped with closures and then filled with water. After that, they are kept at room temperature for 24 hours inverted. There must not be any indication that the containers are leaking93.
- Collapsibility Test:
The containers that must be squeezed to release the substance are subjected to a collapsibility test. When crushed inward at room temperature, the container must yield at least 90% of its contents and remove the material at a specified flow rate94.
- Transparency Test:
Suspension is put into five empty containers to its nominal capacity. Then, these containers are contrasted with the water-filled containers95.
- Metal Containers for Eye Ointments:
Metal collapsible tubes must pass this test in order to be approved for use with metal particles. The ointment base should be placed inside the empty tubes. After that, it is sealed and kept overnight. The appropriate filter paper in a bacteriological filter assembly is then heated until it reaches the ointment base's melting point. The ointment base is expelled from the tubes and subsequently passed through heated filter paper under vacuum. Chloroform is then used to clean the filter paper. After drying, the filter paper is examined under oblique light with a magnifying glass96.
CONCLUSION
In conclusion, a detailed examination of packaging procedures demonstrates the crucial part it plays in a variety of sectors, including food and consumer goods, pharmaceuticals, and electronics. Beyond being aesthetically pleasing, good packaging is an essential tool for protecting, preserving, and promoting products. Due to environmental concerns, the importance of sustainable packaging solutions has increased, leading companies to choose eco-friendly materials and designs. In order to maximise packing effectiveness and minimise waste, teamwork is necessary along the whole supply chain, from manufacturers to retailers. In the future, successful package techniques will revolve upon establishing a balance between usability, sustainability, and beauty. In conclusion, packaging is a vital and dynamic part of contemporary commerce, and it is crucial to continually assess and adapt it in order to take advantage of the opportunities and problems presented by a market that is undergoing rapid change. Environmental concerns and customer demand for eco-friendly options have significantly shifted packaging industry focus in recent years towards sustainable packaging options. Due to this change, recyclable materials have been adopted, packaging waste has decreased, and innovative designs that prioritise environmental responsibility have all resulted. In conclusion, a thorough examination of packaging procedures reveals its crucial function in different businesses. Packaging is a potent marketing tool that shapes consumer perceptions and purchasing decisions in addition to protecting goods during transportation and storage. The packaging sector is primed for greater revolution as technology develops. Smart packaging options, individualised designs, and improved traceability are becoming more and more common, providing both customers and businesses with more information and convenience. In summary, packaging continues to be a keystone of industry that is always changing and whose future is being shaped by innovation, sustainability, and consumer-centered design.
ACKNOWLEDGEMENT:
I would like to express my special thanks of gratitude to our principal Sir, Ashokrao Mane Institute of Pharmacy, Ambap. I am really thankful to them to Mr. Sardar S. Shelake Sir for the patience and support in every Methodological aspect of this several other works. Thank you for your honest advices, your help in overcoming so many obstacles, and above all thank you for all your interest. Secondly I would also like to thanks my friends who helped me a lot in finalizing this project within the limited time frame. Last but not the least, my parents are also an important inspiration for me so with due regards, I express my gratitude to them also.
CONFLICT OF INTEREST STATEMENT:
The authors declare that they have no conflicts of interest regarding the publication of this review paper. All authors certify that they have no financial or personal relationships with other people or organizations that could inappropriately influence (bias) their work or this manuscript.
REFERENCES
-
- European Pharmacopoeia Commission. European Pharmacopoeia. 5th ed. Council of Europe: Strasbourg Cedex, France; 2004.
- Potter NN, Hotchkiss JH. Principles of food packaging. InFood Science: Fifth Edition 1995 (pp. 478-513). Boston, MA: Springer US.
- Patil MS, Nitave SA. RECENT TRENDS AND FUTURE OF PHARMACEUTICAL PACKAGING TECHNOLOGY.
- Sabee MM, Uyen NT, Ahmad N, Hamid ZA. Plastics packaging for pharmaceutical products. Reference Module in Materials Science and Materials Engineering; Elsevier: Amsterdam, The Netherlands. 2021.
- Das PS, Saha P, Das R. Pharmaceutical packaging technology: a brief outline. Research Journal of Pharmaceutical Dosage Forms and Technology. 2018;10(1):23-8.
- Eurostat. Packaging waste statistics [Internet]. 2018 [cited 2018 Mar]. Available from: http://ec.europa.eu/eurostat/statisticsexplained/index.php/Packaging_waste_statistics
- United States Environmental Protection Agency. Advancing sustainable materials management: 2014 tables and figures. Assessing trends in material generation, recycling, composting, combustion with energy recovery and landfilling in the United States [Internet]. 2014 [cited 2018 Apr]. Available from: https://www.epa.gov/sites/production/files/2016-11/documents/2014_smm_tablesfigures_508.pdf
- Vannela R. Are we “digging our own grave” under the oceans? Biosphere-level effects and global policy challenge from plastic (s) in oceans.
- Vannela R. Are we “digging our own grave” under the oceans? Biosphere-level effects and global policy challenge from plastic (s) in oceans.
- Gustavsson J, Cederberg C, Sonesson U, Van Otterdijk R, Meybeck A. Global food losses and food waste.
- Pålsson H, Finnsgård C, Wänström C. Selection of packaging systems in supply chains from a sustainability perspective: the case of Volvo. Packaging technology & science. 2013;26(5):289-310.
- Wikström F, Verghese K, Auras R, Olsson A, Williams H, Wever R, Grönman K, Kvalvåg Pettersen M, Møller H, Soukka R. Packaging strategies that save food: A research agenda for 2030. Journal of Industrial Ecology. 2019 Jun;23(3):532-40.
- Svanes E, Vold M, Møller H, Pettersen MK, Larsen H, Hanssen OJ. Sustainable packaging design: a holistic methodology for packaging design. Packaging Technology and Science: An International Journal. 2010 Apr;23(3):161-75.
- Olsmats C, Dominic C. Packaging scorecard–a packaging performance evaluation method. Packaging Technology and Science: An International Journal. 2003 Jan;16(1):9-14.
- de Koeijer B, Wever R, Henseler J. Realizing product?packaging combinations in circular systems: Shaping the research agenda. Packaging Technology and Science. 2017 Aug;30(8):443-60.
- Sustainable Packaging Coalition. Definition of Sustainable Packaging Version 2.0 [Internet]. 2011 [cited 2018 Apr]. Available from: https://sustainablepackaging.org/wp-content/uploads/2017/09/Definition-of-Sustainable-Packaging.pdf
- The Consumer Goods Forum. Global Protocol on Packaging Sustainability 2.0 [Internet]. 2011 [cited 2018 Apr]. Available from: https://www.theconsumergoodsforum.com/wp-content/uploads/2017/11/CGF-Global-Protocol-on-Packaging.pdf
- Pålsson H, Hellström D. Packaging logistics in supply chain practice–current state, trade-offs and improvement potential. International Journal of Logistics Research and Applications. 2016 Sep 2;19(5):351-68.
- Kumar S, Saini M. Pharmaceutical packaging materials. Int J Sci Eng Comput Technol. 2016 Mar 1;6(1):6-8.
- World Health Organization. Guidelines on packaging for pharmaceutical products, Annex 9 (WHO Technical Report Series, No. 902) [Internet]. 2002 [cited 2024 Jun 22]. Available from: http://www.who.int/medicines/areas/quality_safety/quality_assurance/regulatory_standards/en/index.html
- WHO Expert Committee on Specifications for Pharmaceutical Preparations, World Health Organization. WHO Expert Committee on Specifications for Pharmaceutical Preparations: Thirty-fourth report. World Health Organization; 1996.
- World Health Organization. Guidelines on packaging for pharmaceutical products: Introductory note. WHO Technical Report Series [Internet]. 2002 [cited 2024 Jun 22]. Available from: http://wwwgmpua.com/World/WHO/Annex9/trs902ann9.pdf
- Singh A, Sharma PK, Malviya R. Eco friendly pharmaceutical packaging material. World Applied Sciences Journal. 2011 May;14(11):1703-16.
- Florence AT. New drug delivery systems. Chemistry and Industry. 1993 Dec(24):1000-4.
- Aulton ME, Taylor K, editors. Aulton's pharmaceutics: the design and manufacture of medicines. Elsevier Health Sciences; 2013.
- Lorenzini GC, Olsson A, Larsson A. User involvement in pharmaceutical packaging design–a case study. InDS 87-8 Proceedings of the 21st International Conference on Engineering Design (ICED 17) Vol 8: Human Behaviour in Design, Vancouver, Canada, 21-25.08. 2017 2017 (pp. 041-050).
- Bharath S. Pharmaceutical technology: Concepts and applications.
- Sustainability Team Discussion Paper. Back to sustainable value creation: How sustainable is packaging? No. 23; 2009.
- Singh A, Sharma PK, Malviya R. Eco friendly pharmaceutical packaging material. World Applied Sciences Journal. 2011 May;14(11):1703-16.
- Cooper JW, Gunn C. Cooper and gunn's dispensing for pharmaceutical students. (No Title). 1975.
- Aulton ME, Taylor KM. Aulton’s Pharmaceutics: The Design and Manufacturing of Medicines. Churchill Livingstone Elsevier.
- Remington JP. Remington: the science and practice of pharmacy. Lippincott Williams & Wilkins; 2006.
- Lachman L, Lieberman HA, Kanig JL. The theory and practice of industrial pharmacy. Philadelphia: Lea & Febiger; 1976.
- Mehta RM. Dispensing Pharmacy, Containers and Closures for Dispensed Products. 4th ed. Delhi: Vallabh Prakashan; 2009.
- Briasco B. Multidisciplinary Approach for the Investigation of Content-Container Interactions.
- Brinkmann S, Vieweg N, Gärtner G, Plew P, Deninger A. Towards quality control in pharmaceutical packaging: Screening folded boxes for package inserts. Journal of Infrared, Millimeter, and Terahertz Waves. 2017 Mar;38:339-46.
- Shedafa R, Tibalinda P, Manyanga V, Sempombe J, Temu M, Masota N, Kaale E, Bonsmann C. Assessment of Moisture Permeability and Closure Systems of High Density Polyethylene Plastic Bottles Used as Primary Packaging Containers for Moisture Sensitive Medicines. Pharmacology & Pharmacy. 2016;7(08):321.
- Janssen R. Recent trends in lyophilized delivery devices and packaging. InLyophilized Biologics and Vaccines: Modality-Based Approaches 2015 May 20 (pp. 361-379). New York, NY: Springer New York.
- Cherry CL, Millward H, Cooper R, Landon J. A novel approach to sterile pharmaceutical freeze-drying. Pharmaceutical Development and Technology. 2014 Feb 1;19(1):73-81.
- Woodward JH. Microscopy, Microbiology and Regulations of Paper and Paperboard Utilized in Pharmaceutical Packaging. Microscopy and Microanalysis. 2015 Aug;21(S3):61-2.
- Lau OW, Wong SK. Contamination in food from packaging material. Journal of chromatography A. 2000 Jun 16;882(1-2):255-70.
- Singh A, Sharma PK, Malviya R. Eco friendly pharmaceutical packaging material. World Applied Sciences Journal. 2011 May;14(11):1703-16.
- Pathak A, Rao NR, Grover P, Sharma V, Malik A, Rawat AP, Singh S, Maurya A. Ecofriendly pharmaceutical packaging material: A review. Materials Today: Proceedings. 2023 Sep 22.
- Pathak A, Rao NR, Grover P, Sharma V, Malik A, Rawat AP, Singh S, Maurya A. Ecofriendly pharmaceutical packaging material: A review. Materials Today: Proceedings. 2023 Sep 22.
- International Organization for Standardization. ISO 8536-7: Infusion equipment for medical use - Part 7: Caps made of aluminium-plastics combinations for infusion bottles. 2009. Available from: http://www.iso.org/iso/iso_catalogue/catalogue_tc/catalogue_detail.htm?csnumber=52178
- International Organization for Standardization. ISO 10985:2009. Caps made of aluminium-plastics combinations for infusion bottles and injection vials — Requirements and test methods. 2009. Available from: http://www.iso.org/iso/iso_catalogue/catalogue_tc/catalogue_detail.htm?csnumber=52179
- Hook P, Heimlich JE. A history of packaging. CDFS-133. [n.d.]. Available from: http://ohioline.osu.edu/cdfact/0133.html
- Diaz E. Microbial biodegradation: genomics and molecular biology. (No Title). 2008 Jan.
- Remington JP. Remington: the science and practice of pharmacy. Lippincott Williams & Wilkins; 2006.
- Nasa P. A review on pharmaceutical packaging material. World Journal of Pharmaceutical Research. 2014 May 22;3(5):344-68.
- Allsopp MW, Vianello G. Poly (vinyl chloride). Ullmann's Encyclopedia of Industrial Chemistry. 2000 Jun 15.
- Palmer RJ. Polyamides, plastics. Kirk?Othmer Encyclopedia of Chemical Technology. 2000 Dec 4.
- Nasa P. A review on pharmaceutical packaging material. World Journal of Pharmaceutical Research. 2014 May 22;3(5):344-68.
- Sabah A, Ahmed I, Arsalan A, Arif A, Tanwir S, Abbas A, Ahmed FR. Features, functions and selection of pharmaceutical packaging materials. International Journal of Pharmaceuticals and Neutraceuticals Research. 2014;1(1):1-2.
- Mehta RM. Dispensing pharmacy, containers and closures for dispensed products. 4th ed. Delhi, India: Vallabh Prakashan; 2009.
- United States Pharmacopeial Convention. United States Pharmacopeia. 2013.
- http://4my3232.blogspot.in/ assesed on 17 may2014
- Marsh K, Bugusu B. Food packaging—roles, materials, and environmental issues. Journal of food science. 2007 Apr;72(3):R39-55.
- Panvalker SG, Piskolti-Caldwell E. Making your packaging environmentally friendly. InInternational Trade Forum 2001 Apr 1 (No. 2, p. 15). International Trade Centre.
- http://www.Glass-packaging- best-for-the-environment.html.
- http://www. 'Green' Packaging is Growing IMTIndustry Market Trends.html.
- http://www.Glass-is-greener-on-the-other-side-says-trade-body.html.
- Sustainability Team. Back to sustainable value creation: How sustainable is packaging? 2009. pp. 2-3.
- International Organization for Standardization. ISO 595-1:1986. Reusable all-glass or metal-and-glass syringes for medical use. 1986. Available from: [http://www.iso.org/iso/iso_catalogue/catalogue_tc/catalogue_detail.htm?csnumber=4696](http://www.iso.org/iso/iso_catalogue/catalogue_tc/catalogue_detail.htm?csnumber=4696)
- http://www.pharmatutor.org/articles/the-pharmaceutical-packaging-article.
- http://www.zorge.com/assets/Documents/Rubber-technology.pdf assesed on 20 may2014.
- http://www.Product Packaging.html.
- International Organization for Standardization. ISO 595-1:1986. Reusable all-glass or metal-and-glass syringes for medical use. 1986. Available from: [http://www.iso.org/iso/iso_catalogue/catalogue_tc/catalogue_detail.htm?csnumber=4696](http://www.iso.org/iso/iso_catalogue/catalogue_tc/catalogue_detail.htm?csnumber=4696)
- Kumar Basniwal P, Jain D. Simvastatin: review of updates on recent trends in pharmacokinetics, pharmacodynamics, drug–drug interaction, impurities and analytical methods. Current Pharmaceutical Analysis. 2012 May 1;8(2):135-56.
- Mehta RM. Dispensing pharmacy, containers and closures for dispensed products. 4th ed. Delhi, India: Vallabh Prakashan; 2009.
- Singh A, Sharma PK, Malviya R. Eco friendly pharmaceutical packaging material. World Applied Sciences Journal. 2011 May;14(11):1703-16.
- Kumar S, Gupta SK. Applications of biodegradable pharmaceutical packaging materials: a review. Middle-East Journal of Scientific Research. 2012;12(5):699-706.
- Sustainability Team. Sustainability team discussion paper. 2009.
- Hook P, Heimlich JE. A History of Packaging. CDFS-133. Available from: http://ohioline.osu.edu/cdfact/0133.html.
- Pumping Up Recycling. Food Business (Report). 1990;14-15.
- Available from: http://www.boramikang.com/processbookwebsite/1research/tea_researchsustain.
- Marsh K, Bugusu B. Food packaging—roles, materials, and environmental issues. Journal of food science. 2007 Apr;72(3):R39-55.
- http://www.Product Packaging.html.
- http:// www.news.thomasnet.com/IMT/archives/2002/10/green_packaging.html.
- http://www.option.com.
- http://www.recycling packaging.html
- http://www.rephouse.html.
- http://www.zorge.com/assets/Documents/Rubber-technology.pdf assesed on 20 may2014.
- Kunal CM, Akhilesh D, Kumar BS. Recent trends in pharmaceutical packaging: A review. Int. J. Pharm. Chem. Sci. 2012 Jul;1(3):1282-92.
- http://www.sha.org/bottle/closures.htm assesed on 20 may2014
- Jenke DR, Stults CL, Paskiet DM, Ball DJ, Nagao LM. Materials in manufacturing and packaging systems as sources of elemental impurities in packaged drug products: a literature review. PDA journal of pharmaceutical science and technology. 2015 Jan 1;69(1):1-48.
- Roberts A, Haighton LA. A hard look at FDA’s review of GRAS notices. Regulatory Toxicology and Pharmacology. 2016 Aug 31;79:S124-8.
- Pareek VI, Khunteta AL. Pharmaceutical packaging: current trends and future. Int J Pharm Pharm Sci. 2014 Jun 6;6(6):480-5.
- Guberac V, Maric S, Lalic A, Drezner G, Zdunic Z. Hermetically sealed storage of cereal seeds and its influence on vigour and germination. Journal of Agronomy and Crop Science. 2003 Feb;189(1):54-6.
- Uddin MS. QUALITY CONTROL TESTS FOR PHARMA-CEUTICAL AEROSOLS. Advances in Natural and Life Sciences Volume: II. 2016:155.
- Uddin MS, Mamun AA, Akter N, Sarwar MS, Rashid M, Amran MS. Pharmacopoeial standards and specifications for pharmaceutical oral liquid preparations. Archives of Current Research International. 2016 Jan 10;3(2):1-2.
- Han Y, Jiang F, Zhao Y. RESEARCH ON AMPOULES INJECTION LIQUID PARTICLES INSPECTION MACHINE BASED ON MACHINE VISION. Journal of the Balkan Tribological Association. 2016 Jun 15;22.
- Sormunen E, Nevala N, Sipilä S. Critical factors in opening pharmaceutical packages: a usability study among healthcare workers, women with rheumatoid arthritis and elderly women. Packaging Technology and Science. 2014 Jul;27(7):559-76.
- Raina H, Jindal A. Packaging of non-injectable liquid pharmaceuticals: a review. Journal of Applied Pharmaceutical Science. 2017 Feb 27;7(2):248-57.
- Mizumachi S, Kojima S, Nakamura A. Transparency test for plastic containers. Eisei Shikenjo hokoku. Bulletin of National Institute of Hygienic Sciences. 1989 Jan 1(107):94-9.
- Solomon P, Nelson J. Profiling extractable and leachable inorganic impurities in ophthalmic drug containers by ICP-MS. Pharmaceutical development and technology. 2018 Mar 16;23(3):247-54


 Nikita Patil*
Nikita Patil*

 10.5281/zenodo.12739482
10.5281/zenodo.12739482