Abstract
This study explores the formulation and optimization of a nanoemulsion for enhanced drug delivery. Nanoemulsions are colloidal dispersions with droplet sizes in the nanometer range offering increased stability and improved bioavailability. The primary focus is on manipulating particle size through two key methods: Ultrasonication and High-pressure homogenization. These techniques are employed to achieve the desired nanoscale range enhancing the formulations efficiency. The impact of pH on nanoemulsion stability and drug release is investigated recognizing its influence on droplet surface charge and interfacial properties. Viscosity measurements are conducted to understand the formulations rheological behavior crucial for its administration and therapeutic effectiveness. The study also assessesdrug content within the nanoemulsion to ensure accurate dosing. In vitro studies are conducted to evaluate the nanoemulsions performance in simulated biological environments. These investigations include drug release kinetics permeability and overall efficacy. The results aim to provide insights intothe nanoemulsions potential as a drug delivery system shedding light on its stability, particle size optimization and impact on drug release profiles. Overall, this research contributes valuable knowledge to the field of pharmaceutical science offering a comprehensive understanding of the key parameters influencing nanoemulsion formulation and its potential applications in drug delivery.
Keywords
Nanoemulsion, components, methods of preparation and characterization.
Introduction
Nanoemulsions are thermodynamically stable transparent (translucent) dispersions of oil and water stabilized by an interfacial film of surfactant and co-surfactant molecules having a droplet size of less than 100 nm [1]. Many studies have shown that nanoemulsion formulations possess improved transdermal and dermal delivery properties in vitro [2-3] as well as in vivo [4-5]. Nanoemulsions have improved transdermal permeation of many drugs over the conventional topical formulations such as emulsions [6-7] and gels [8-9]. However, the application of the nanoemulsion to the skin is in convenient due to low viscosity [10]. To increase their viscosity and to make them more suitable for the transdermal application, gelling agents can be used [11]. However, formulation such as microemulsion, self- emulsifying formulations, self-micro emulsifying formulations contains high concentration of surfactant, which limits its use due to regulatory problems as well as toxicity concerns [12]. However, nanoemulsion offer advantages of lipid-based systems while overcoming the limitation of requirement of high surfactant concentration. Nanoemulsions are heterogeneous mixture of oil in water, kinetically stable, translucent or milky. They are stable without any apparent flocculation or coalescence during the long-term storage due to their nanometers sized droplets (typically less than 200 nm) [13]. Nanoemulsion is one of the promising technologies to enhance the oral bioavailability of poorly soluble drugs as it provides ultra-low interfacial tensions and large o/w interfacial areas. Nanoemulsions have a higher solubilization capacity than simple micellar solutions especially for hydrophobic drugs. Nanosized droplets would influence the transport properties of drug, an important factor in sustained and targeted drug delivery. Nanoemulsion has been reported to make the plasma concentration profiles and bioavailability of drugs more reproducible [14].

Fig: 1 Structure Of Nanoemulsion
Nanoemulsions are metastable systems, meaning they have a tendency to break down over time due to a variety of destabilization mechanisms, such as gravitational separation, coalescence, flocculation, and Ostwald ripening [15]. However, emulsions may be formulated to remain stable for a desired period. Addition of stabilizers and co- adjuvant molecules may have important effects on physical properties and stability of nanoemulsions since molecular interactions strongly influence structure and rheological behavior. In delivery of nutraceuticals, vitamins, drugs, antimicrobials, colors or flavors, the physicochemical and structural properties of nanoparticles formed must be controlled regarding the desired application [16,17]. Food protein stabilized nanoemulsions have been studied as delivery systems because they have excellent emulsifying properties, good binding capacity for hydrophobic bioactive compounds, and excellent gelation properties.
Components of nanoemulsion
The main components of nanoemulsion are oil, emulsifying agents, and aqueous phases [18]. Oils can be of any type like castor oil, corn oil, coconut oil, evening primrose oil, linseed oil, mineral oil, olive oil, peanut oil, etc. A mixture of oil and water may yield a crude temporary emulsion, which upon standing, will separate in two distinct phases due to the coalescence of the dispersed globules. Emulgents or emulsifying agents can impart stability to such systems. Emulgents are broadly classified as surfactants like spans and tweens, hydrophilic colloids such as acacia and finely divided solids, e.g., bentonite and vee gum. An emulgent, in addition to its emulsifying properties, should be nontoxic and its taste, odor and chemical stability should be compatible with the product. Some of the desirable properties of an emulgent are:
- It should be able to reduce the surface tension to below 10 dynes/cm
- It should be adsorbed rapidly around dispersed phase globule to form a complete and coherent film to prevent coalescence,
- It should help in building up an adequate zeta potential and viscosity in the system so as toimpart optimum stability.
- It should be effective in a fairly low concentration. Emulgents form monomolecular,multimolecular or particulate films around the dispersed globules [19].
Factors to be considered during preparation of nanoemulsion
- Surfactant must be selected carefully such that an ultralow interfacial tension may be achieved which is primary requirement to produce nanoemulsion.
- Concentration of surfactant must be high enough to stabilize the microdroplets to produce nanoemulsion.
- The surfactant must be flexible or fluid enough to promote the formation of nanoemulsion.
SCREENING OF EXCIPIENTS:
OILS \LIPIDS.
The solubility of the drug in the oil phase is an important criterion for the selection of oils. Oils representone of the most important excipients in the Nanoemulsion development, not only due to the potential to solubilize marked amounts of lipophilic drugs, but also due to increase in the fraction of lipophilic drug transported via the intestinal lymphatic system, thereby increasing absorption of drugs from the GI tract depending on the molecular nature of the oils. The w/o Nanoemulsion are better choice for hydrophilic drugs, however lipophilic drugs are preferably solubilized in o/w Nanoemulsion.
List of oils\lipids used in nano emulsion formulation

Surfactants
Surfactants lower interfacial tension to aid dispersion process and provide a flexible film that can readily deform around droplets. Their lipophilic character provides the correct curvature at the interfacial region for the desired Nanoemulsion type i.e. for o/w, w/o or continuous [20,21]. Surfactants with low HLB value [22–23], such as Spans are generally considered for the development of w/o Nanoemulsion, however the high HLB value [24–25] surfactants, like Tweens are preferred when o/w Nanoemulsion system is desired.
List of surfactants used in nanoemulsion formulation.

Co-surfactants
Transient negative interfacial tension is not achieved by the use of a single surfactant, usually necessitating the addition of a cosurfactant. In the absence of cosurfactant, a highly rigid film is formed by the surfactant and thus produces Nanoemulsion over only a very limited range of concentration. The presence of cosurfactants allows the interfacial film sufficient flexibility to take up different curvatures required to form Nanoemulsion over a wide range of composition [26].
List of co-surfactants used in nanoemulsion formulation.

Application of nano emulsion in different drug delivery systems: Oral delivery
Nanoemulsion can bring a great revolution in oral drug delivery system. It has overcome several limitations of the traditional systems. Drug solubility, rate of absorption and targeted drug delivery were always the matters of concern during designing oral dosage forms. Nano emulsified drug delivery system has come out with a one-step solution for all of these problems. In case of drug solubility, both hydrophilic and lipophilic drugs can be solubilized in either O/W or W/O nanoemulsion which in turn ensures a better dissolution because of extremely small size of the particles having both hydrophilic and lipophilic units. Furthermore, these small particles can easily penetrate through the epithelial layer to ensure good rate of absorption of the drug.
Topical delivery
Topical drug delivery has some advantages over the oral route which include no drug loss by first pass metabolism, no damage of the drug in GI environment, no gastric irritation, no unpleasant taste or difficulty to administer and no need of disintegration and dissolution step. The main difficulty regarding topical drug delivery is the skin barrier which prevents the drug entering the systemic circulation. Nanoemulsion-based topical drug delivery can significantly overcome this barrier. Usually, drugs penetrate through the skin in three routes which are hair follicle, sweat duct and directly through the stratum corneum. The small sized nanoparticles in nanoemulsions can pass through the pores easily. Moreover, the hydrophobic and hydrophilic units facilitate to penetrate through the hydrophobic stratum corneum as well as the hydrophilic sweat ducts [27].
Fig:2 Comparison of nano emulsion with the conventional transdermal formulations in case of crossing skin barrier.

Ophthalmic delivery
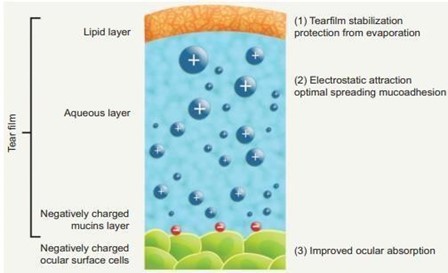
Conventional eye drops as ophthalmic drug delivery result in poor bioavailability and pharmacological action because of lacrimal secretion and nasolacrimal drainage in the eyes. Tear drainage of the eyes transports the significant part of the administered drug via the nasolacrimal duct to the gastrointestinal tract. As a result, it may be absorbed, sometimes causing side effects. The drug needs to be in contact with the eyes for a longer period of time to overcome this problem. Other preparations like ointment or aqueous gels cause blurred vision. From this point of view, dilutable nanoemulsions are potent drug delivery for ophthalmic administration because of their numerous advantages as sustained effect and high ability of drug penetration into the deeper layers of the ocular structure and the aqueous humor [28].
Fig:3 Nano Emulsion As Ophthalmic Delivery
Parenteral delivery
Drugs with low solubility are always considered unsuitable for parenteral administration, however, with the help of nano emulsification techniques, they can now be formulated as parenteral dosage form. The use of biodegradable surfactants ensures good pharmacological action with no interference to the regular biological activities of the body. Recent research shows that Carbamazepine, a widely used anticonvulsant drug which is poorly soluble, can be prepared as nanoemulsion by spontaneous emulsification method containing 2 mg/mL where about 95% drug is released within 11h [29]. Another study shows that IV preparation of thalidomide (0.01% – 0.05% w/w) by spontaneous emulsification releases 95% drug within 4h [30].
Method of preparation of nano emulsion:
High energy methods:
High-energy methods use intense mechanical forces to disrupt droplets into smaller droplets, and typically involve the use of mechanical devices [31,32]. The methods in this category have a drawback, which is that they need high energy to prepare nanoemulsions and therefore, they are unfavorable for many industrial applications [33]. The formation of nanoemulsion droplets directly depends on controllable formulation parameters such as the amount of energy, the amount of surfactant and the nature of the components[34].
High pressure homogenizer:
In a standard procedure, materials are passed among the narrow gap of homogenizer in high pressure (50– 200 Mpa). This high pressure causes a strong disruptive force such as shearing, collision and cavitation. Intensive turbulency and hydraulic shear cause coarse emulsion change to nanoemulsion. The droplet size depends on the number of cycles, the pressure and temperature of the system. The more the number of cycles and the pressure, the smaller the size of droplets produced. In addition, this size also depends on emulsion composition (e.g., organic and aqueous phase and surfactant), emulsifier’s characteristic (e.g., adsorption kinetic, interfacial tension depression, and stabilizing properties) and physicochemical condition of different phases (e.g., viscosity and interfacial tension). High pressure homogenization can be processed in high temperature (hot HPH technique) or in low temperature (cold HPH technique), which then later is used for processing temperature labile drugs [35]. Comparing to other procedures, HPH has many advantages such as easy scale up, avoidance of organic solvents and small process time.
Fig.4 High Pressure Homogenizer.

Microfluidizer:
Oil and aqueous phase are mixed together and enter the homogenizer to produce coarse emulsion. This coarse emulsion enters the microfluidizer to produce stable nanoemulsions. In the microfluidizer, a high-pressure pump is used. This pump, which works in high pressure up to 2000 psi, forces the produced emulsion to pass through the interaction chamber, which has some microchannels. Therefore, the emulsion’s droplet will be very small [36,37]. The diameter of nanoemulsions droplets depends on the pressure of the operation and the number of microchannels in interaction chamber. The produced nanoemulsion can be filtered through a 0.2 mm filter under nitrogen to remove large droplets and have a uniform nanoemulsion. Generally, inertial forces in turbulent flow along with cavitation cause droplet disruptions in the microfluidizer. The advantage of microfluidizer is that the distribution of droplets in nanoemulsion is narrower than that of other emulsifying devices. However, micro fluidization is unfavorable in specific cases, such as high pressure and longer emulsification time, since it leads to re- coalescence of emulsion droplets and an increase in EDS. The temperature of nanoemulsions at the exit of the interaction chamber is linearly dependent on pressure and emulsification time. Higher temperature leads to a decrease in viscosity and interfacial tension and facilitates droplet breakup. The most important problem in high-energy emulsification is the increase in temperature which leads to re- coalescence of emulsion droplets and increase in EDS. To alleviate this and to have small droplets, a cooling jacket is used.

Fig:5 Microfluidizer.
Ultrasonication
Ultrasound is very effective in decreasing the droplet size; however, it is appropriate for small batches. The process efficiency depends heavily on ultrasonication time in different amplitudes. In this equipment, ultrasound energy large droplets disrupt to smaller ones. In ultrasonication, the temperature is linear function of the time. Ultrasonication has a similar behavior to microfludization at a high temperature. The ultrasonication time has an important effect in droplet size. As the time increases, the amount of energy increases as well, leading to more droplets to be disrupted and, therefore, to a decrease in EDS. Increasing the residence time over the optimum limit has no effect on EDS, but wastes energy. Therefore, one should not expect that droplet disruption increases and droplet sizes decreases when the amount of energy increases. The input energy should maintain at a level at which the EDS is the lowest. Usually there is no over-processing in ultrasound emulsification. This is because residence time of emulsion in emulsification region, which is equivalent to sonication time, is high in equipment, while the residence time in interaction chamber in the microfluidizer is about millisecond [38]. Despite the simplicity of the ultrasonication, it results in a less than optimal heterogeneous distribution of nanodroplet size. Moreover, the ingredients would suffer from the damage due to high-energy output. The distribution of the size of droplets depends on coarse emulsion input to ultrasound. If the coarse emulsion entered to sonication chamber has larger EDS and vaster distribution, the produced nanoemulsion will have droplets with larger diameter.

Fig.06 Ultrasonicator.
Spontaneous emulsification:
This process is based on the diffusion upon the dilution of the system causing the movement of water- miscible components (solvent, surfactant and co-surfactant) from an organic phase into the aqueous phase. Spontaneous emulsification generally involves the addition of an organic phase (containing oil and hydrophilic surfactant) into an aqueous phase (containing water and co-surfactant). The rapid migration of water-miscible components into aqueous phase causes an immense turbulence in the interface of two phases and a large increase in the oil-water interfacial area. It leads to the spontaneous formation of oil droplets surrounded by aqueous phase through a budding process. Nanoemulsions can also be prepared by dilution of microemulsions or cubic liquid crystals with water. During dilution with water, the co-surfactants diffuse from the oil-water interface to an aqueous phase. These make the micelles no longer thermodynamically stable, obtaining nanoemulsions. Moreover, the Spontaneous emulsification method is used in the pharmaceutical industry to obtain nanoemulsions as carriers for lipophilic drugs in aqueous media. Systems prepared using this approach are usually referred to in the literature as self-nanoemulsifying drug delivery systems (SNEDDS) [39].
Phase inversion method:
Phase Inversion Method: Fine dispersion is obtained by chemical energy resulting of phase transitions occur through emulsification method. The adequate phase transitions are produced by changing the composition at constant temperature or by changing the temperature at constant composition. The phase inversion temperature (PIT) method was introduced based on the principle of changes of solubility of polyoxy ethylene type surfactant with temperature. This surfactant becomes lipophilic with increase in temperature because of dehydration of polymer chain. At low temperature the surfactant monolayer has a great positive spontaneous curvature forming oil swollen micellar solution phase [40].
Characterization of prepared nanoemulsion:
Particle size and zeta potential measurement:
The formulation (0.1 ml) was dispersed in 50 ml of water in a volumetric flask and gently mixed by inverting the flask. Globule size and zeta potential of the nanoemulsion were determined by Zetasizer HSa 3000 that analyses the fluctuations in light scattering due to the Brownian motion of the particles. Light scattering was monitored at 25°C at a 90° angle. [41]
Stability studies:
Temperature stability:
Shelf life as a function of time and storage temperature was evaluated by visual inspection of the nanoemulsion system at different time period. Nanoemulsion was diluted with purified distilled water to determine the temperature stability of samples. Samples were kept at three different temperature ranges (4°C, room temperature) and observed for any evidences of phase separation, flocculation or precipitation.
Centrifugation:
In order to estimate metastable systems, the optimized nanoemulsion formulation was diluted with purified distilled water. Then nanoemulsion was centrifuged at 10,000 rpm for 30 minutes at room temperature and observed for any change in homogeneity of nanoemulsions. [42]
Ph determination:
The pH of 10% w/w aqueous solution of each Nanoemulsion formulation was measured by direct immersion of the pH meter electrode standardized using pH 4 and 7 standard buffers before use. [43]
Viscosity:
Viscosity of nanoemulsion was determined using 1 ml of the formulation and speed of the spindle was adjusted to 100 rpm and a shear rate of 100 s-1 was applied at 37°C for 10 min. Viscosity of the optimized nanoemulsion was determined using R/S CPS plus Rheometer. Drug content:
Drug content of the nanoemulsion formulation was carried out by dissolving 1 ml of the formulation in 10 ml of methanol. This formulation was then placed in shaking incubator (LSI-2005 RL, Lab Tech Co., Korea) (50 rpm at 37°C) for 30 min. After 30 min supernatant was collected and analyzed using UV spectrophotometer (UV-1700 Pharma Spec, Shimadzu, Japan) at 210 nm against methanol as blank [44].
Morphological Analyses:
Morphology and structure of the nanoemulsion were studied using Transmission Electron Microscopy (TEM) LEO 912AB EFTEM. To perform the TEM observations, samples were placed on a formvar carbon-coated copper grid (200 mesh in1) and then stained with 1% phosphotungstic acid. The excess phosphotungstic acid on the sample was gently wiped off using filter paper and examined after drying for about half an hour at room temperature. [45]
In-Vitro Drug Diffusion Studies:
Franz diffusion cell is used to obtain the drug release profile of the nanoemulsion formulation in the case of formulations for transdermal application. The membrane was mounted between the donor and receptor compartments. Cylindrical glass tube open at both ends with an exposed surface area of 3.14 cm was used as diffusion cell. A dialysis membrane was allowed to hydrate in distilled water for 24 hrs. Dialysis membrane was fixed to one end of the cylinder with rubber band. 1 gm of gel was spread over the cellophane membrane. Precautions were taken to ensure uniform thickness of gel over the membrane and to remove all air bubbles between the gel and the membrane. With the help of another cylinder tube dialysis membrane should be in contact with the receptor media. The cell was immersed in a beaker containing 25ml of 5% v/v methanolic phosphate buffer pH 7.4 (receptor media). The system was maintained at 37±2°C. Precaution was taken to keep the buffer below the rubber band. The buffer was stirred with a magnetic stirrer during the entire 8 hrs release study. Samples of 2ml were withdrawn at different time intervals for a period of 8 hrs from the receptor compartment and replaced with an equal volume of buffer at 37±2°C. The samples after diluting suitably were analyzed spectrophotometrically, at a wavelength of 368.20 nm for tenoxicam content. [46]
CONCLUSION:
In conclusion, this paper presents a comprehensive overview of nanoemulsion as an innovative drug delivery system and explores its various applications in diverse drug delivery contexts. Additionally, it critically examines the screening process for excipients essential in the preparation of nanoemulsions. The discussion delves into both high-energy and low-energy methods employed for nanoemulsion preparation, encompassing techniques such as high shear homogenization, ultrasonication, phase inversion, spontaneous emulsification, and micro fluidization. Furthermore, the paper extensively covers the characterization of nanoemulsions, providing insights into crucial parameters such as particle size, stability studies, pH levels, viscosity, drug content, morphology analyses, and in vitro diffusion studies. This thorough examination of nanoemulsion properties ensures a nuanced understanding of their behavior and performance, contributing significantly to the field of drug delivery systems. By synthesizing information on nanoemulsion applications, excipient selection, preparation methods, and comprehensive characterization, this paper serves as a valuable resource for researchers and practitioners. It facilitates a deeper comprehension of the intricacies involved in utilizing nanoemulsions for drug delivery, fostering advancements and informed decision-making in pharmaceutical research and development.
REFERENCES:
- Chen H, Chang X, Wen T, Du D, Li J, X H, Yang X. Nanoemulsion based hydrogel.
- Craig DQM, Barker SA, Banning D, Booth SW. An investigation into the mechanisms of self-emulsification using particle size analysis and lowfrequency dielectric spectroscopy. Int J Pharm. 1995; 114: 103 –110.
- Gonzalez E, Cruz C, Nicolas R, Egido J,HerreroBeaumont G. Long-term effects of nonsteroidal anti-inflammatory drugs on the production of cytokines and other inflammatorymediators by blood cells of patients with osteoarthritis: 1994; 41:171-178.
- Gosh MN. Fundamentals of Experimental Pharmacology. Kolkata, India: Hilton andCompany;2005:192.
- Kazimiera A,Katarzyna Z, Agnieszka H, Adam Biocompatible nanoemulsions of dicephalic aldonamide-type surfactants:Formulation,structureand temperature influence. Journal of Colloid and Interface Science 2009; 334: 87–95.
- Kemken J, Ziegler A, Muller BW. Influence of supersaturation on the pharmacodynamic effect of bupranolol after dermal administration using microemulsions as vehicle. Pharm Res.1992; 9:554-558.
- Kreilgaard M, Kemme MJB, Burggraaf J,Schoemaker RC, Cohen AF. Influence of microemulsion vehicle on cutaneous bioequivalence of a lipophilic model drug assessed by micro dialysis and pharmacodynamics. Pharm Res. 200118:593-599.
- Kreilgaard M, Pedersen EJ, Jaroszewski JW.NMR characterization and transdermal drug delivery potentials of microemulsion systems. J Control Rel;2000; 69:421-433.
- Kreilgaard M., Dermal pharmacokinetics of microemulsion formulation determined by invitromicro dialysis. Pharm Res. 2001; 18: 367-373.
- Lawrence MJ, Rees GD. Microemulsion-based media as novel drug delivery systems. Adv Drug Deliv Rev 2000; 45:89-121.
- Klang V, Matsko NB, Valenta C, Hofer F. Electron microscopy of nanoemulsions: an essentialtool for characterization and stability assessment. Micron 2012; 43:85- 103.
- Wakerly MG, Pouton CW, Meakin BJ, Morton FS. Self-emulsification of vegetable oil-non- ionic surfactant mixtures. ACS Symp Ser, 1986;311: 242–55.
- Chen H, Khemtong C, Yang X, Chang X, Gao J. Nanoization strategies for poorly water- soluble drugs. Drug Discov Today, 2011;16(7–8): 354–60.
- Kotta S, Khan AW, Pramod K, Ansari SH, Sharma RK, Ali J. Exploring oral nanoemulsions forbioavailability enhancement of poorly water-soluble drugs. Expert Open Drug Deliv, 2012;9(5):585–98.
- Maali A, Mosavian MH. Preparation and application of nanoemulsions in the last decade (2000–2010). Journal of dispersion science and technology. 2013 Jan 1;34(1):92-105.
- Komaiko JS, McClements DJ: Formation of food-grade nanoemulsions using low-energy preparation methods: a review of available methods. Comprehensive Rev. Food Sci. Safety 2016, 15:331-352. * This is a very interesting review that discuss the advantages and basis of low energy preparation methods.
- Esmaeili A, Gholami M: Optimization and preparation of nano capsules for food applications using two methodologies. Food Chem. 2015, 179:26-34.
- Gasco MR, Gallarate M, Pattarino F (1991) In vitro permeation of azelaic acid from viscosizedmicroemulsions. Int J Pharm 69:193–196.
- Sharma SN, Jain NK (1985) A text book of professional pharmacy. Vallabh Prakashan, 1st edn,p 201.
- D.O. Grigoriev, R. Miller, Curr. Opin. Colloid Interface Sci. 14 (2009) 48–59.
- M. Huang, T.S. Horwitz, C. Zweiben, S.K. Singh, J. Pharm. Sci. 100 (2011) 4617–4630.
- F. Shakeel, N. Haq, F.K. Alanazi, I.A. Alsarra, J. Mol. Liq. 182 (2013) 57–63.
- D.J. McClements, E.A. Decker, J. Weiss, J. Food Sci. 72 (2007) R109–R124.
- J. Weiss, E. Decker, D. McClements, K. Kristbergsson, T. Helgason, T. Awad, Food Biophys. 3(2008) 146–154.
- S. Baboota, F. Shakeel, A. Ahuja, J. Ali, S. Shafiq, Acta Pharma. 57 (2007) 315–332.
- W. Warisnoicharoen, A.B. Lansley, M.J. Lawrence, Int. J. Pharm. 198 (2000) 7–27.
- Bernardi DS, Pereira TA, Maciel NR, Bortoloto J, Viera GS, Oliveira GC, et al. Formation andstability of oil-in-water nanoemulsions containing rice bran oil: in vitro and in vivo assessments. J Nanobiotechnol 2011; 44:1 – 3.
- Ammar HO, Salama HA, Ghorab M, Mahmoud AA. Nanoemulsion as a potential ophthalmic delivery system for dorzolamide hydrochloride. AAPS Pharm Sci Tech 2009; 10:808 – 19.
- Kelmann RG, Kuminek G, Teixeira HF, Koester LS. Carbamazepine parenteral nanoemulsionsprepared by spontaneous emulsification process. Int J Pharm 2007; 342:231 – 9.
- Koester LS, Ara ú jo FA, Teixeira HF, Kelmann RG, Ara ú jo BV, Finatto RB. Development and characterization of parenteral nanoemulsions containing thalidomide. Eur J Pharm Sci 2011; 42:238 –15.
- Sole, I., Maestro, A., Gonzalez, C., Solans, C., and Gutierrez, J.M. (2006) Langmuir, 22: 8326–8332.
- Sole, I., Maestro, A., Pey, C.M., Gonzalez; Solans, C., and Gutierrez, J.M. (2006) Colloids andSurfaces A, 288: 138–143.
- Tadros, T., Izquierdo, P., Esquena, J., and Solans, C. (2004) Advances in Colloid and InterfaceScience, 108–109: 303–318.
- Anton, N., Benoit, J.-P., and Saulnier, P. (2008) Journal of Controlled Release, 128: 185– 199.
- Wissing, S.A., Kayser, O., and Muller, R.H. (2004) Advanced Drug Delivery Reviews, 56:1257–1272.
- Constantinides, P.P., Chaubal, M.V., and Shorr, R. (2008) Advanced Drug Delivery Reviews,60: 757–767.
- Chen, H., Khemtong, C., Yang, X., Chang, X., and Gao, J. (2011) Drug Discovery Today, 16:354–360.
- Jafari, S.M., He, Y., and Bhandari, B. (2007) Journal of Food Engineering, 82: 478–488.
- Jintapattanakit A. Preparation of nanoemulsions by phase inversion temperature (PIT) method.Pharmaceutical Sciences Asia. 2018 Jan 1;45(1):1-2.
- Sharma N, Mishra S, Sharma S, Deshpande RD, Sharma RK. Preparation and optimization of nanoemulsions for targeting drug delivery. Int. J. Drug Dev. Res. 2013 Oct;5(4):0975-9344.
- Kanke PK, Pathan IB, Jadhav A, Usman MR. Formulation and evaluation of febuxostat nanoemulsion for transdermal drug delivery. Journal of Pharmaceutical and Biosciences/Jan Mar. 2019;7(1).
- Modi JD, Patel JK. Nanoemulsion-based gel formulation of aceclofenac for topical delivery. International Journal of Pharmacy and Pharmaceutical Science Research. 2011;1(1):6-12.
- Kassem MA, Ghalwash MM, Abdou EM. Development of nanoemulsion gel drug delivery systems of cetirizine; factorial optimization of composition, in vitro evaluation and clinical study. Journal of Microencapsulation. 2020 Aug 17;37(6):413-30.
- Laxmi M, Bhardwaj A, Mehta S, Mehta A. Development and characterization of nanoemulsionas carrier for the enhancement of bioavailability of artemether. Artificial cells, nanomedicine, and biotechnology. 2015 Sep 3;43(5):334-44.
- Sharma P, Namdev A, Agrawal D, Khinchi M, Soni S. Formulation and Evaluation ofNanoemulsion Gel of Tenoxicam for Topical Application. Asian Journal of Pharmaceutical Research and Development. 2015 Jan 1:44-53.
- Lawrence MJ, Rees GD. Microemulsion-based media as novel drug delivery systems. Adv Drug Deliv Rev 2000; 45:89-121


 Kirankumar D. S.*
Kirankumar D. S.*
 Nagaraja T. S.
Nagaraja T. S.









 10.5281/zenodo.11092399
10.5281/zenodo.11092399