Abstract
Based on the provided data on the toxicity of various ligands with varying MW, lipkinski’s rule of five violations, bioavailability scores, lead likeness violations were analyzed and the following conclusions can be drawn: Rutin, Narcissin, and Canthin-6-one exhibit significant toxicity, particularly in terms of carcinogenicity and hypertoxicity. Quercetin, Myricetin, Isorhamnetin, Kaempferol, Feru loylty ramine, Ferulic acid, Gallic acid, P- Coumaric acid, and Apigetrin show varying levels of toxicity, but generally are less toxic compared to Rutin, Narcissin, and Canthin-6-one. Methergine, Vanillic acid, Melilotic acid, and Kaempferol-3-rhamnogalactoside appear to be relatively safer options with low toxicity. PHBA HYDRAXOBENZOIC ACID demonstrates low toxicity but some potential for respiratory toxicity. Among the tested ligands several exhibit low predicted toxicity levels, indicating their potential safety for the therapeutic or industrial application. Ligands such as quercetin, myricetin, isorhamnetin, kaempferol, and ferulic acid demonstrate moderate toxicity level, suggesting the need for cautious consideratiom in their usage. Overall, while some ligands like Rutin, Narcissin, and Canthin-6-one pose significant health risks, others present moderate to low toxicity levels. Further studies may be needed to fully understand the mechanisms and implications of their toxicity in different contexts.
Keywords
Antiasthamatic medication, phytochemicals, phenols, Alkaloids, Terpenoids, 7DFL-receptors.
Introduction
Asthma
The National Institute of Health defines asthma as a chronic inflammatory disorder of airways in which cellular ements play a major role particularly mast cells, T-lymphocytes, eosinophils, epithelial cells, and neutrophils[1,2]. Asthma is an inflammatory disease that targets the airway's narrowing thereby resulting in the change of eosinophils, mastcell, lymphocytes and cytokine levels. It is characterized by the exacerbation of coughing, dysponea, wheezing and chest tightness. The individual with asthma iswell known to have high level of IgE that bind to the receptor of most cell and inflammatory products. The interaction between antigen and antibody IgE result in the activation inflammatory cellular reaction. There by releasing theme diator such as histamine, and prostaglandins the altimately lead to the contraction of airway smooth muscles [3-5]. Asthma can be triggered by various factors such as viral respiratory infections, certain chemicals, certain medication, airborne allergens, occupational sensitizers, smoke, air pollutant. Stress and anxiety or an extreme emotional arousal may also trigger asthmatic attacks. They are characterized into two important forms:
- Extrinsic Asthma: It is caused by allergic response such as house dust, animal fur or various foods. Such cases account for about 10-20%.[6,7]
- Intrinsic Asthma: It is caused by the genetic, structural problems, infection. Both physiological and psychological. Such as accounts for about50-60%[6,7]. The pharmacological management of asthma largely depends on steroidal anti-inflammatory agents. There is no scientifically proven cure of asthma but it can be controlled by certain pharmaceutical drug or by some medicinal plants. Natural treatments of asthma are meant to be complement or an addition in the treatment of it[8].During asthma attack the changes lead to stop air moving easily through airways, recurring symptom, reversible airflow bstruction, bronchospasm, It is characterized by airways inflammation cold cough, wheeze, chest tightness and dysponea. There are many herbs that are useful as pharmaceutical drug in the treatment of asthma. symptoms of asthma are not same for everyone. It includes: wheezing when exhaling, trouble sleeping caused by shortness of breath, chest tightness, coughing and pain on chest. Symptoms are worsened at night, in response to exercise and in early morning.
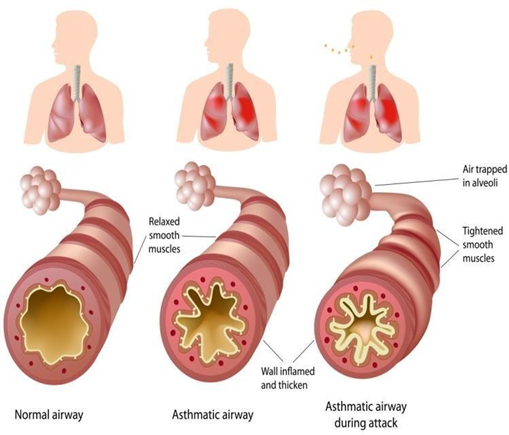
FigNo.1: Normaland Inflamed Bronchial Tube Due to Asthma
Causes of Asthma
It is thought to be caused by environmental factors and genetic interaction. These factors influence its responsiveness and severity.
1. Environmental factor: It includes cold air, allergens, smoke, and chemicals.
2. Genetic factor: Family history is a risk factor for asthma. If one identical twin is suffering from asthma, the probability of the other having the same disease is approximately about25%. It has been estimated that25genes were associated with asthma [9].
3. Medical condition: Those who are suffering from eczema or hay fever are at high risk of developing asthma.
4. Diagnosis: It can be diagnosed by-
a. Physical examination: Where your doctor checks breathing with the stethoscope.
b. Breathing test: Pulmonary function test measures air flow into and out of your lungs.
c. Spirometry:- The device which measures the speed of air.
1.1.2. Asthma classification includes:
1. Mild persistent: The symptoms last more than two weeks but not daily.
2. Moderate persistent: The symptoms occur daily.
3. Severe persistent: The symptoms occurs ever al times every day and mostly in nights (extremely worsen in night).
1.1.3. Treatment of asthma:
Asthma cannot be cured but it can be controlled by various pharmaceutical drugs can be used to quick relief but it may associate with certain side effects. Quick relief asthma treatment includes Bronchodilators (it can be taken as a nebulizer or inhaler, Anti inflammatories, anti-cholinergics, biological therapy drugs). So, to overcome the side effects that associated with treatment of asthma medicinal plants/herbs are used in the combination. Some of the medicinal herbs are turmeric, Ginkgobiloba, Allium sativum, Boswelliaserrata, Piperlongum, Lavandula officinalis, Clerode ndrum, A. vasica, Aervalanata[10].
1.2 Aerva lanata
Aerva lanata (Linn.) Juss. Ex Schult. belongs to the family Amaranthaceae, is one of the important plant grow in the warmer parts of India ascending to 1,000 m. In Sanskrit A. lanata is known as paash aanabheda, gorakshaganjaa, satkabhedi, aadaanpaak. Aervalanata is a common weed which grows wild everywhere in the plains of India. The root has a camphor-like aroma. The dried flowers which look likes of spikes, are sold under the commercial names Buikallan and Boor. It isone of the plants included in Dasapushpam, the ten sacred flowers of Kerala. It is commonly known as sirupeelarin Tamil or Siddha. [11] The plant is extensively used in urinary disorders like Ashmari (Urinary calculi), Mootrakrichra (Dysuria), Mootravikara etc by most of the Ayurveda and Siddha practitioners in southern India, in the name of Pashanabheda. As the plant bears almost all the properties similar to that of the original source of Pashanabheda. [12] Herbs are perennial, 5– 50cm tall. Stem branched from base; branches ascending or stoloniferous, white lanose. Leaves opposite or nearly whorled, sessile, grayish green, subulate, linear, 1–2.5 cm, abaxially whitelanose, adaxially glabrous, base attenuate, sometimes vaginate.[13] Flowers are small size, sessile, greenish or du white in colour, clustered with spikes. The phytoconstituents reported from Aervalanata plant are flavanoids, tannins, anthra-quinons, alkaloid, phenol, proteins, amino acids and carbohydrates.[14] The aerial part of the Aervalanata was studied by using adult albino mice of either sex. A. lanata is rich in lupeol, flavonoids, alkaloids. The ethanol extract of A. lanata showed a anti-asthmatic, anti-microbial activity[15].

FigNo.2: Aervalanata Plant
Molecular docking:
Molecular docking is a process through which small molecules are docked into the macro molecular structures for scoring its complementary values at the binding sites.It is a vibrant research area with dynamicutility instructure -baseddrug- designing,lead optimization ,biochemical pathway and for drug designing being the most attractive tools. It involves placing molecules in appropriate configurations to interact with a receptor. Molecular Docking is a natural process which occurs within seconds in a cell. In molecular modeling the term refers to the study of how two or more molecular structures fit together. Molecular docking aims to achieve an optimized conformation for both the protein and ligands and relative orientation between protein and ligand so that the energy of the overall system is minimized. In Molecular Docking, Drug designing can be achieved throught approaches (i.e.)
-
-
- Structure Based Drug Design: Drug design that relies on the knowledge of the 3-D structure of the bio molecular target is known as Structure based drug design.
- Ligand Based Drug Design: An approach used in the absence of the receptor molecule and it relies on the knowledge of molecules that bind to the biological target is known as Ligand based drug design.
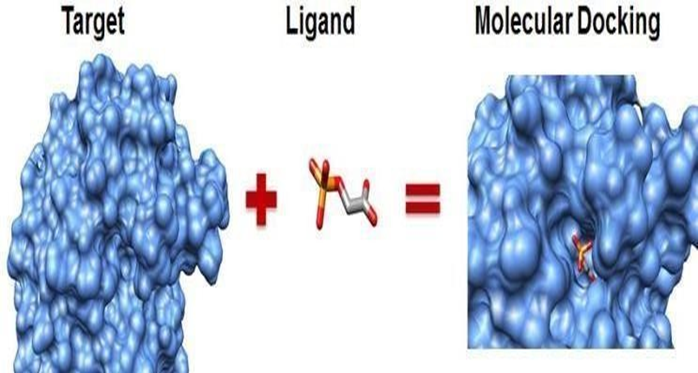
Fig.No.3 Molecular Docking
1.3.1. Experimental methods to study molecular docking:
Numerous experimental techniques are available to study molecular recognition. These include X- ray crystallography, NMR, electron microscopy, site directed mutagenesis, co- immune precipitation etc...They allow us to experimentally solve the detailed 3- dimensional structures of biomoleculesin their association form which is a necessary step in identifying crucial residues, study the strength of interaction forces, their energetics, understand how molecular structures fit together, and investigate mechanisms of action.
Fig.No.4: Computational Docking
1.3.2. Definition of computational docking:
Computational docking (also called in-silico molecular docking or just docking) is a computational science aiming at predicting the optimal binding orientation and conformation of interacting molecules in space, and to estimate the stability of their complex.
1.3.3. Application of Computational Docking:
- Computational docking is an essential component in modern drug discovery.
- Over the last few decades, it has been routinely and successfully applied in most pharmaceutical and biotech companies for a large number of applications.
- It covers the entire drug discovery field, including structure-activity relationships, lead finding and lead optimization.
- Some typical applications of molecular docking will be presented in the section entitled "Uses of Docking in Research"[16]
Fig.No.5: Computer Programmers Used for Docking Studies
Literature Survey:
- Immunomodulatory (2022):The anti-asthmatic effect of A. lanata might be contributed by the suppression of edema, pro-inflammatory cytokines and IgE antibodies, and elevation of aquaporin expression levels, suggesting future study and clinical trials to propose it as a candidate to treat allergic asthma. The anti-oxidant phytochemicals present in A. lanata might be responsible for such potential.
- Anti-bacterial activity (2018): Antimicrobial activity of Aerva lanata whole plant ethyl acetate and methanol extracts against Bacillussubtilis,B.cereus,S.aureus,E.coli,Salmonellatyphi, Shigellasonnei, Shigellaflexneriae, Shigellaboydii, Klebsiella, A.nigerfumigatus. The findings indicated that Ethyl acetate and Ethanol extracts of stems have high anti-bacterial activity when compared to conventional drugs. The entire plant Aervalanata was extracted with hydroalcohol, then fractionated withn-butanol and ethylacetate. At various dilutions, the fractions were tested for antibacterial efficacy against Escherichia coli, Clostridium species, Proteus species, and S. aureus. Antibacterial activity against microbiological infections were tested on the extracts.
-
- Anti-diuretic activity (2018): The amount of urine voided and the electrolyte concentration in urine were both influenced by Ecboliumli gustrinum. According to the findings, the roots of Aerva lanata and Ecboliumli gustrinumare diuretic. Furosemide was used as the standard medication in an experiment on male wister strain albino rats. Oral dosages of 20 mg/kg and 200 mg/kg were used for the standard and test, respectively. The urine output, pH, salt, potassium, and chloride amounts in the urine are all measured to determine the diuretic action.
- Nephroprotective activity (2015) : When employed against mercuric chloride, the extract, which was ethanolic in nature, demonstrated nephroprotective effect. In the kidneys and damage to the liver of the groups that were removed and treated, there was an increase in glutathione, Vitamin C, and antioxidant enzymes such glutathione oxidase, dismutase, catalase, and methyl transferase.[22]This analysis was also considered for studying the nephroprotective activity present in the cisplatin and gentamicin by inducing acute injuries in albino rats irrespective of their sex. In the entire process, the dosage of 75,150, and 300 mg/kg expressed a dosedependent decrease in the raised blood urea and serum creatinine levels. Also furtherhelped in normalising the histopathological reactions.
2.5. Toxicity and Safety (2013)
Studies have shown that Aerva lanata extracts are generally safe at therapeutic doses. Acute toxicity studies indicate a high LD50, suggesting a wide margin of safety.
2.6. Antioxidant activity (2012): Antioxidant compounds can be found in a variety of natural sources, including plants and microorganisms. Antioxidant properties have also been reported in many Aerva species. The aerial parts of A. lanata had significant antioxidant activity in both aqueous and metabolic extracts. Aqueous extract of A. lanata inhibited 2, 2-diphenyl-1- picrylhydrazyl (DPPH) extreme with an IC50 value of 110.74 g/mL ,according to another study.
2.7. Molecular Docking (2009)
Molecular docking predicts the preferred orientation of a molecule when bound to a protein. Morris et al. highlighted the use of AutoDock as a powerful tool for docking studies. Docking helps identify potential drug candidates by estimating binding affinities and interactions
2.8. Molecular Dynamics (MD) Simulations (2002)
MD simulations provide insights into the dynamic behavior of biomolecular systems. Karplus and McCammon illustrated the application of MD in understanding protein-ligand interactions and conformational changes, improving drug efficacy
Aim and Objective:
Aim: To perform virtual screening of AervaLanata for the Antiasthmatic Activity
Objective:
- To sketch the chemical constituents of Aervalanata.
- To study the molecular docking of the chemical constituents of Aervalanata.
- To Study the ADMET properties of selected phytoconstituents.
Plan of Work:
-
- Literature survey.
- Downloading and installing all the required software programs.
- Molecular docking study.
- Preparation of phytoconstituents.
- Selection of preparation of proteins for docking.
- Virtual-screening.
- Study of ligand-protein interaction.
- ADMET study.
- ADME evaluation using swiss ADME.
- Toxicological study.
Experimental Work:
3.1 Protein Preparation:
RCSB (Research Collaboratory for Structural Bioinformatics) Protein Data Bank was used to retrieve the three-dimensional structure of receptor PDB-ID:7DFL download the structure in .pdb format from the online data base and was rectified using auto dock software which is already present in the PyRx software. Preparation of receptor was done with the help of Discovery studio. The pdb format isopened in the discovery studio and then press Ctrl+H and then remove the pre-associated ligand presentin the receptor also hetero atoms and water molecules present in structure were cleaned and the active sites were identified and then saved in the working folder as pdb file.
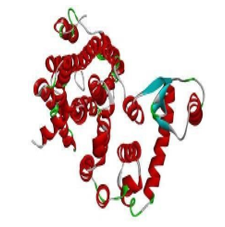
FigNo.6: Structure of antiasthmatic enzyme receptor (PDB-ID:7DFL
Ligand Preparation:
Library of active phytochemicals of Aerva Lanata plant species which is responsible for antiasthmatic activity were retrieved from literature. PubChem compound data base (https://pubchem.ncbi.nl m.nih.gov) was used for the retrieval of structures in PDB (Protein data bank) format. Preparation of ligand file used the software’s and online library from the PubChem for their specification Smiles notation were copied from PubChem database and then structure was generated. From this smile notation in Chem Sketch afterwards this smile notation was used to retrieve ligands to3DPDB format with the help of Avogadro software.
Downloading Software Program:
Chemsketch:
ChemSketch is a molecular modeling program used to create and modify images of chemical structures. It allows molecules and molecular models displayed in two and three dimensions, to understand the structure of chemical bonds and the nature of the functional groups.[9]
Avagadro: Avogadro is a molecule editor and visualizer designed for cross platform use in computational chemistry, molecular modeling, bioinformatics, materials science, and related areas. It helps to convert mol file into PDB format.[9]
PyRx:
PyRx is Virtual Screening software for Computational Drug Discovery that can be used to screen libraries of compounds against potential drug targets. It helps users in every step of this process - from data preparation to job submission and analysis of the results. [22]. Now the PyRx software is used for to obtain the docking score or binding score. TheVinaWizard module was started and selected the ligand and the Macromolecule and converted into .pdbqt formats. The grid was selected and the screening was carried out.
Swiss ADME:
Swiss ADME software (www.swissadme.ch) of Swiss institute of bio-informatics (http://www.sib.swiss) was accessed in a web server that displays the Submission page of Swiss ADME in Google was used to estimate individual ADME behaviors of the compounds from the plant. The input zone itself contains a molecular sketcher based on Chem Axons Marvin JS(http://www.chemax on.com) that allowed the user to draw and edit 2D chemical structures. The structures are transferred as a list to the right-hand side of the submission page, which is the actual input for computation. The list is made to contain one input molecule per line with several inputs, defined by simplified molecular input line entry system (SMILES) and the results are presented for each molecule in tables, graphs and also an excel spreadsheet.[23]
BIOVIA discovery studio:
BIOVIA Discovery Studio brings together over 30 years of peer-reviewed research and world class insilico techniques such as molecular mechanics, free energy calculations, bio therapeutics develop ability and more into a common environment. It provides researchers with a complete toolset to explore the nuances of protein chemistry and catalyse discovery of small and large molecule therapeutics from target ID to Lead Optimization. With Discovery Studio you can: Investigate and test hypotheses in silico prior to costly experimental implementation, thus reducing the time and expense involved in bringing products to market Drive scientific exploration from target identification to lead optimization with a wealt of trusted life science modelling and simulation tools. [24]
RESULT:
Docking Studies:
The in silico study showed phytoconstituents of the Aervalanata having potent Antiasthamatic activity. These studies can be helpful to design molecule with better specificity at receptor level and be safe. The compound Chlorpheniramine shows the best affinity towards 7dfl antiasthamatic receptor. From this Docking results identified the best molecule interact with the receptor these selected one molecule used for the docking score -8.3.
4.2 Analyzing the ADMET properties and screening of ligands:
Absorption, Distribution, Metabolism and Excretion (ADME) are significant properties which defines pharmacokinetic and pharmacodynamic profile of the drugs. Thus, these properties of each ligand, were determined using Swiss ADME.
4.3 Toxicity study of selected from the plant Aerva lanata:
The plant has many medicinal properties, however, it shows some toxicity reactions after long term usage. The plant possesses a wide variety of pharmacological activities. Different parts of plants have different functions. The phytochemicals present in the plant contribute to a wide variety of therapeutic activities. Different combinations of different parts of plants can be studied and bring out more properties. Further research should be conducted to fully understand the mechanism of action and evaluate its efficacy and how safely it can be applied in humans. From the analysis of ADMET properties (shown in table1),it was observed that Narcissin, Rutinand Kaempferol-3-rhamnoglucoside were the compounds which didn’t followed Lipinski’s rule; which is a significantly required property for orally administered drugs. Consequently, these 3 compounds were excluded from the further study. The docking of the receptor 1dlr with chemical constituents of Aerva lanata has been done by using. The table shows the binding affinity and inhibition constant of 18 compounds including standard. In silico studies revealed that all the chemical constituents show good binding affinity toward the target protein. Protox3.0 is a powerful tool in the realm of computational toxicology, offering predictive insights into the potential toxicity of various chemical compounds, particularly ligands. It analyses the structural properties of provided ligands to forecast their toxicity profiles. Thus, it was used to determine the toxicity profile of ligands.
TableNo.1: Docking Score of Aerva lanata phytoconstituent with Antiasthamatic receptor (PDB-ID:7DFL)
|
Sr. No
|
Ligand Name
|
Ligand Structure
|
Docking Score
|
|
1.
|
Rutin
|

|
-8.3
|
|
2.
|
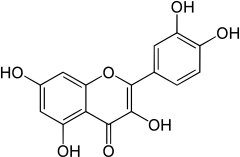
Quercetin
|
|
-5.1
|
|
3.
|
Myricetin
|

|
-7.3
|
|
4.
|
Isorhamnetin
|
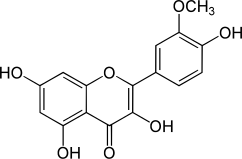
|
-8.7
|
|
5.
|
Kaempferol
|
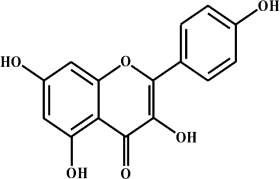
|
-7.3
|
|
6.
|
Narcissin
|
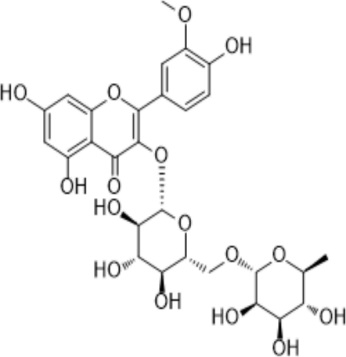
|
-7.9
|
|
7.
|
Methergine (Methylergonovine)
|
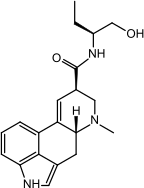
|
-6.9
|
|
8.
|
Norisoboldine
|
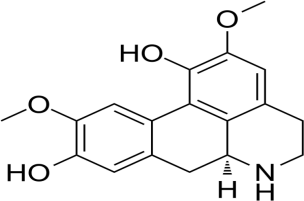
|
-6.2
|
|
9.
|
Kaempferol-3-rhamnogalactoside
|
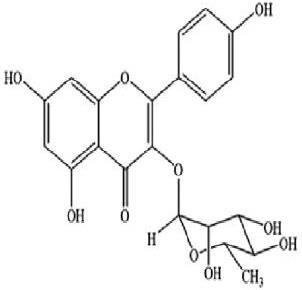
|
-8
|
|
10.
|
Methergine
|
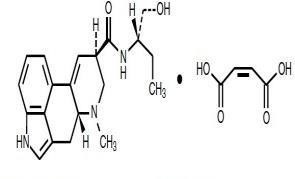
|
-6.9
|
|
11.
|
Ferulic acid
|
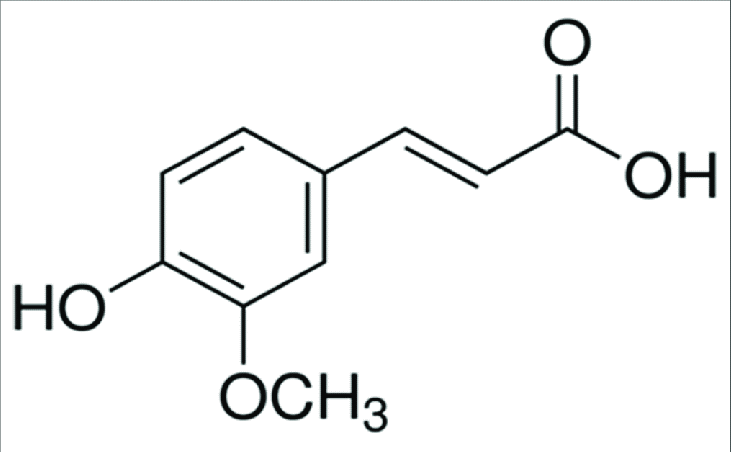
|
-6.3
|
|
12.
|
Gallic acid
|
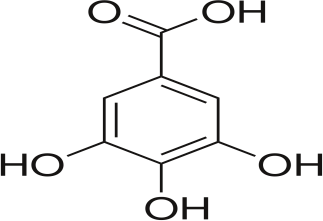
|
-6.2
|
|
13.
|
Canthin-6-one
|
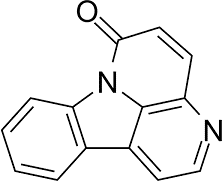
|
-6.5
|
|
14.
|
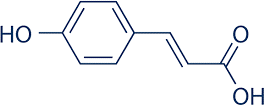
p-Coumeric acid
|
|
-5.7
|
|
15.
|
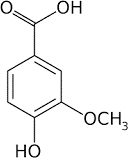
Vanillic acid
|
|
-5.3
|
|
16.
|
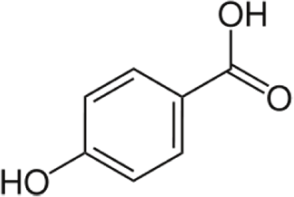
PHBA(p-Hydroxybenzoicacid)
|
|
-6.4
|
|
17.
|
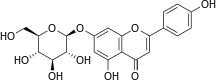
Apigetrin
|
|
-7.8
|
|
18.
|
Chlorpheniramine (Standard)
|
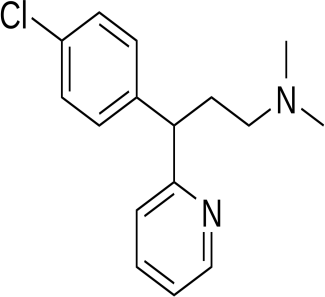
|
-8.3
|
5.2Visualization of docking interaction:
Docking analysis is a complex process which involves analysis of various ligand-receptor interactions like hydrogen bonding interactions, hydrophobic interactions, π-π stacking, metalco- ordination etc. Here are some ligands were analyzed for their receptor interactions as:
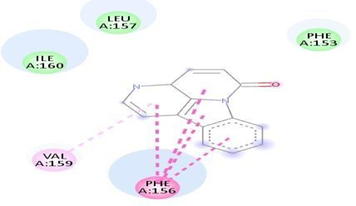
Fig.No.7: Aminoacid interaction of chlorpheniramine with7DFL
Above fig shows the amino acid interaction of standard chlorpheniramine with 7DFL gives bindingscore-8.3
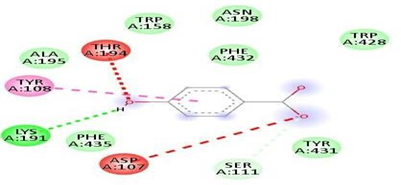
Fig.No.8: Aminoacid interaction of rutin with 7DFL
Above fig shows the amino acid interaction of standard rutin with 7DFLgives bindingscore-8.3
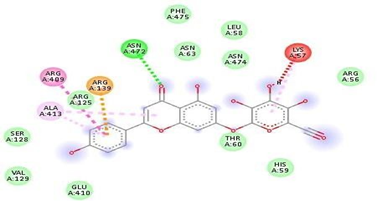
Fig.No.9: Aminoacid interaction of canthin-6-one with 7DFL
Above fig shows the amino acid interaction of standard Canthin-6-one with 7DFL gives bindingscore-6.5.7
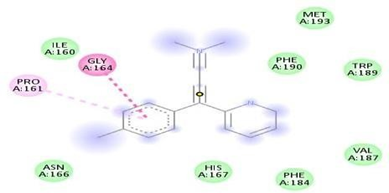
Fig.No.10: Amino acid interaction of ferulic acid with 7DFL
Above fig shows the amino acid interaction of standard ferulic acid with 7DFL gives bindingscore-6.3
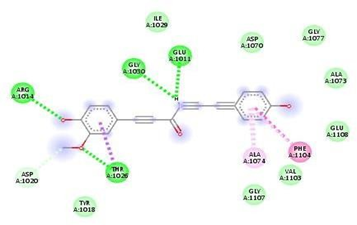
Fig.No.11: Amino acid interaction of gallic-acid with 7DFL
Above fig shows the amino acid interaction of standard gallic acid with 7DFL gives binding Score -6.2.

Fig.No.12: Aminoacid interaction of isorhamnetin in with7DFL
Above fig. shows the amino acid interaction of std. isorhamnetin with7DFL gives binding score-8
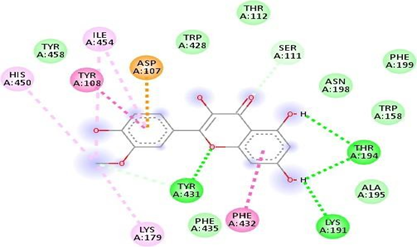
Fig.No.13: Aminoacid interaction of kaempferol with 7DFL
Above fig shows the amino acid interaction of standard kaempferol with 7DFL gives bindingscore-7.3
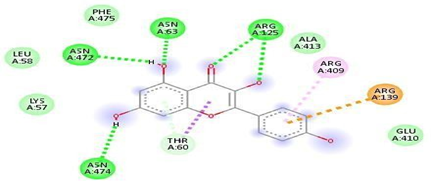
Fig.No.14: Amino acid interaction of kaempferol-3-rhamnogalactoside with7DFL.
Above fig shows the amino acid interaction of standard kaempferol-3-rhamnogalactoside with7DFLgives binding score -8.0
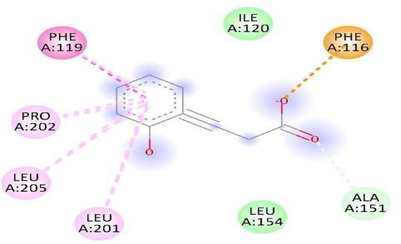
Fig.No.15: Aminoacid interaction of methergine with 7DFL
Above fig shows the amino acid interaction of standard methergrine with 7DFL gives binding Score-6.9.
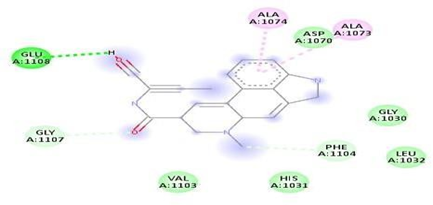
FigNo.16: Aminoacid interaction of myricetin with7DFL Above fig shows the amino acid interaction of standard myricetin with 7DFL gives bindingScore-7.3
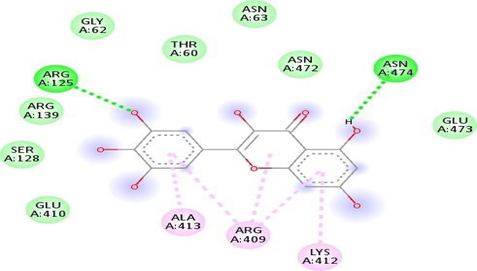
FigNo.17: Aminoacid interaction of narcissin with7DFL Above fig shows the amino acid interaction of standard Narcissin with 7DFL gives bindingscore-7.9
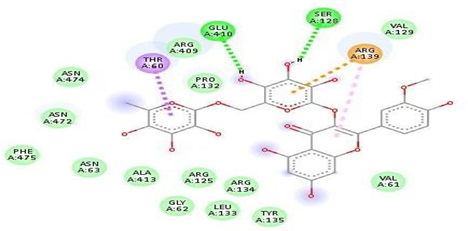
Fig.No.18: Aminoacid Interaction Of P-Coumeric Acid With7DFL
Above fig shows the amino acid interaction of standard p-coumericacid with 7DFL gives binding score-5.7
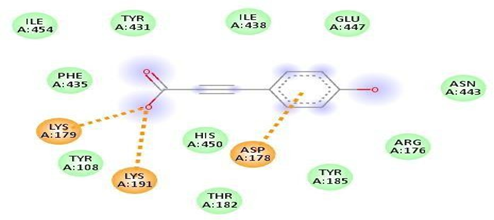
Fig.No.19: Aminoacid interaction of PHBA with7DFL
Above fig shows the amino acid interaction of standard PHBA with 7DFL gives binding score-6.4
Table2: Chemical and Physical properties of selected ligands (Swiss ADME)
|
Sr.no
|
Ligand
|
MW
|
Rotateable bonds
|
Hbond
acceptor
|
#HBonddonrs
|
Lipinskivioations
|
Bioavailability Score
|
Lead like ne ss# violations
|
|
1.
|
Rutin
|
610.52
|
6
|
16
|
10
|
3
|
0.17
|
1
|
|
2.
|
Quercetin
|
302.24
|
1
|
7
|
5
|
0
|
0.55
|
0
|
|
3.
|
Myricetin
|
318.24
|
1
|
8
|
6
|
1
|
0.55
|
0
|
|
4.
|
Isorhamnetin
|
316.26
|
2
|
7
|
4
|
0
|
0.55
|
0
|
|
5.
|
Kaempferol
|
286.24
|
1
|
6
|
4
|
0
|
0.55
|
0
|
|
6.
|
Narcissin
|
624.54
|
7
|
16
|
9
|
3
|
0.17
|
1
|
|
7.
|
Methylergonov
ine
|
455.5
|
7
|
7
|
5
|
0
|
0.56
|
1
|
|
8.
|
Norisoboldine
|
313.35
|
7
|
4
|
3
|
0
|
0.55
|
0
|
|
9.
|
Kaempferol-3- rhamno
glucoside
|
714.62
|
10
|
19
|
13
|
3
|
0.17
|
2
|
|
10.
|
Methergine
|
455.5
|
7
|
7
|
5
|
0
|
0.56
|
1
|
|
11.
|
Ferulic acid
|
194.18
|
3
|
4
|
2
|
0
|
0.85
|
1
|
|
12.
|
Gallic acid
|
170.12
|
1
|
5
|
4
|
0
|
0.56
|
1
|
|
13.
|
canthin-6-one
|
220.23
|
0
|
2
|
0
|
0
|
0.55
|
1
|
|
14.
|
p-Coumericacid
|
164.16
|
2
|
3
|
2
|
0
|
0.85
|
1
|
|
15.
|
Vanillic acid
|
168.15
|
2
|
4
|
2
|
0
|
0.85
|
1
|
|
16.
|
PHBA(p-Hydroxybenzoi cacid)
|
138.12
|
1
|
3
|
2
|
0
|
0.85
|
1
|
|
17.
|
Ethylvanillin
|
166.17
|
3
|
3
|
2
|
0
|
0.85
|
1
|
|
18.
|
Apigetrin
|
432.38
|
4
|
10
|
6
|
1
|
0.55
|
1
|
Observation:
Most ligands, such as Quercetin, Kaempferol, and Myricetin, demonstrate favorable drug-likeness based on Lipinski's rule with minimal violations and good bioavailability scores. Compounds like Rutin and Kaempferol-3-rhamnoside show higher molecular weights and multiple violations, suggesting lower oral bioavailability. Overall, several phytoconstituents of Aerva lanata exhibit promising properties for further investigation as anti-asthmatic agents.
Table3: Chemical And Physical Properties of Selected Ligands (Swiss ADME)
|
Sr no
|
Ligand
|
MW
|
Consensus Log P
|
Metabolism
|
Excretion
|
GI
absorption
|
BBB permeant
|
|
1.
|
Rutin
|
610.52
|
-1.29
|
Yes
|
No
|
Low
|
No
|
|
2.
|
Quercetin
|
302.24
|
1.23
|
No
|
Yes
|
High
|
No
|
|
3.
|
Myricetin
|
318.24
|
0.79
|
No
|
Yes
|
Low
|
No
|
|
4.
|
Isorhamnetin
|
316.26
|
1.65
|
No
|
Yes
|
High
|
No
|
|
5.
|
Kaempferol
|
286.24
|
1.58
|
No
|
Yes
|
High
|
No
|
|
6.
|
Narcissin
|
624.54
|
-0.8
|
Yes
|
No
|
Low
|
No
|
|
7.
|
Methylergo -novine
|
455.5
|
1.41
|
Yes
|
No
|
Low
|
No
|
|
8.
|
Norisoboldine
|
313.35
|
2.39
|
No
|
No
|
High
|
No
|
|
9.
|
Kaempferol rh amnoglucosd
|
714.62
|
-2.43
|
No
|
No
|
Low
|
No
|
|
10.
|
Methergine
|
455.5
|
1.41
|
Yes
|
No
|
Low
|
No
|
|
11.
|
Ferulic acid
|
194.18
|
1.36
|
No
|
No
|
High
|
Yes
|
|
12.
|
Gallic Acid
|
170.12
|
0.21
|
No
|
No
|
High
|
No
|
|
13.
|
canthin-6-one
|
220.23
|
2.39
|
No
|
Yes
|
High
|
Yes
|
|
14.
|
p-Coumericacid
|
164.16
|
1.26
|
No
|
No
|
High
|
Yes
|
|
15.
|
Vanillic acid
|
168.15
|
1.08
|
No
|
No
|
High
|
No
|
|
16.
|
PHBA(p-Hydroxybenzoic acid)
|
138.12
|
1.05
|
No
|
No
|
High
|
Yes
|
|
17.
|
Ethylvanillin
|
166.17
|
1.35
|
No
|
No
|
High
|
Yes
|
|
18.
|
Apigetrin
|
432.38
|
0.55
|
Yes
|
No
|
Low
|
No
|
Observation:
Several ligands like Kaempferol, Ferulic acid, and p-Coumaric acid show favourable GI absorption and BBB permeability, suggesting good bioavailability and CNS accessibility.. The study highlights that certain phytoconstituents from Aerva lanata possess promising drug-like properties, supporting its traditional use in asthma management.
Table4: Toxicity Study of Selected Ligands:
|
SR NO.
|
Ligands
|
Predicted toxicity
|
Carcinogi city
|
AMES
Toxicity
|
Hypertoxicity
|
Respiratory Toxicity
|
|
1.
|
Rutin
|
4
|
inactive
|
active
|
Inactive
|
Inactive
|
|
2.
|
Quercetin
|
2
|
inactive
|
moderate
|
Inactive
|
inactive
|
|
3.
|
Myricetin
|
2
|
inactive
|
moderate
|
Inactive
|
inactive
|
|
4.
|
Isorhamnetin
|
2
|
inactive
|
moderate
|
Inactive
|
inactive
|
|
5.
|
Kaempferol
|
2
|
inactive
|
moderate
|
Inactive
|
inactive
|
|
6.
|
Narcissin
|
4
|
inactive
|
active
|
Inactive
|
inactive
|
|
7.
|
Methergine
|
3
|
inactive
|
inactive
|
Inactive
|
active
|
|
8.
|
Feruloyltyra
Mine
|
2
|
moderate
|
inactive
|
Inactive
|
moderate
|
|
9.
|
Kaempferol-3- Rhamnogalact Oside
|
5
|
inactive
|
inactive
|
Inactive
|
inactive
|
|
10.
|
Ferulic Acid
|
2
|
moderate
|
active
|
Moderate
|
active
|
|
11.
|
Gallic Acid
|
2
|
inactive
|
inactive
|
Inactive
|
moderate
|
|
12.
|
Canthin-6-One
|
4
|
active
|
active
|
Active
|
active
|
|
13.
|
P-Coumer Ic
Acid
|
3
|
inactive
|
inactive
|
Moderate
|
moderate
|
|
14.
|
Vanillic Acid
|
7
|
inactive
|
inactive
|
Inactive
|
inactive
|
|
15.
|
Phba
|
1
|
inactive
|
inactive
|
Moderate
|
moderate
|
|
16.
|
Melilotic Acid
|
2
|
inactive
|
inactive
|
Inactive
|
inactive
|
|
17.
|
Apigetrin
|
3
|
active
|
inactive
|
Inactive
|
inactive
|
Observation:
Most ligands, particularly flavonoids like Quercetin, Myricetin, and Kaempferol, exhibit low predicted toxicity (scores between 1–3), inactive carcinogenicity, and inactive or moderate AMES toxicity. They also show no significant hypertoxicity or respiratory toxicity, suggesting a favorable safety profile. A few compounds like Vanillic Acid show higher predicted toxicity but remain non-carcinogenic and safe in respiratory aspects.
5. DISCUSSION:
The anti-asthamatic action of Aerva lanata is probably mediated by a number of mechanisms. Numerous bioactive substances, such as flavonoids, alkaloids, and terpenoids, have been found by phytochemical study and may be responsible for the plant's antiallergic qualities.
• Molecular Weight (MW): A compound's pharmacokinetic characteristics, including absorption, distribution, metabolism, and excretion (ADME), can be influenced by its molecular weight. In general, smaller molecules have better dispersion and absorption characteristics than bigger ones. Nevertheless, depending on the particulars of the molecule, there might be an exception.
• Lipophilicity (iLOGP): Pharmacokinetics and drug design both heavily rely on lipophilicity. Moderately lipophilic compounds frequently have the best distribution and absorption characteristics. Low lipophilicity may result in inadequate tissue penetration, whereas high lipophilicity may cause poor aqueous solubility. For drugs to function as well as possible, lipophilicity and hydrophilicity must be balanced.
• Absorption from the Gastrointestinal Tract: This signifies the probability of a substance being taken up by the systemic circulation from the gastrointestinal tract. Good bioavailability is indicated by high GI absorption, but low absorption may call for formulation techniques to improve absorption, such as pro drugs or the use of permeation enhancers.
• Blood-Brain Barrier(BBB):
A compound's capacity to pass through the blood-brain barrier and enter the central nervous system is determined by its BBB permeability. Certain medications must cross the blood-brain barrier (BBB) in order.
6. CONCLUSION:
Based on the provided data on the toxicity of various ligands with varying MW, lipkinski’s rule of five violations, bioavailability scores, lead likeness violations were analyzed and the following conclusions can be drawn:
1. Rutin, Narcissin, and Canthin-6-one exhibit significant toxicity, particularly in terms of carcinogenicity and hypertoxicity.
2. Quercetin, Myricetin, Isorhamnetin, Kaempferol, Feruloyltyramine, Ferulic acid, Gallic acid, P- Coumaric acid, and Apigetrin show varying levels of toxicity, but generally are less toxic compared to Rutin, Narcissin, and Canthin-6-one.
3. Methergine, Vanillic acid, Melilotic acid, and Kaempferol-3-rhamnogalactoside appear to be relatively safer options with low toxicity.
4. PHBA demonstrates low toxicity but some potential for respiratory toxicity.
5. Among the tested ligands several exhibit low predicted toxicity levels, indicating their potential safety for the therapeutic or industrial application.
6. Ligands such as quercetin, myricetin, isorhamnetin, kaempferol, and ferulic acid demonstrate moderate toxicity level, suggesting the need for cautious consideratiom in their usage.
Overall, while some ligands like Rutin, Narcissin, and Canthin-6-one pose significant health risks, others present moderate to low toxicity levels. Further studies may be needed to fully understand the mechanisms and implications of their toxicity in different contexts.
7. Future Scope:
• There are multiple areas in which future research directions in the field of plant-derived antasthamatic treatments will focus.
• First off, pinpointing the precise molecular targets and signaling pathways of bioactive substances would help us better understand how they work and make it easier to create focused therapies.
• Second, evidence-based practice depends on carrying out well planned clinical trials to evaluate the safety, effectiveness, and ideal dosage schedules of plant-based antiasthamatic treatments in arrange of patient populations.
• Furthermore, investigating synergistic interactions between conventional antiasthamatic and phytoconstituents or other natural substances may improve the effectiveness of treatment and reduce.
8. ACKNOWLEDGEMENT
The authors are thankful to the principal of Priyadarshini J.L. College of Pharmacy. The resources and academic environment played a vital rolein the successful completion of this research work.
REFERENCES
- Lee J, McDonald C. Review: Immunotherapy improves some symptoms and reduces long-term medication use in mild to moderate asthma. Ann Intern Med.2018 Aug21;169(4):JC17
- Tesfaye ZT, Gebreselase NT, Horsa BA. Appropriateness of chronic asthma management and medication adherence in patients visiting ambulatory clinic of Gondar University Hospital: across-sectional study.World Allergy Organ J. 2018;11(1):18.
- Salo PM, Cohn RD, Zeldin DC. Bedroom Allergen Exposure Beyond House Dust Mites. CurrAllergyAsthma Rep. 2018 Aug 20;18(10):52.
- SciricaCV, Celedón J C. Genetics of asthma: potential implications for reducing asthma disparities.Chest.2007 Nov;132(5 Suppl):770S-781S.
- Piloni D, Tirelli C, Domenica RD, Conio V, Grosso A, Ronzoni V, Antonacci F, Totaro P,Corsico AG. Asthma-like symptoms: is it always a pulmonary issue? MultidiscipRespirMed.2018;13:21.
- AggarwalB, MulgirigamaA, Berend N. Exercise-induced broncho constriction:prevalence, pathophysiology, patient impact, diagnosis and management. NPJ Prim Care Respir Med. 2018Aug 14;28(1):31.
- YiiAC,Soh AZ, Chee CBE, Wang YT, Yuan JM,Koh WP. Asthma, Sinonasal Disease, and the Risk of Active Tuberculosis. JAllergy ClinImmunol Pract. 2019Feb;7(2):641-648.e1.
- D'Amato M, Molino A, Calabrese G, Cecchi L, Annesi-Maesano I, D'Amato G. The impact of cold on the respiratory tract and its consequences to respiratory health. ClinTranslAllergy.2018;8:20.
- Burrows B, Barbee RA, Cline MG, Knudson RJ, Lebowitz MD. Characteristics of asthma among elderly adults in a sample of the general population. Chest. 1991 Oct;100(4):935-42.
- MartinAJ, LandauLI, PhelanPD.Lung function in young adults who had asthma in childhood.Am Rev Respir Dis.1980 Oct;122(4):609-16.
- Ragavendran P, Sophia D, Arul Raj C, Gopalakrishnan VK.Functional group analysis ofvariousextractsofAervalanata(L.,)by FTIR spectrum.Pharmacologyonline.2011;1:358-64
- AdepuA, NaralaS, GanjiA, Chilvalvar S.A review on natural plant: Aervalanata. IntJPharmaSci.2013;3( 6):398-402.
- Bitasta M, Madan S. Aervalanata: A blessing of mother nature. J PharmacognPhytochem.2016;5(1):92-101.
- Sharif M, Anjum I, Shabbir A, Mushtaq MN. Immuno modulatory and antiinflammatory effects of Aervalanata inovalbumin induced allergic asthmatic mice. JEthnopharmacol.2022;289:115 087
- Shirwaikar A, Issac D, Malini S. Effect of Aervalanata on cisplatin and gentamicin models ofcuterenal failure.JEthnopharmacol.2004;90:81–6.
- ElieCohen(Synergix), ClaudeCohen (Synergix), DinaSchneidman (WolfsonandNussinovgroup,Tel-AvivUniversity-Israel), ttps://www.drugdesign.org/chapters/molecular-docking/
- T.A. Selvakumar, P. Rajasekar Aerva lanata mediated phyt of abrication of silver nanoparticles and evaluation oftheir antibacterial activity against wound associated bacteria J. Taiwan Inst.Chem.Eng., 78 (2017), pp. 539-551
- G. Kumar, L. Karthik, K.V. Rao Phytochemical composition and in vitro antioxidant activity of aqueous extract of Aerva lanata (L.) Juss. exSchult. Stem(Amaranthaceae) AsianPacificJ.Trop.Med.(2013), pp.180-187.
- D.M. Simona, F. Carmen, Z. Franco, N. Antonella, A. Lina, R. Gennaro, I
- Maria Antioxidant activity and chemical components as potential anticancer agents in the olive leaf(Ole aeuropaeaL.cvLeccino.)DecoctionAnti-Cancer Agent Med.Chem.,14(2014),pp.1376-1385.
- O.I. AruomaFree radicals, oxidative stress, and antioxidants in human health and diseaseJ. Am.OilChem. Soc., 75 (1998), pp.199-212
- M. Yamunadevi, E.G. Wesely, M. Johnsona chromatographic study on the glycosidesofAervalanata LChinese J. Nat. Med., 9 (3) (2011), pp.0210-0214 "Chemsketch".Archived fromtheoriginalon3July2014.Retrieved 21August 2014.
- Hanwell, Marcus D; Curtis, Donald E; Lonie, David C;Vandermeersch, Tim; Zurek, Eva;Hutchison,GeoffreyR(2012)."Avogadro: Anadvanced semantic chemical editor, visualization, and analysis platform".
- "Chemsketch".Archived from the original on 3July2014.Retrieved 21August 2014.
- Yair T, et al. “Protein-Protein and Protein-Ligand Docking.” Protein Engineering - Tech and App. First edition may 2013;187
- Ncbi nationl library of medicine (gene), National institute of health, (https://www.ncbi.nlm.nih.gov/gene ) accessed on dated,20, Jan 2024
- Swiss model, Swiss institute of bioinformatics,( https://swissmodel.expasy.org/ ) accessed dated,20, Jan 2024
- Protein data bank, RCSB, (https://www.rcsb.org/) accessed on dated,17, Jan 2024
- Biovia, Dassault system, (https://www.3ds.com/products/biovia), accessed on dated,17, Jan 2024
- Guedes IA, et al. “Receptor-ligand molecular docking.” Biophys Rev. 2014 Mar;6(1):75-87.
- Pyrx, Source fordge (https://pyrx.sourceforge.io/ ) , accessed on dated,17, Jan 2024
- Kumar CJ, et al. “Computer Aided Molecular Docking Studies on a Series of Benzothiazepines as Potential Anti Convalescent Agents.” Int. J. Pharm. Sci. Rev. Res., 47(2), 2017; 14-17
- Baroroh U, et al. “ Molecular interaction analysis and visualization of protein-ligand docking using Biovia Discovery Studio Visualizer.” Indonesian journal of comput biol.2; 2023;22-30
- Javed MR. “CADD and Molecular Dynamic Simulations: Potential Impacts to Conventional Medicines.” Comb Chem High Throughput Screen. 2022;25(4):658-659.
- Sliwoski G, et al. “Computational methods in drug discovery.” Pharmacol Rev. 2013 Dec 31;66(1):334-95.
- Karthikeyan M, Deepa K, et al. (2010). "Bronchodilator and anti-asthmatic activity of Aerva lanata in experimental models." Journal of Ethnopharmacology.
- Warrier PK, et al. (1994). Indian Medicinal Plants – A Compendium of 500 species. Orient Longman.
- Singh A, Meena H, et al. (2015). "Antioxidant and anti-inflammatory activities of Aerva lanata." Pharmacognosy Research.
- Rao CM, et al. (2012). "Evaluation of Aerva lanata extract on histamine-induced bronchospasm in guinea pigs." International Journal of Green Pharmacy.
- Patel M, et al. (2013). "Toxicity studies of Aerva lanata." Journal of Medicinal Plants Research.
- Ravindra, R., Hiremath, S. P., & Patil, M. B. (2012). Antiasthmatic activity of Aerva lanata Linn. against histamine and acetylcholine induced bronchospasm in guinea pigs. Indian Journal of Pharmacology, 44(1), 123–126.
- Gandhi, G. R., & Ignacimuthu, S. (2014). Evaluation of anti-asthmatic and anti- inflammatory properties of Aerva lanata in experimental animal models. Journal of Ethnopharmacology, 151(3), 1185–1190.
- Sathiyanarayanan, L., & Arulmozhi, S. (2007). Ethnobotanical studies of medicinal plants used by Malayali tribes in Salem district of Tamil Nadu, India. African Journal of Traditional, Complementary and Alternative Medicines, 4(2), 179–182.
- O. Gaulton, A., et al. ChEMBL: a large-scale bioactivity database for drug discovery. Nucleic Acids Research, 2012, 40(D1), D1100-D1107.
- P. Sterling, T., & Irwin, J.J. ZINC 15 – Ligand Discovery for Everyone. Journal of Chemical Information and Modeling, 2015, 55(11), 2324-2337.
- Parish, T., & Stoker, N.G. Mycobacteria protocols. Methods in Molecular Biology, 1998, 101, 1–342.
- Ponraj, P., & Anbarasu, A. Molecular docking and simulation studies of TB target proteins with natural compounds. Asian Pacific Journal of Tropical Disease, 2014, 4, S674–S678.
- R. Cheng, F., Li, W., Zhou, Y., et al. admetSAR: a comprehensive source and free tool for assessing chemical ADMET properties. Journal of Chemical Information and Modeling, 2012, 52(11), 3099-3105.
- S. Rathi, E., Mehta, P., Yadav, A. Drug discovery and design using in silico techniques: a brief overview. Asian Journal of Pharmaceutical and Clinical Research, 2015, 8(1), 15-19.
- Schnecke V. Kuhn LA. Virtual Screenitig with Solvation and Ligandinduced Complementarity, Perspect. Drug Discov. 2000, 20:171-190


 Dinesh Kawade*
Dinesh Kawade*
 Sejal Kshirsagar
Sejal Kshirsagar





































 10.5281/zenodo.15241829
10.5281/zenodo.15241829