Abstract
Pharmaceutical cocrystal is an approach to physicochemical and mechanical properties of a drug substance the cocrystal are composed of conformer and API which are mainly used for solubility and dissolution mechanical properties and stability of drug. this review will provide information about what is cocrystal, how they are prepared, what is the advantages behind the pharmaceutical cocrystal preparation, the whole cocrystal development pathway in which all the steps involved in the cocrystal formation from selection of API to the after preparation scale up process are given. the concept of multidrug cocrystal are given, then the different method of crystallization the main focused on this review on herbal cocrystal it provide information about herbal cocrystal. Case studies of varies herbal cocrystal have been discussed. The chart of herbal drug along with their conformer and the method of preparation and application of cocrystal. we can achieve successful drug delivery through co-crystallization. the pharmaceutical industry will beneficial through successful development of co-crystallization of herbal drug substance.
Keywords
Pharmaceutical cocrystal, API, conformer, solubility, dissolution, herbal cocrystal.
Introduction
The solubility process is a ability to amount of solute dissolve in solvent to form a solution below a particular situation of gravity and temperature solubility play a vital role in dissolution procedure to complete a movement for required response and solubility of drug for the better bioavailability The solubility and dissolution is an important aspect in the rate determining step in absorption of drug most of the drug developed in pharmaceutical industry are insoluble in water and therefore to enhancing the solubility of practically insoluble drug was most challenging aspect For enhancing the solubility of drug and bioavailability we design a pharmaceutical cocrystal Mainly solubility is the maximum amount of drug dissolve in given amount of solvent or conc of solute in saturated solution at a certain temp ad pressure of certain chemicals good solubility influence the therapeutic efficacy of pharmaceuticals therefor in order to improve solubility and dissolution rate formulation scientist often use various approaches such as polymorphic and amorphous form solid dispersion and inclusion complex co-crystallization alters the molecular interaction and composition of pharmaceutical material the different technique such as traditional PH, particle size ,solid dispersion, hydrotropy and now a day advance technique such as micronization, nano suspension, homogenization salt formation spray drying ,holt melt extrusion, solvent evaporation and conventional technique1 .
2. What are the Co-crystal
Pharmaceutical cocrystal is defined as multicomponent complex non covalently bonded in which one is API and other is co-former. Cocrystal having several types of interaction from which they can formed hydrogen bond pie, bond and wander wall forces. Very first known cocrystal quinhydrone was studied by Friedrich Wholer in 1844 for drug to give its pharmacological action it should be absorbed from the site of administration Cocrystal is a crystal line form that contain many compounds in the crystal. A cocrystal is a multiple component crystal in which all component are solid under ambient condition when in their pure form2.
3. Advantages of Pharmaceutical Cocrystal
1. it is the stable crystalline form as compare to amorphous solid.
2. It can enhance the solubility of poorly water-soluble drug.
3. It can enhance bioavailability due to enhanced solubility3
4. Co crystal formation technique may be used for purification step4.
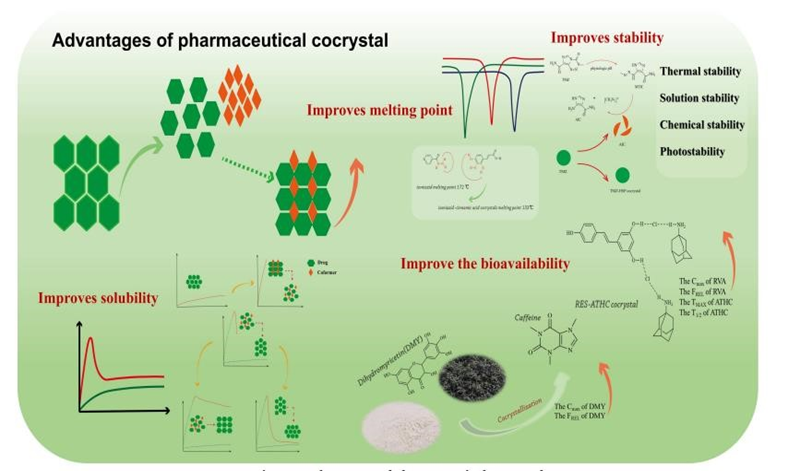
Figure 1 Advantages of Co-crystal
4. How are Cocrystals Prepared?
Supramolecular hetero synthons, such as carboxylic acid–aromatic nitrogen carboxylic acid–amide and alcohol–pyridine, are now widely reported and appear to favor the production of cocrystals. In addition to slow evaporation from a solution containing stoichiometric proportions of the components (cocrystal formers), other effective methods for creating cocrystals include sublimation, growth from the melt, and grinding two or more solid cocrystal formers in a ball mill. Indeed, the 1840s saw the introduction of dry grinding, and as previously mentioned, the more contemporary approach of solvent-drop grinding seems to be an especially successful preparation technique. The phase that is obtained is typically not reliant on the synthetic methodology2.
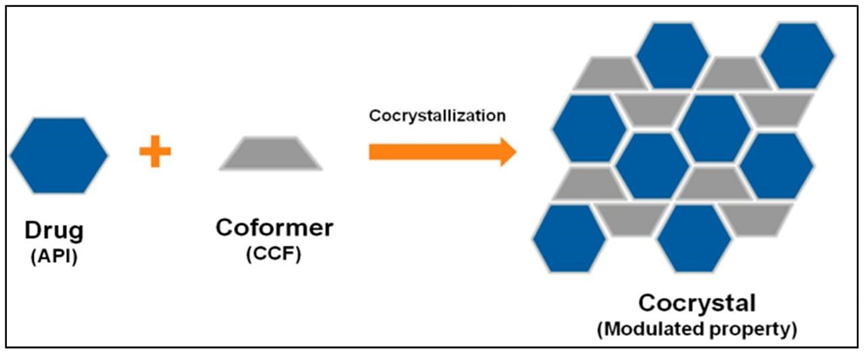
Figure 2 Co-crystal Formation
5. The Cocrystal Development Pathway
The development pathway of pharmaceutical co-crystals has been encompassed into eight stages These included
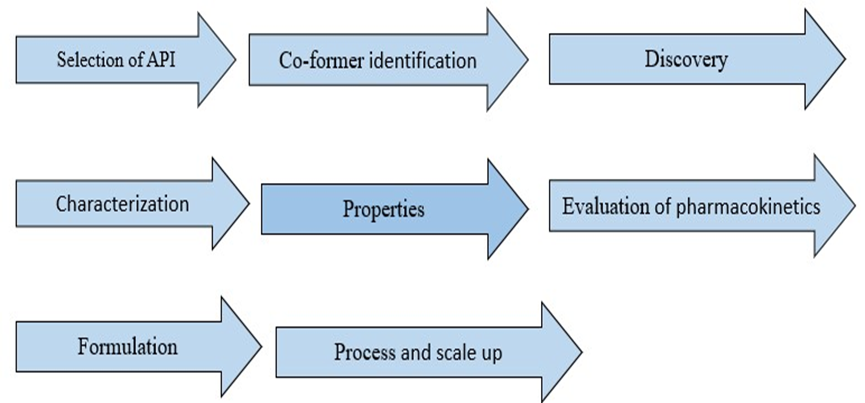
Selection of API: Preparation pharmaceutical co-crystal selection of an API is an important step.
Co-former identification: Initially complementary co former to a drug molecule candidate is selected from a library. the pharmaceutical co crystal, the co-former is selected from mostly the list provided by FDA and each ingredient added to food in the united state list approved by FDA.
Discovery: A stage during which co-crystal synthesis is carried out. Methods such as slow solvent evaporation, slurry mediated transformation and mechanical grinding (both neat and solvent-drop or liquid assisted) are used. Other methods used during cocrystal screening are also identified.
Characterization: A stage during which physical and chemical properties are assessed is carried out using different techniques. Differential scanning calorimetry (DSC), thermogravimetric analysis (TGA), infrared and Raman spectroscopy, powder X-ray diffraction (PXRD), single crystal X-ray diffraction (SCXRD), and solid-state nuclear magnetic resonance (ss-NMR), are used to characterize novel co-crystals. Properties: This was described as a stage during which further steps would be decided. In case the produced cocrystal shows enhance desired properties such as aqueous solubility and dissolution rates, pharmacokinetics, formulation, reproduction, and scale-up will be performed. Co crystallization also enables the improvement of properties
Properties: It was said that decisions about next measures would be made at this point. Pharmacokinetics, formulation, replication, and scale-up will be carried out in the event that the generated cocrystal exhibits improved desired qualities such aqueous solubility and dissolution rates. Additionally, cocrystallization permits the enhancement of characteristic5.
Evaluation of pharmacokinetics: A vital stage in the development of new drugs is pharmacokinetic evaluation. In this phase, the drug's action site flow and performance are investigated. The basis for determining the dosage of any medication is this method of drug administration. A common definition of PK includes the study of bodily functions like drug distribution, metabolism, absorption, and excretion. Changes in the thermodynamics of APIs lead to modifications in the physical features that supramolecular systems, such co-crystals, provide. Through mechanisms described in the preceding paragraph, which include but are not limited to the timely exploration of drug quantity (concentration) in biological fluids, tissues, and excreta, these in turn impact not only drug bioavailability but also drug PK6.
Formulation: A co-crystal's dosage form formulation is not an easy procedure to carry out. Stability of co-crystals with together by hydrogen bonds becomes risky when excipients that also contain hydrogen bonding groups are present. In order to reduce the potential source of interactions with the co-crystal, selecting the appropriate excipients is essential. One of the main concerns is the stability of co-crystals in formulation. This is due to the fact that H bonds stabilize co-crystals and that using excipients that already have H bonds carries a number of dangers, particularly when those excipients are intended to improve a drug's formulation qualities. Furthermore, the behavior of pharmaceutical co-crystals differs from that of their pure API due to their improved physicochemical features. require a modification regardless of how stable they are in relation to H-bonds7.
Process and scale-up: Scale-up of the co-crystal produced by traditional solution methods of co-crystallization such as solvent evaporation and slurry present challenges. Good quality co-crystals are obtained from solution crystallization, a method usually used to purify chemical substances such as APIs. Not only the problem of solvent cost and solubility of the individual component but also the tendency to crystallize which is different for these components reduce the chances of reproducing the same cocrystals with the desired yields. For many co-crystals prepared using simple and common solidstate approaches known as mechanochemical approaches, there are challenges with required high mechanical stress and the difficulty in achieving a homogeneous final product for larger-scale processes8.
6. Multi Drug Co-crystallization (MDCS)
The ability to increase therapeutic efficacy and enhance disease management has made the combination of many medications in a single oral dose form a preferred approach for drug development. This also has the benefit of lowering product development costs and improving patient adherence. Pharmaceutical co-crystals containing combination medications offer these and other advantages. Though they have received some recognition from various solid-state scientists, very little has been written about them in the literature, despite their potential. These hybrids are crucial, nevertheless, given the current rise in complex medical problems and the mounting push to find novel APIs. Multidrug co-crystals are dissociable solid crystalline supramolecular structures, according to Thipparaboina9.
7. Preparation Method of Crystallization Solvent based crystallization solvent evaporation:
In this solvent evaporation method is based on the dissolution of API and conformer in a single solution or mixed solution and the cocrystal product is obtained through slow evaporation of the solution. this method involved nucleation and growth of API and
CCF in a solvent solution which is facilitated by slow evaporation of solvent to provide
oversaturation solution for cocrystal single crystal. The crystal must be collected before the solution evaporates and dry to confirmed2
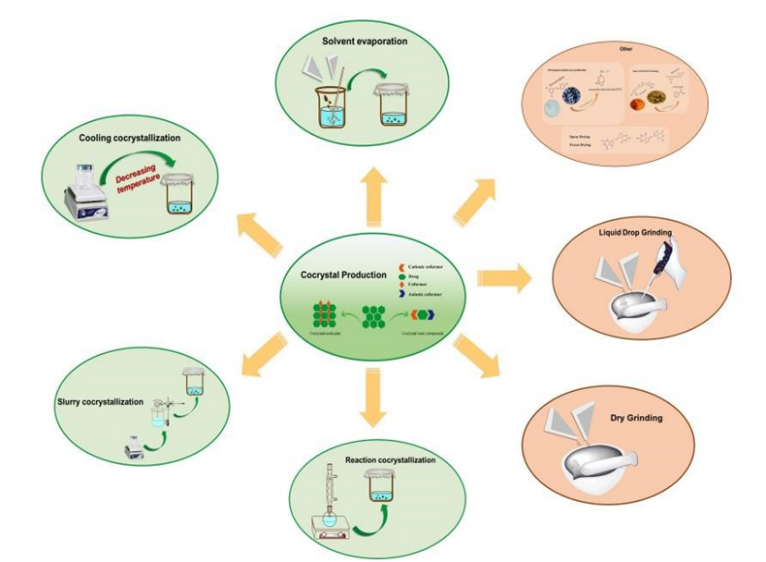
Figure 3 Preparation method of Co-crystallization
Colling co-crystallization: in this method of crystallization cocrystal are prepared by temperature variation. the component is fully dissolved in the solvent by heating the mixed component and the solvent and then this component reaches to the saturation state in the solution by decreasing temperature and the cocrystal are formed in this process. The single crystal of apixaban- quercetin-2 acetonitrile was obtained by this method10
Slurry co-crystallization: With the slurry process, one of the raw ingredients is supersaturated and the other two are combined in a solvent at a set molar ratio. In realworld scenarios, the technique can also be accomplished by suspending CCF or introducing API to the solution. Despite being a solution approach, it doesn't require full dissolving of API in solution in comparison to alternative techniques, the ultimate Solubility and relative concentration determine conversion rate. of CCF, API, and cocrystal nucleation and growth of cocrystal11
Reaction co crystallization: this process of crystallization the solubility if two compartment with the different and equimolar solvent evaporation possible result of single component crystallization reaction or precipitation cocrystallization can be use to solve problem, resevetrol is non flavonoid compound produced by many plants such as grapes having antiviral antioxidant property isoniazid is first synthetic drug that can act as a classical treatment of tuberculosis12. the isoniazid and reservetrol were prepared by reaction cocrystallization method.
Ultrasound assisted co crystallization: Typically, ultrasound is employed in conjunction with slurry and solution co crystallization techniques. The ultrasonic cavitation energy technique can shorten the metastable zone and induction period, so causing the formation of cocrystals at a lower saturation state. In sequence to enhance resveratrol's antiviral impact on amantadine hydrochloride (ATHC) and experience the mutual benefits of the two parts, an enhanced technique for co crystallization of ATHC and resveratrol were suggested. Wu, Zhi-Yong, and others prepare the Using a liquid drop method, resveratrol and ATHC cocrystal were formed ultrasonic solvent and grinding techniques. The filtrate was gradually evaporated for three to five days at ambient temperature, and the appropriate crystals for X-ray single-crystal diffraction were chosen from the acquired crystals with point blocks for detecting11.
Supercritical fluid technology:
Three distinct techniques have been successfully used by supercritical fluid technology, which mostly uses supercritical CO2, to manufacture cocrystal based on various supercritical CO2 characteristics: solvent degree of atomization and antisolvent. Supercritical liquid Technology is not frequently employed as a medicinal crystal in crystal form. It mostly makes advantage of the CO2 in the solvent's solvent capacity to suspend CCF and API in a solvent or straight in CO2 as a suspension. This may lessen the use for harmful organic solvents. By altering the CO2's thermodynamic conditions (temperature, pressure, etc.), It is possible to adjust its density and solvent capacity to regulate the fine turn to control the formation of cocrystal equipment. Curcumin- resveratrol was prepared by co crystallization with supercritical solvent technology.
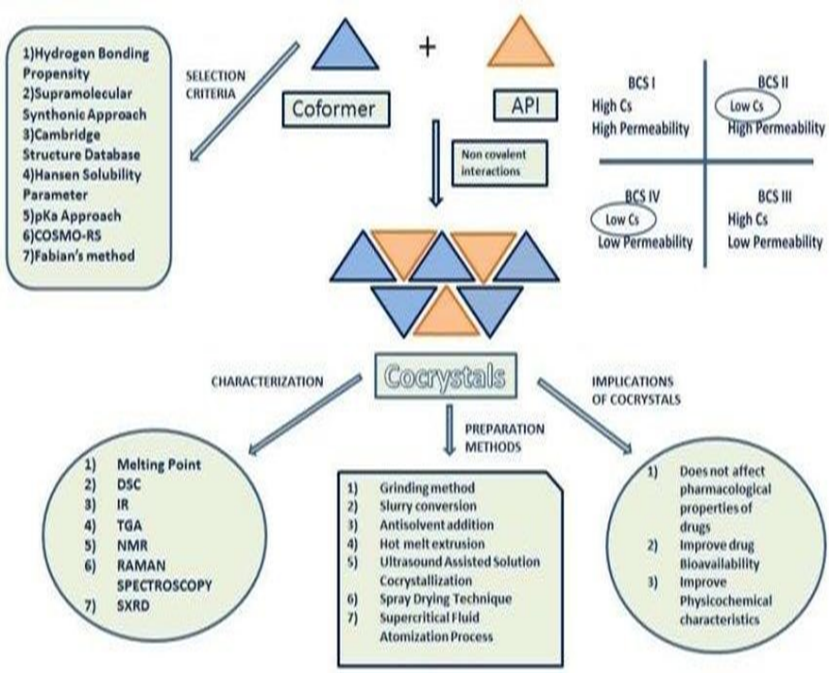
Figure 4 Summary of Pharmaceutical cocrystal
8. What is Herbal Cocrystal
In the development of new drug of herbal medicines active ingredients solubility and oral bioavailability are the main factors which are important to be considered in the development of new drug. Pharmaceutical cocrystal provide excellent opportunity to developed new drug in the herbal medicines pharmaceutical cocrystal improved the physical and chemical properties of drug without changing the initial structure if drug the herbal cocrystal of betulin was prepared by extraction of butylene from birch bark exhibit varies type of pharmacological activity including antiviral anti-inflammatory anti-HIV or anti-cancer effect the major problem with the development of cocrystal is bioavailability several strategies are applied to improved solubility and dissolution rate. in such a way many cocrystal are prepared which are herbal esculetin, cocrystal of isoniazid -gentisic acid13.
9. Case Studies:
Pharmaceutical cocrystal of curcumin ascorbic acid
Curcumin is a phytochemical belonging to BCS class IV which is having poor solubility and poor permeability so it can face some limitation in practical application .the pharmaceutical cocrystal is one of the best method to enhancing the solubility of class IV drug the cocrystal was developed to improve the solubility and permeability in this the cocrystal were prepared by solvent evaporation by using methanol as a solvent 1:1ratio L-proline and piperine is used as conformer .to enhanced solubility and permeability in -silico screening through molecular docking proof highly effective and efficient for the conformer selection the molecular ratio of curcumin and L-proline (1:1) and curcumin and piperine(1:1) for improving solubility and permeability gives very good results after 8 hours pure curcumin exhibited 20% of cumulative drug relese while by preparing the cocrystal of curcumin L-proline achieved about 71% of cumulative drug release . and the permeability of pure curcumin is 0.0142mg/ml and curcumin piperine combination show about 0.2545mg/ml this is one of the major advantages while formulation of pharmaceutical cocrystal the study is conducted on cocrystal for stability according to ICH guideline. the tablet formulation of curcumin cocrystal have the stability about 3 months. this is finding by SEM, FTIR, DSC the effective approach in coformer selection to enhanced solubility, permeability and stability of drug through the varies BCS classes14.
Saturated solubility study
Phase solubility method was used to evaluate the saturation solubility of curcuminascorbic acid combinations in triplicate. An excessive quantity (50 mg) each of ascorbic acid, curcumin, and physical combinations of the two; also, the solvent-evaporated curcumin-ascorbic acid mixture was each separately diluted in 10 milliliters of pH 1.2 and pH 6.8 buffer, and distilled water. Moreover, the scatterings were agitated in an orbital shaker for 72 hours at 37°C. Following equilibrium, the samples were centrifuged for 15 minutes at 2000 rpm. Afterward, the supernatant Passed through
Whatman filter paper number 45 for filtering, and absorbances were determined at ? max 425 nm with a Shimadzu UV- 1800 Spectrophotometer (Shimadzu, Japan) UV visible spectrophotometer 14
|
Sr.no
|
Compound
|
Solubility mg/ml
|
Reported solubility mg/ml
|
|
1
|
Curcumin
|
0.07928
|
<0>
|
|
2
|
Curcumin-Lproline co
crystal
|
0.47658
|
-
|
Pharmaceutical cocrystal of betulin and terephthalic acid
Betuline is isolated from bichaccording the development method it is purified by recrystallization from ethanol terephthalic acid was of analytical grade. for the preparation of betuline terephthalic acid mixture of dii molar ratio (1:1:2:1) were ground in an mortar or in SPEX8000 mixer mill the mixture mix homogeneously in mortar for 5 min by addition of 1 ml of solvent and grinding in this case mixer mill ,ball mill was used I a 60 ml steel jar with ball of same material and 6mm in diameter .in this cocrystal of terephthalic acid were prepared by liquid assisted grinding method ,formation of cocrystal conformed by powder X ray diffraction method IR spectroscopy and also by thermal analysis method HPLC analysis shown the cocrystal of betulinterephthalic acid cocrystal the solubility was increased as compaired to initial botulin which is having pharmacological activity such as anti-HIV anti-cancer anti-bacterial15. Isoniazid-gentisic acid cocrystal
Isoniazid is used as an antitubercular agent it is the line constituent used in triple therapy it is being used to counter tuberculosis since 1952. Isoniazid having poor chemical stability in its solid state as it associated with hydrazide group co-crystallization with gentisic acid having antioxidant activity may produce solid form which increased pharmaceutical properties the nature of the functional group of isoniazid and the coformer result in success in cocrystal formation of cocrystal (INH -gentisic acid ) was characterized by solid state NMR, DSC,PXRD and single crystal-XRD the synthesis cocrystal was tested for the inhibition of synthetic free radical scavenging activity against DPPH16 . which is better than that of the ascorbic acid used as a standard the solubility and the flowability properties of synthesized cocrystal are optimized. the cocrystal of INH gentisic acid was prepared by dissolving 1:1 molar ratio of conformer and INH in methanol. both the given solution are mixed and heated at 55 degree for 15 min with stirring and kept the solution at room temperature slow evaporation lead to the formation of white small cocrystal after the seven days which are filter and dry after washing17the cocrystal of isoniazid and gentisic acid increased in the storage stability and use in treatment of tuberculosis17 .
10. List of Herbal Cocrystal along with their Co former and Method of Preparation18
|
Drug
|
Co former
|
Method of preparation
|
|
Baicalein
|
Nicotinamide
|
Slow evaporation, rotary evaporation cogrinding
|
|
Betulinic acid
|
Ascorbic Acid
|
Solubilization of dry and conformer by using isopropyl alcohol followed by slow evaporation technique
|
|
Emodin
|
Nicotinamide
|
Slow or rapid solvent evaporation method
|
|
Naringenin
|
Isonicotinamide
Picoline acid betaine
|
Slurry method, liquid diffusion method
|
|
Hesperetin
|
Picolinic acid, nicotinamide and caffeine
|
Solvent drop grinding technique
|
|
Chrysin
|
Cytosine and thiamine hydrochloride
|
Mechanochemical technique using solvent drop grinding method
|
|
Luteolin
|
Isoniazid and caffeine
|
Liquid assisted grinding method
|
|
Myricetin
|
Piracetam
|
Slow evaporation, solvent grinding method
|
|
Curcumin
|
N- acetylcysteine
|
Crystallization with supercritical solvent technique
|
|
Scoparone
|
3,5, -difluorobenzoic acid urea, pyrimethamine succinimide
|
Solvent crystallization method
|
|
Caffeine
|
Hydroxybenzoic acid
|
Solution mediated phase transformation
|
|
Quercetin
|
Caffeine: methanol iso nicotinamide theobromine dihydrate
|
Slurry method
|
|
Caffeine[form1]
Caffeine[form2]
Carbamazepine
|
Pterostilbene
|
- Grinding method and solvent based method
- Vapour diffusion experiment
- Grinding method and solvent based method
|
|
Genistein
|
Isonicotinamide
|
Slow evaporation technique
|
11. Application of Pharmaceutical Co-crystal
1. Pharmaceutical cocrystal are molecular complex that consist of an active pharmaceutical ingredient (API) and pharmaceutical acceptable conformer that is solid under ambient condition
2. Crystal engineering strategies that rely on intermolecular between the API and conformer have proof efficient in the design and synthesis of cocrystals
3. Resent endorsements by the food and drug administration of the USA and the European Medicines Agency further validated the use of cocrystal as potential alternative solid form for drug development19.
4. pharmaceutical cocrystal are helpful in sustained release of drug.
5. pharmaceutical cocrystal are also beneficial in increase solubility1 and bioavailability20.
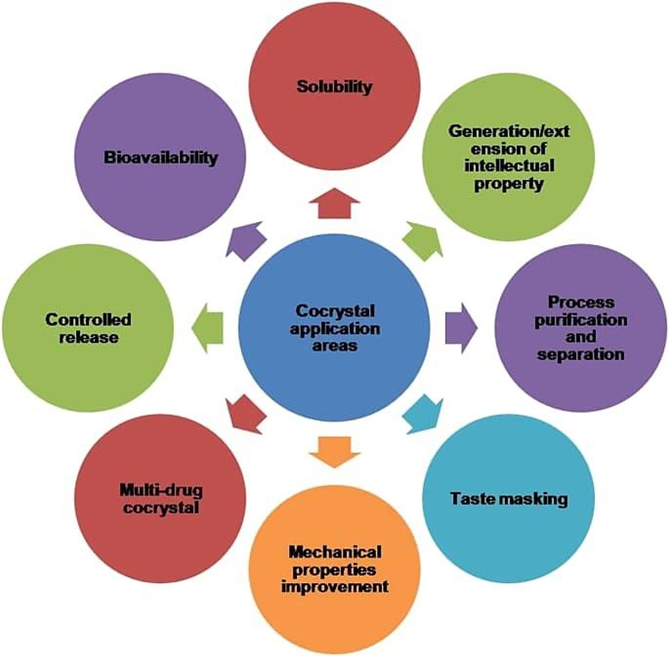
Figure 5 application of pharmaceutical cocrystal
CONCLUSION:
Cocrystal have potential to address hurdles in drug delivery system. they are the multicomponent crystal and may improve the physicochemical and mechanical properties of drug multi drug cocrystal is the novel approach may be useful for combination therapy and increase therapeutic efficacy of drug the life cycle of old API may increase by pharmaceutical cocrystal crystal engineering help the understanding of intermolecular forces involved in the hydrogen bonding between molecule .there is more options to prepare pharmaceutical cocrystal which can provide new option to treat the diseases cocrystal offer one of the opportunity to successful drug delivery so it is beneficial to prepare for pharmaceutical industry.
REFERENCES
- Hiendrawan, S.; Hartanti, A.; Veriansyah, B.; Widjojokusumo, E.; Tjandrawinata, R. Solubility Enhancement of Ketoconazole via Salt and Cocrystal Formation. Int. J. Pharm. Pharm. Sci. 2015, 7, 160–164.
- Shan, N.; Zaworotko, M. J. The Role of Cocrystals in Pharmaceutical Science. Drug Discov. Today 2008, 13 (9), 440–446. https://doi.org/10.1016/j.drudis.2008.03.004.
- Guo, M.; Sun, X.; Chen, J.; Cai, T. Pharmaceutical Cocrystals: A Review of Preparations, Physicochemical Properties and Applications. Acta Pharm. Sin. B 2021, 11 (8), 2537–2564. https://doi.org/10.1016/j.apsb.2021.03.030.
- advantages of pharmaceutical cocrystal. Bing. https://www.bing.com/images/search?q=advantages+of+pharmaceutical+cocrysta l&FORM=HDRSC3 (accessed 2023-12-05).
- Wouters, J.; Quéré, L. Pharmaceutical Salts and Co-Crystals; Royal Society of Chemistry, 2011.
- Dooner, H.; Mundin, G.; Mersmann, S.; Bennett, C.; Lorch, U.; Encabo, M.; Escriche, M.; Encina, G.; Smith, K. Pharmacokinetics of Tramadol and Celecoxib in Japanese and Caucasian Subjects Following Administration of Co-Crystal of Tramadol-Celecoxib (CTC): A Randomised, Open-Label Study. Eur. J. Drug Metab. Pharmacokinet. 2019, 44 (1), 63–75. https://doi.org/10.1007/s13318-0180491-9.
- Pharmaceutical Cocrystal of Piroxicam: Design, Formulation and Evaluation - PMC. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5651061/ (accessed 202312-10).
- Pharmaceutical co?crystal: An alternative strategy for enhanced physicochemical properties and drug synergy - Ngilirabanga - 2021 - Nano Select - Wiley Online Library. https://onlinelibrary.wiley.com/doi/full/10.1002/nano.202000201 (accessed 2023-12-06).
- Žegarac, M.; Lekši?, E.; Šket, P.; Plavec, J.; Dev?i? Bogdanovi?, M.; Bu?ar, D.-K.; Dumi?, M.; Meštrovi?, E. A Sildenafil Cocrystal Based on Acetylsalicylic Acid Exhibits an Enhanced Intrinsic Dissolution Rate. CrystEngComm 2014, 16 (1), 32–35. https://doi.org/10.1039/C3CE42013B.
- Deepika; Maurya, P. K. Health Benefits of Quercetin in Age-Related Diseases. Molecules 2022, 27 (8), 2498. https://doi.org/10.3390/molecules27082498.
- Deng, Y.; Zhang, Y.; Huang, Y.; Zhang, M.; Lou, B. Preparation, Crystal Structures, and Oral Bioavailability of Two Cocrystals of Emodin with Berberine Chloride. Cryst. Growth Des. 2018, 18 (12), 7481–7488. https://doi.org/10.1021/acs.cgd.8b01257.
- Meng, T.; Xiao, D.; Muhammed, A.; Deng, J.; Chen, L.; He, J. AntiInflammatory Action and Mechanisms of Resveratrol. Molecules 2021, 26 (1), 229. https://doi.org/10.3390/molecules26010229.
- Ma, X.; Peng, Y.; Cui, J.; Liu, T. A Review on Cocrystal of Active Ingredients in Traditional Chinese Medicine. Tradit. Med. Res. 2023, 8 (4), 19. https://doi.org/10.53388/TMR20220816002.
- Singh, M.; Takawale, S.; Patil, R.; Chaudhari, S.; Pathan, A. Development and Characterization of Co-Crystals Assisted with In-Silico Screening for Solubility and Permeability Enhancement of Curcumin.
- Mikhailovskaya, A. V.; Myz, S. A.; Bulina, N. V.; Gerasimov, K. B.; Kuznetsova, S. A.; Shakhtshneider, T. P. Screening and Characterization of Cocrystal Formation between Betulin and Terephthalic Acid. Mater. Today Proc. 2020, 25, 381–383. https://doi.org/10.1016/j.matpr.2019.12.096.
- Bhutani, H.; Singh, S.; Jindal, K. C. Drug-Drug Interaction Studies on FirstLine Anti-Tuberculosis Drugs. Pharm. Dev. Technol. 2005, 10 (4), 517–524. https://doi.org/10.1080/10837450500299982.
- Mashhadi, S. M. A.; Batsanov, A. S.; Sajjad, S. A.; Nazir, Y.; Bhatti, M. H.; Yunus, U. Isoniazid-Gentisic Acid Cocrystallization: Solubility, Stability, Dissolution Rate, Antioxidant and Flowability Properties Studies. J. Mol. Struct. 2021, 1226, 129388. https://doi.org/10.1016/j.molstruc.2020.129388.
- Qiao, N.; Li, M.; Schlindwein, W.; Malek, N.; Davies, A.; Trappitt, G. Pharmaceutical Cocrystals: An Overview. Int. J. Pharm. 2011, 419 (1), 1–11. https://doi.org/10.1016/j.ijpharm.2011.07.037.
- Morissette, S. High-Throughput Crystallization: Polymorphs, Salts, CoCrystals and Solvates of Pharmaceutical Solids. Adv. Drug Deliv. Rev. 2004, 56 (3), 275–300. https://doi.org/10.1016/j.addr.2003.10.020.
- Solubility Advantage of Amorphous Drugs and Pharmaceutical Cocrystals | Crystal Growth & Design. https://pubs.acs.org/doi/abs/10.1021/cg200492w (accessed 2023-12-10).
- https://onlinecourses.swayam2.ac.in/aic22_ge21/preview


 Kalyani Avhale *
Kalyani Avhale *
 Dr. Kawade Rajendra M.
Dr. Kawade Rajendra M.






 10.5281/zenodo.14278837
10.5281/zenodo.14278837