Millions of lives are saved annually by vaccines, which offer significant protection against infectious diseases. The recent COVID-19 pandemic underlined the necessity of vaccination in providing mass-scale immunization against outbreaks. However, because of their enormous molecular size and low stability at room temperature, vaccine distribution presents a special set of difficulties. For the effective development of vaccines, advanced biomaterials and delivery systems including nano- and microscale carriers are increasingly essential. We present an updated overview of current developments in the creation of nano- and micro-scale carriers for the controlled delivery of vaccines in this review, with an emphasis on carriers that work well with the nucleic acid-based treatments and vaccines that have been developed in the wake of the recent pandemic. We first describe delivery systems at the nanoscale, with an emphasis on nanoparticles, and then we discuss systems at the microscale. Self-assembled peptides have demonstrated exceptional properties for medication targeting and vaccine delivery. It is possible to purposefully design peptide molecules so that they will self-assemble into particular nanoarchitectures in response to variations in the conditions under which they assemble, such as temperature, ionic strength, pH, and interactions between host (drug) and guest molecules. The resultant supramolecular nanostructures have a variety of mechanical and physicochemical properties and include hydrogels, nanovesicles, nanofibers, nanotubes, and nanoribbons. By including adhesion ligands, receptor recognition ligands, or peptide-based antigens into their design—often in a multivalent display—these compounds can be engineered for cell-specific targeting. Depending on how they are made, self-assembled peptide nanostructures can be advantageous in terms of adjuvanting, shear-thinning viscoelastic characteristics, encapsulating hydrophobic pharmaceuticals, stability against enzyme degradation, and/or sustained drug release.
needle-free delivery, edible vaccines, microneedles, microparticulates, Infectious disease ; Vaccine ; Biosafety material ;Vaccine delivery ; Release kinetics etc.
(a) managing the vaccine's release profile from the delivery device.
(b) directing immune system targeting to the right cell types to provide the best response; and
(e) creating formulations suitable for non-invasive nasal (or nasal) researchral coronal) distribution.
The objective of controlled vaccine delivery product development research are:
1. to cause a single-contact immunological response that is long enough to be protective. 2. amplify the body's immune reaction to the vaccination without having any negative side effects.
3. combine numerous vaccinations into one formulation.
Biodegradable microspheres encapsulating vaccines can help achieve many, but not necessarily all, of these goals. According to Alonso et al. (1993), Stevens (1993), Alonso et al. (1994), Singh et al. (1992), these microspheres have the power to elicit an initial and a sustained immune response. Without causing a reaction or having any negative effects on the materials they are composed of, they have shown effective in enhancing the response to model antigens such ovalbumin (O'Hagan et al., 1991). many vaccinations can be incorporated into a single microsphere formulation, or many vaccines can be created by mixing microspheres encasing distinct antigens. Additionally, Peyer's patches in the small intestine can be targeted by the vaccine using microspheres, allowing.
2. INTRODUCTION OF IMMUNITY BY VACCINE:
The World Health Organization defines immunization as the process of administering a vaccine to a person in order to make them resistant to or immune to a disease. The vaccination boosts immunity, preventing a person from contracting infections or illnesses later on.
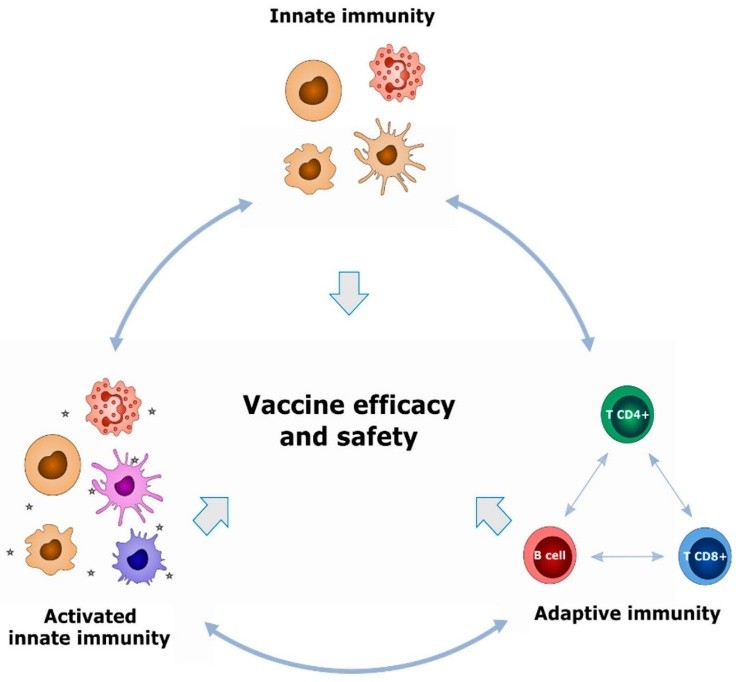
Vaccination is used to prime the immune system to resist the infectious agent, thereby protecting the individual against infection. Effector cells (also known as cell mediated immunity, or CMI) or effector chemicals (such as complement, cytokines, antibodies, and so on) might provide this resistance. Immunization also causes the immune system to form a "memory" of exposure to antigens on the virus, enabling it to react to those antigens quickly on repeated encounters. Vaccines are made of several different substances. Certain vaccinations, including oral typhoid, BCG, and polio, use pathogen strains that have been attenuated and rendered non-infectious. Some use inactivated or destroyed pathogens (rabies and the Salk polio vaccine, for example). Then there are vaccinations, like tetanus, that are based on the chemical components of the virus.
1.The immune system's cell types and the effector responses they produce, which modify the immune response, are briefly outlined.
2. Immune recognition, or the capacity to discriminate between various "non-self" forms and amino acid sequences, as well as between "self" and "non-self," and
3. The cellular and molecular processes that transpire during the activation of an immune response.
2.1 CELL TYPES:
Types of cells Cells who chathe a target, they assault (they do split into two factions). Phagocytes and auxillary cells are two examples of these that are not particular. Invading microorganisms are swallowed and digested by phagocytes, which include cosinophils, macrophages, and mononuclear phagocytes. Auxillary cells that mediate the lysis of invading neutrophils, release substances that cause inflammation, and stimulate the production of lymphocytes include basophils, mast cells, and platelets. The functions unique to a target are carried out by the second group of cells. They can differ in that they can be effectors of self molecules and different types of non-self molecules of cognition. Lymph is in charge of the two primary subgroups. in the setting of bone marrow, "B-cells" e delAg; conversely, they produce antibodies (Ab). These may eventually acquire the function from the "plasma cells alter-producingher."
2.2. REATION OF EFFECTOR:
The above-discussed phagocytes and auxillary cells are the initial line of defense against an infection. When auxillary cells come into contact with foreign material, they release inflammatory chemicals that excite and draw phagocytes to the infection site. The substance is then consumed by these cells. The infectious agent is eliminated from circulation and reduced to tiny fragments after phagocytosis. Peptide fragments generated during this step are crucial for the production of a T cell response. Macrophages operate as antigen-presenting cells (APCs) when they carry out this task. These cells operate as effectors as well. They carry metabolic organelles that produce a large quantity of free radicals, which destroy microorganisms. They also have a wide range of lysosomal enzymes that can break down different kinds of substances.
2.3. RECOGNITION BY THE IMMUNE SYSTEM:
Two degrees of discrimination are involved in immune recognition, as demonstrated by the separation of immune cells into Ag-specific and non-specific kinds. At the molecular level, specific immunological recognition is achieved by lymphocytes identifying and binding particular regions of the Ag molecule (epitopes) through receptors on their cell membrane. An Ag-specific reaction is produced in response to this identification, which is aimed at the identified epitope. Unlike non-specific identification, this method may identify a remarkably wide range of materials with different molecular "shapes" and physical characteristics.
The T and B cell receptors function differently. The surface immunoglobulin, also known as the B cell receptor (BCR), has the ability to interact with an epitope's three-dimensional shape. For this reason, the BCR is frequently referred to as a "conformation reading" receptor. In contrast, the T Cell receptor (TCR) is a "sequence-reading" receptor. It reacts, in contrast to the BCR, to pieces of processed Ag that are "shown" to it in conjunction with Major Histocompatibility Complex (MHC) proteins. The peptide fragments are held in a stretched-out configuration for examination by various TCRs by these MHC proteins, which are produced on the surface of antigen-presenting cells (APC). It is the actual amino acid sequence in this case.
2.4. THE SEQUENCE OF EVENTS :
The flow of things It is now possible to see, in some detail, what happens when a vaccine formulation is administered. When injected into the body, macrophages and other "professional" phagocytic cells identify it as "foreign" at first. Its protein chain is broken down into pieces upon ingestion by, for example, macrophages, and some of these pieces are incorporated onto MHC proteins. This creates the MHC-peptide complexes for a variety of TCRs to investigate. Ag is also transported by phagocytes to lymphoid tissue, the site of B and T cell aggregation. The immune response is started when the ligands for these cells' receptors are identified. The following are the events pertaining to B cells. The antigen is taken up by the B cell.
them.
3. DESIGNING RATIONALE AND REQUIREMENTS OF AN EFFICIENT VACCINE DELIVERY SYSTEM:
Keeping the events described above in mind, it is possible to enumerate some design requirements for vaccine delivery systems.
3.1 PARTICULAR ANTIGEN:
Specific antigen As phagocyte uptake plays a crucial role in initiating an immune response, the vaccine formulation ought to be phagocytosis-friendly. Pinocytosis, which is at least ten times less efficient than phagocytosis, is the process by which soluble material is taken up. The formulation can be made into a particle form to improve absorption efficiency. The preference for particulate Ag can also be attributed to the fact that soluble material is mobile. Should the injected vaccination widely disperse from the injection site, it may be sufficiently diluted to evade the immune system's attention. Particulate matter moves in a more constrained manner. Ultimately, the mere existence of particulate matter serves as a crucial cue for phagocytic cells.
3.2 BOOSTER DOSES:
Additional dosages Vaccines that must be administered in divided doses are ineffective if only the initial dosage is administered. An additional response to splitting up One ded dosages is that "too little Ag is ps fough dose tolerance." dose tole the immunological systerious condition that arises from an excessively high dosage. In this instance, the nighean of the first causes the immune system to become resistant. after borrowing The biological activities that occur after firman pization can be used to explain the greater efficacy of divided dosages. Specifically, when the Ag binds to the B cell receptor (BCR), the cell receives signals to divide. As can be observed, a bolus dosage.
3.3 ROUTE OF IMMUNIZATION:
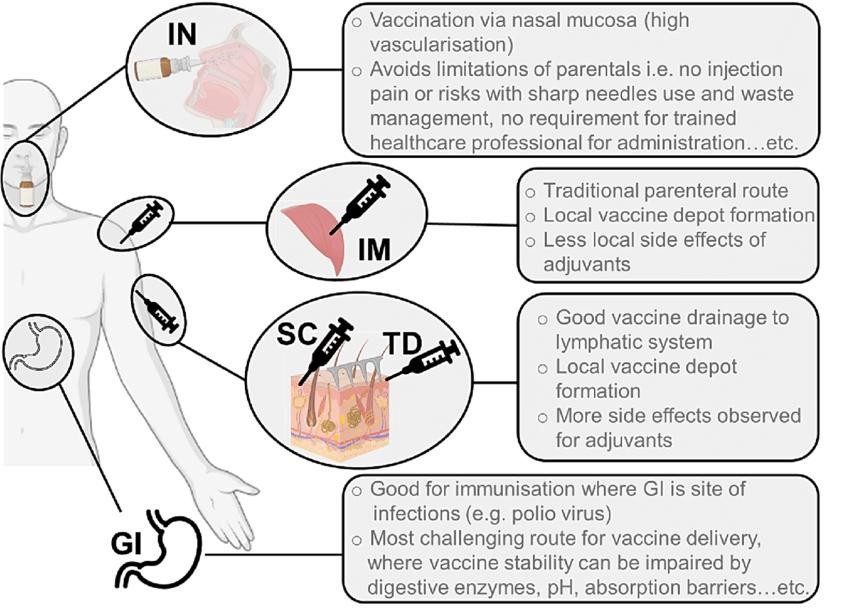
Fig: Route Of immunization.
3.4 ADJUVANTICITY: EFFECT OF VEHICLE
An "adjuvant" is typically added to conventional vaccination formulations when the vaccine is given as a killed pathogen or as isolated protein(s) or polysaccharide(s) rather than a live, attenuated organism. Through a number of methods, including encouraging antigen-presenting cells to absorb the antigen, encouraging phagocytic cells to consume it, and extending the Ag's half-life in the body if it is insufficient to elicit an immune response, adjuvants enhance the immune response. It is also anticipated that a vaccine delivery system will possess adequate adjuvanticity to enhance the immune response to the administered antigen.
3.5. STABILITY OF ANTIGEN:
The vaccination antigen may adapt by changing its conformation if it is exposed to environmental stressors like temperature, pH, or non-aqueous media. The epitopes intended to elicit the B cell response may reorganize as a result of this. Ag must be kept in a conformation that preserves the three-dimensional structure of the immunogenic B cell epitopes because the efficiency of such a formulation would be severely jeopardized. In practical terms, this means that freezing temperature, physiological pH, and aqueous environment are not strictly adhered to, but rather the Aglow's stability requirements must be followed. Thus, the distribution system's setup and storage requirements should guarantee that the Ag is preserved .
4.PREPAPERATION OF MICROSPHERES CONTROL DELIVERY OF VACCINE:
Given that most vaccinations are highly potent and not widely available, preparation methods must be made to accommodate a few milligrams of the encapsulating polymer and a few micrograms of the vaccine. Additionally, the preparation process greatly affects the created microspheres' properties, so when choosing a preparation method, the desired qualities should be kept in mind. Among these appealing qualities .
1) optimal antigen loading: Ag to polymer ratio should be such that the maximum amount of of Ag is included in the smallest possible quantity of polymer. This lowers the mass of the substance that needs to be given.
2. Microsphere size: In addition to the adjuvanticity consacrations, a significant prerequisite of the The microsphere system can be syringed using a hypodermic needle. consistency and repeatability between batches.
3. Within the group.
4. Minimal material waste.
5. The Ag's minimal contact to denaturing environments.
Microspheres must be sterile, just like any other parenteral product. A comprehensive asceptic processing procedure or a final sterilizing step can guarantee this. Regarding safety standards further, the processing solvent and excipients employed should either be non-toxic or demonstrate that they have been eliminated from the finished product. The formulation of lactide-glycolide microspheres for vaccination distribution has involved a number of techniques that are common to pharmacists but that have been appropriately tailored to the material, processing circumstances, and consistency requirements. While they haven't been assessed separately, the first few that have been covered here are more receptive to process control and scaling up. Here, they are addressed as Instrumental Methods.
4.1) INSTRUMENTAL METHOD:
- Spray Drying :
This technique involves suspending spray-dried protein in an organic solvent (tetrahydrofuran or methylene chloride) solution containing the polymer(s). A peristaltic pump then pumps this into a spray drier. The solution is atomized by dry air at high pressure and 37°C inlet air temperature. As the solvent evaporates from the droplets, the polymer forms a matrix encasing the protein. Thus, it is possible to prepare spherical particles with the specified size range and loading. According to Gander et al., the spray-drying method can microencapsulate TT (Gander and al., 1993). The primary disadvantage of this approach is the comparatively large batch size needed for processing given the constraints of the equipment that is now available.

Fig: Spray drying Process.
-
- Air Suspension Coating:
Wurster coating of suspended aqueous droplets containing Ag by spraying a poly(lactide-co-glycolide) (PLGA) solution can be used to create microcapsules (Deasy, 1988). With this technique, particles bigger than 50 um in size are created.
- Press Grinding:
Grinding by press Using this technique, spray-dried polymer and spray-dried protein are combined, then the mixture is compacted at room temperature into a cylindrical disc.

The resulting pellet is then pulverized in a grinder with dry ice, and the particles are gathered and sieved to the proper size (Wang, et al., 1990). Unfortunately, antigen entrapment is not anticipated to be effective due to the low compressibility of lactide-glycolide polymers.
- Coacervation : Phase Separation
Phase Separation of Coacervation Except for the work of Nihant et al. (1995), the synthesis of Ag-loaded microspheres by non-aqueous coacervation-phase separation has not gained much traction. This group has described the steps involved in microencapsulation, which involves utilizing silicone oil to create phase separation while coacervating a PLGA solution in dichloromethane (DCM) with aqueous solutions of BSA or a-thrombin. At first, the Ag-polymer solution system equilibrates so that the solution encircles the Ag. At the polymer solution/Ag contact, the addition of the non-solvent silicone oil causes desolvation, which separates the polymer from its solution. A shell is subsequently formed as a result of this being adsorbed around the Ag.
4.2) EMUSIFICATION METHOD :
Emulsification techniques are based on the idea of supplying energy to the Ag solution (also known as the "water" or aqueous phase) and the polymer solution in an organic solvent (sometimes known as the "oil" phase). Elimination of the oil phase results in the deposition of the polymer around the Ag. The diverse strategies used to achieve this have led to the development of the following methods:
A) Oil in Oil Emulsion :
Oil-in-oil mixture Using sonication, dry protein is first distributed throughout a polymer solution in dichloromethane to create an emulsion of two non-aqueous phases. After that, this suspension is injected into a well-stirred emulsion comprising silicone oil, dichloromethane, and a surfactant like Span-85 using a syringe fitted with a 22G needle. As the silicone oil dichloromethane mixture equilibrates, droplets of this suspension develop. The dichloromethane is then extracted from the antigen-polymer-dichloromethane droplets by adding petroleum ether dropwise after that. The resulting microspheres are then vacuum-dried after being filtered and cleaned with petroleum ether (Wang, et al., 1991). The process is different from the previously described precipitation approach in that the dichloromethane is extracted gradually.
B.Oil in water emulsion:
An oil-in-water mixture This process entails emulsifying a dry protein suspension in a polymer solution—typically in dichloromethane—with an aqueous solution that contains sodium oleate or another appropriate stabilizer. Microspheres are gathered, cleaned, and vacuum-dried after dichloromethane is eliminated using appropriate techniques like rotary evaporation (Cowsar et al., 1985; Watts et al., 1990). The polymer precipitates at the dichloromethane-water interface as a result of solvent exchange during the interaction with the aqueous phase. The slow withdrawal of the solvent not only provides the forming microspheres a form, but it also stabilizes them.
c.Multiple emulsion:
This is the method of choice, utilized by most workers in the field of vaccine delivery. In 1970 a patent issued to Vranken and ClayeP described a microencapsulation process involving multiple emulsion (Vranken & Claeys, 1970). This was adapted for the entrapment of a water-soluble peptide by Ogawa and colleagues (Ogawa et al., 1988). According to this procedure an aqueous solution of the core material is dispersed in a solvent of a hydrophobic film forming polymer. This w/o emulsion is then stirred in a larger volume of water to form w/o/w emulsion. This complex emulsion is then added to a third solvent which is a precipitant for the polymer and miscible with both water and organic solvent. Under these conditions the solvent contained in the polymer droplets is extracted into the aqueous medium. This results in the entrapment of the core substance within the hardened microspheres and hence microencapsulation by "solvent extraction". In the same patent a similar microencapsulation process was described whereby removal of the hydrophobic polymer is effected by evaporation rather than extraction. This method has since been adapted to suit the needs of different workers in the area of vaccine delivery. The constituents of the multiple emulsion do not vary much in different reports. Dichloromethane is used to dissolve the encapsulating polymer, and Ag dissolved in phosphate-buffered saline or water is emulsified with the polymer solution. This primary emulsion is emulsified with a large volume of water, which contains an emulsifying agent (usually, poly vinyl alcohol). The complex emulsion is stirred overnight to allow dichloromethane to evaporate.
Emulsions can be prepared using standard emulsification equipment such as homogenizers, colloid mills, impellers, sonicators or an ordinary laboratory magnetic stir bars. In selecting the proper emulsification equipment, one must consider the size and the extent of Ag loading of microspheres that one would like to produce, as well as the volume and the viscosity of the organic phase.
4.3) SOLVANT EXTRACTION PRECIPITATION:
Usually through homogenization or sonication, the antigen is first suspended in a polymer solution. After that, this solution is injected into silicone oil containing Span-85 or another surfactant using a syringe fitted with a 22G needle. After agitating the silicone oil for a while, petroleum ether is added to remove dichloromethane and precipitate the microspheres (Wang et al., 1990). Needless to say, the antigen is exposed to extremely harsh conditions, viz, shearing stress as well as bydrophobic environment.
4.4. ROTATARY EVAPORATION:
Protein in powder form is added to a polymer solution and the resulting dispersion is rotary evaporated to remove the dichloromethane. The rotatory motion has the effect of imparting a spherical shape to the drying droplets, which ultimately Inse all the solvent and form a dry monolithic sphere in which the Ag is dispersed. These microspheres are collected, washed with water and vacuum dried (Wang et al., 1990). Small particles that are essential for targeting to APC are difficult to obtain with this procedure.
5) EVOLUTION OF VACCINE LOADED MICROSPHERES :
5.1 Particle size:
Light microscopy makes it simple to assess the particle size of microspheres, whereas scanning electron microscopy (SEM) provides more detailed information. A scanning electron microphotograph of a batch of microspheres is shown in Figure . SEM is useful for evaluating the surface morphology of microspheres as well. Particle size and distribution can also be ascertained using common micromeritics tools like laser diffractometers and Coulter Counters. This application also makes use of laser-based technology that uses the "time of transition" methodology, which is used to estimate sedimentation rates. An example of a batch of microspheres manufactured with a mean particle size of 0.945 µm is shown in fig.
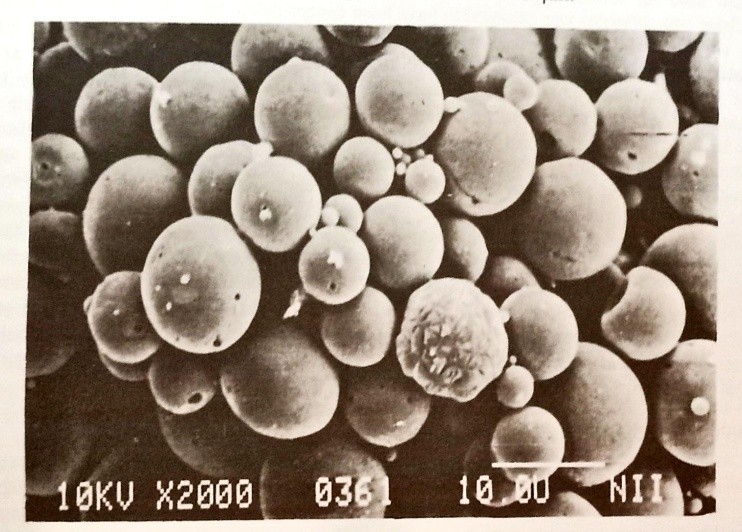
Fig: Scanning electron micrograph of PLGA Microspheres
5.2) Antigen loading:
Antigen loading Different groups have used different techniques to determine antigen entrapment. The 'difference approach' was a major component of early quantitation attempts. This includes calculating the quantity inside the microspheres and the amount used for microencapsulation; the amount outside the microspheres was presumed to have been encapsulated based on the difference between the two estimations. Because these processes need extrapolations, they are relatively prone to inaccuracy. Currently, the majority of groups use a technique for removing encapsulated Ag and measuring the amount that is recovered. The typical process for extraction is as follows. A sample of microspheres is dissolved in an appropriate solvent, such as acetonitrile or dichloromethane, and the protein is extracted using water or an appropriate buffer, and the protein is then quantified using an appropriate protein estimation technique. It ought to be determined.
5.3. Encapsulated antigen's structural integrity :
Polyacrylamide gel electrophoretic analysis (PAGE) may be utilized to identify significant disruptions in order to validate the structural integrity of the antigen in the microspheres (Gilley et al., 1992; Alonso et al., 1994). PAGE may not be able to identify protein aggregation in the presence of microencapsulation when denaturing conditions are met (i.e., when a surface active agent like SDS is present). Combination Controlled Delivery of Vaccines 157 can be detected by native PAGE or gel-permeation chromatography, which can reveal changes in molecular size with greater accuracy. The integrity of immuno-epitopes can be put in evidence by Western blot.
5.4. In.Vitro research on antigen Release studies :
On in vitro Ag release have mostly used as an indicator on the length of immune response as an quality control matrix.
These offer important insights into formulation parameters that can be changed to produce a range of release rates and burst effects, as well as aid in understanding the nature of release. In order to conduct these investigations, the majority of workers disperse the microspheres in an appropriate buffer, preferably in a vial, and stir them for extended periods of time at 37°C. Periodically, samples are taken out and subjected to an appropriate technique for analyzing Ag release (Alonso et al., 1994; Uchida et al, 1994). An illustration showing the release of an anti-fertility compound,

Fig : In vitro release profile of LHRH- DT vaccine intrapped in 50-50 PLGA microspheres of main size 1.06 un.
5.5) Preparation of Antigenicity:
The Preparation Is Antigenic In vivo estimation of the Ag delivery kinetics from a controlled release vaccine delivery device is often challenging. This is due to the extremely small dose range and the infrastructure needed for this kind of study. As a result, researchers would rather assess how the vaccination affects the immunological response produced in suitable animal models. Rather than the pharmacokinetics of Ag delivery, the pharmacodynamic profile of the response is used as an indication of efficiency in the biopharmaceutics of vaccination formulations. As was previously said, the responses that a vaccine delivery system evokes from B cells and T cells indicate how effective it is. Simple immunoprecipitation procedures are among the approaches utilized to estimate Ab titres.
6) PROPERTIES OF VACCINE LOADED MICROSPHERES:
6.1) How antigens are released ?
Instead of diffusing out of PLA PGA and PLGA microspheres, the encapsulated antigen is released through a process known as "bulk-erosion" (Shah et al., 1992, Kenley et al, 1987; Li et al., 1990). The release happens in two stages: first, the weakly held Ag is released, and then the remaining part is released when the polymer matrix breaks down. By adjusting the time interval between the two stages of antigen release from microspheres, the release kinetics of the encapsulated antigen can be tailored to meet the needs. For this objective, three variables can be changed: 1. the molecular weight of the polymer(s); 2. the copolymer's chemical makeup; and 3. the size of the microspheres.
6.2) Control of release rate by polymer molecular weight :
The subsequent phase of the encapsulated antigen's release is significantly influenced by the molecular weight of the polymer matrix (Park et al., 1994). The hydrolytic cleavage of ester bonds in polymer chains, which happens non-enzymatically, is the cause of microsphere structural erosion. The molecular weight of a polymer determines how many ester bonds it contains. Higher molecular weight polymers or longer chain lengths—that is, more ester bonds—in microspheres naturally take longer to break down and postpone the second stage of antigen release from microsphere structures. However, one must remember that a batch of polymer is made up of a diverse population of polymer chains that are grouped around a day 13 through day 20, following which there was an ongoing leak. In the formulation including PLA of MW 80 KD, the lag period was observed to be shorter, ranging from 6 to 10 days. Lower molecular weight PLA (less than 10 KD) did not cause a lag period. Rats were used in release tests for the third polypeptide, bPRL, to see if the outcomes of in vitro research could be associated with the in vivo scenario. Radio-immunoassay was used to measure the circulating hormone released from an implanted film composed of PLA 10 KD. The outcomes matched those of in vitro research. The hormone was said to be released continuously, with no apparent pause in between.
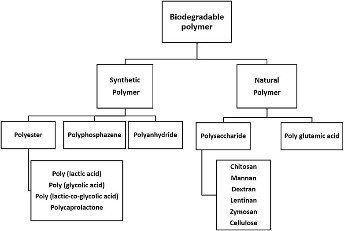
6.3) Copolymer composition regulates antigen release:
Biodegradable polymers used in vaccine delivery.:
These polymers degrade by non-enzymatic hydrolysis, and the hydrophilic character of the polymer speeds up this process. Being more hydrophilic, PGA deteriorates more quickly than PLA. Because the former makes up a larger percentage of the PLGA copolymer, the microsphere formations collapse earlier and quickly release their antigen content. In PLGA copolymers, monomer ratios are often expressed as lactic glycolic acid. In vitro cumulative percent tetragastrin release from microspheres with varying polymeric compositions was demonstrated by Hutchinson (1982). Utilized were low molecular weight PLA (less than 10 KD) and PLGA 50:50, 67:33, 75:25. According to reports, the release of the peptide was delayed in microspheres composed of co-polymers with a larger percentage.
6.4) Control cell targeting by size of Microspheres:
The longer it takes for a particle to collapse and discharge its contents, the larger the particle size (that is, the smaller the specific surface). To present Ag to T-cells and demonstrate the adjuvanticity of the microspheres, macrophages and other APC must engulf antigen-containing microspheres. The majority of the Ag content would be discharged as soluble Ag if the microspheres' size is unsuitable for phagocytosis, which would lead to ineffective Ag presentation to lymphocytes and a reduced production of immunological response. Delivering a portion of the microsphere system as particles that are suited for phagocytosis is crucial for this reason. Gilley et al. confirmed this size to be less than 10 um, reporting a study.
6.5. Improving microsphere phagocytic uptake:
On an experimental basis, uptake is being enhanced by coating the surface of antigen-loaded microspheres with chemoattractants or stimulators of phagocytic cells. For this reason, interleukin-2 (IL-2) has been employed to promote the growth of T helper cells and draw macrophages to the injected particles (Stevens et al., 1993). Additionally, attempts have been made to produce microspheres by encasing the Ag in addition to a conventional adjuvant like alum. Naturally, this did not result in any significant advantages. Conversely, it was discovered that injecting microspheres in combination with an adjuvant that activates phagocytes improved the body's reaction to the encapsulated Ag (Esparza & Kiessel, 1992).
6.6) Interpreting result of immunogenicity studies:
6.1 - Adjuvanticity:
Eldridge et al. (1991) examined the possibility of potentiating the antibody response by immunizing with either free or microencapsulated SEB toxoid in order to test the adjuvanticity of PLGA microspheres. They created PLGA 50:50 microspheres that ranged in size from 1 to 10 µm, and they used intraperitoneal injection to immunize animals with either free or encapsulated Ag. Compared to the free toxoid, the antibody titres against the toxoid in microspheres were significantly higher. When the research was extended to day 90 for secondary immunization, the immunopotentiation supplied by the microspheres kept the level of immunity at 32–64 times that of the free toxoid. O'Hagan et al. (1991) found similar outcomes in their work using microsphere-entrapped ovalbumin, although relatively few researchers choose to disclose comparisons.
6.2. Evaluation of immune status:
assessment of the immune system The research by Alonso et al. (1993), which was previously mentioned, also showed that antibody titres obtained using enzyme immunoassays are not necessarily a reliable indicator of a vaccine's efficiency. Determining whether other immune response components are appropriately engaged and estimating the anti-serum's bioneutralization potential are critical tasks. This was succinctly demonstrated by the finding that, despite no discernible difference in serum antibody levels between the mice injected with TT-microspheres and the group given alum-adsorbed TT, the former group's serum samples had a significantly higher toxin neutralizing capacity at all points of analysis. Consequently, it would be excellent to carry out "challenge" experiments where experimental animals may be.


 Rohini Biramane *
Rohini Biramane *
 Ulka Mote
Ulka Mote









 10.5281/zenodo.14235670
10.5281/zenodo.14235670