Abstract
There are several or many drug which banned since 2016. These are many 344 drug which FDC were prohibited. The Government which banned 156 FDC-fixed dose combination including antibiotics, NSAID agent including painkillers, multivitamins and combination dose of fever, menstrual cramp of many treatments used drug. They have prohibited biggest risk. The Paracetamol , Mefenamic acid and Phenylephrine hydrochloride is widely used but they produced many adverse drug reactions and Toxicity. The Union of Healthcare and Family welfare ministry key -FDC issued gazed notification some popular dose Paracetamol and Mefenamic acid these Active Pharmaceutical Ingredients produce therapeutice effects but some hepetoxicity, overdose , Paracetamol poisoning , liver failure. In case of Mefenamic acid cause the following Ulcers, Bleeding, Holes in the stomach, Triggers drug reactions with eosinphilia, Dress syndrome, Blurred vision, Heart stroke, CNS toxicity. Phenylephrine hydrochloride which produce the Hallucinations, Seizure.
Keywords
Ulcers, Bleeding, Paracetamol, Acid and Phenylephrine.
Introduction
Although acetaminophen, also known as Paracetamol, was discovered a century ago, its usage as an over-the-counter (OTC) medication did not start until the 1960s, and it is currently the most often used OTC medication.[1] Because paracetamol is so widely available and accessible, overdosing on medication is a common way for people to poison themselves worldwide.[2] It is widely accessible as a stand-alone drug as well as a part of a large number of over-the-counter and prescription drug combinations. Although it is safe when taken as directed, one of the most frequent overdoses that poison centers receive reports of is Paracetamol poisoning. 401 deaths related to paracetamol or paracetamol combination products were reported in 2009 by the National Poison Data System of the American Association of Poison Control Centers.[3]Acute liver failure caused by paracetamol is currently the most common cause and the second Mefenamic acid is an anti -inflammatory drug of non steroidal Set (NSAIDS), has been approved as a drug only in the UK and European recipes since the early 1960s, and is often used to treat Dysmorphic and severe bleeding menstruation (HMB). It will be. This use has been confirmed by 5-7 clinical trials, and the recommendation of the command that has been released 8 and 9 is the clinical clinic of Moody acid compared to other NSAIDs from 10 to 12. It does not imply important advantages. Although NSAIDs are mentioned as treatment options in current guidelines, Mefenamic acid is not specifically recommended for the treatment of dysmenorrhea 13 or HMB 14 . The tendency of Mefenamic acid overdose to cause central nervous system (CNS) toxicity, particularly seizures, has been noted in a number of case reports and small case series, 15–22 but the differential neurotoxicity of individual NSAIDs between overdose and routine use, as well as the influence of other risk factors on the development of neurotoxicity have not been previously reported. It is now recognised that when considering the risks and benefits of a drug, it is necessary to take into account not only the risks associated with normal therapeutic use but also the risks arising from overdose. In the European Union, Directive 2010/84/EU now requires Member States to operate pharmacovigilance systems that collect information on suspected adverse reactions resulting from the use of medicinal products outside (and within) marketing authorisation, including those occurring following overdose 23 . One of the potential valuable methods to do is the use of data collected by the center of poison when advising doubts. Therefore, this study compares the frequency of neurotomy between mephenamic acid and other NSAIDs, after overdose using data collected by a British poison center. , It was implemented to study the impact on the reported doses. Most clinical ophthalmic examinations as well as the objective, Retinal and disc imaging involves the administration of Topical Gourdins in control of optimal administration. To obtain a good and fast, a conduct in phenylephrinehydrochlo (an ? receptor agonist) and a tropic amide (muscaric antagonist Receptor T or) are often combined. Phenylephrine can cause pronounced cardiovascular side effects, including increases in systolic and diastolic blood pressure (BP) and change in heart rate (HR), when given systematic.1,2 On the other hand, topical It is believed that the tropicamide is safe and does not cause any unwanted cardiovascular in adults. To date, there is no conclusive evidence concerning the diovascular side effects of the phenylephrine car, 2.5% or 10%, the eye drops alone or in combination with a tropicamide. (n = 100) against the tropicamide, 1% (n = 50) did not find an increase inbporhrup at 30 minutes after administration in one or the other group. 3 On the other hand, a certain number of series of cases reported an increase in the two BP and HR after the topical administration of phenylephrine, 2.5% and 10%, eaverytes. 2.4 emmunting evidence indicated that not only short and long -term increases in the PA but also Variation of short -term PA, for example, 24 -hour ambulatory BP The measure is associated with the theater, the progression, and severity of cardiovascular events, target damage to organs and mortality, 5 It is important to assess potential side effects of topical phenylephrine administered on BP and HR. For example, carotid intima-media thickness and left ventricular mass index, two measures of target organ damage associated with hypertension, can be significantly elevated over a 24-hour period in individuals with elevated systolic blood pressure.6 Therefore, it is important to know whether topical phenylephrine can cause an increase, even if only temporarily, in systolic blood pressure. Neither blood pressure nor heart rate are routinely monitored in ophthalmology or optometry settings. What else should you do before using topical phenylephrine? The need for security is increased. Against this background, we conducted a systematic review and meta-analysis of the literature to determine the safety of topical phenylephrine and to synthesize available data on the extent of cardiovascular side effects
History
Harmon Northrop Morse first synthesized paracetamol by reduction of p-nitro phenol using tin in glacial acetic acid in 1878. However, paracetamol was not used therapeutically for another 15 years. In 1893, paracetamol was discovered in human urine by taking phenacetin. In 1899, Paracetamol was so It is considered an acetalide metabolic. 1948, Brody and Axel rod have decided it The analgesic effect of acetalide was related to itself Activated metabolic product Paracetamol. The product was first marketed in 1955 by McNeill Laboratories under the trade name Tylenol Children's Elixir to relieve pain and fever in children. [5] Epidemiology of toxicity The number of cases of liver failure in India is lower than that of paracetamol toxicity.
History of Mefenamic acid Toxicity:
In the India 7 December 2023 The Indian
Pharmacopoeia commission issued drug safety about the painkiller meftal spas as it trigger the DRESS syndrome. These medications can cause acidity and ulcers since they inhibit particular enzymes which reduce mucous production in the stomach. NSAIDs also reduce the blood flow to the kidneys, due to which they have to work harder. Due to the imbalance of two important enzymes, there is an increased clotting tendency which can cause heart attack and strokes," said Dr.Tayal.
In the national library of medicine( NLM ) shows the report toxicity and shows other than the therapeutic effect are following:
In the FDC Dicyclomine and Mefenamic acid produce the other than therapeutic acid dicyclomine is used for the irritable bowel syndrome Mefenamic acid is used for the analgesic, NSAID, menstrual cramp, reduce inflammation is quite effective in dysmenorrhea. Therefore following case are given : A 30-year-old woman presented with excessive use of dicyclomine and Mefenamic acid tablets over the past 10 years. She started using them in 2007 for dysmenorrhea. A year after starting, she began using it to relieve tension and headaches caused by stress. In 2014, she started taking 2-3 tablets a day to relieve stress caused by marital disputes, and in 2018, she increased the dosage to 10-15 tablets a day, demonstrating tolerance. She reported a strong desire to take the tablets, indicating craving.She experienced anxiety, restlessness, and headaches for 2-3 days after cessation of treatment, indicating withdrawal symptoms. When her husband found out in 2017, it caused conflict with her family, which left her feeling depressed, suffering from periodic headaches, anxiety and difficulty sleeping. On mental status examination, she reported subjective effects of depressed mood and anxiety. When the signal was exposed or disappeared, she developed tachycardia, palpitations, and diaphoresis, and objectively exhibited numbness. His BMI was 18.2 kg/m2, his hemoglobin was 10.4 g/dL, and other routine physical and laboratory tests were within normal limits. He was diagnosed with non-dependent drug addiction with adjustment disorder (International Classification of Diseases-10). She was initially treated on an outpatient basis and then during her hospital stay she was given 20 mg of fluoxetine daily and 0.5 mg of clonazepam daily with recommendations to discontinue administration. Thanks to these measures, she returned to a normal physiological state 1 week after her stay in our department. During the 1-year follow-up, she denied misusing dicyclomine tablets and Mefenamic acid, but her husband suspected she was taking the tablets intermittently and did not acknowledge this. Our patient was started on dicyclomine and Mefenamic acid tablets to treat dysmenorrhea and subsequently to reduce stress. Since this caused therapeutic benefit, it acted as a positive reinforcement for drug use. Later, relief from the symptoms of cancellation acted as a negative reinforcement for drug use. The transition from daily drug use to drug abuse can be neurobiologically conceptualized as a transition from positive to negative reinforcement mechanisms [3]. The abuse potential may be due to dicyclomine anticholinergic effects and its ability to cross the blood-brain barrier. Dicyclomine abuse has been reported in previous literature [4,5], but to the authors' knowledge, no literature on Mefenamic acid abuse has been found. Our patient met criteria for craving, withdrawal, and tolerance for substance use disorder. This suggests that even non-addictive substances such as dicyclomine may be addictive if misused. Therefore, further research into the abuse of anticholinergic drugs, particularly dicyclomine, is warranted. We also suggest the therapeutic use of anticholinergic drugs, especially dicyclomine, combined with close vigilance and effective counseling of patients and their families to prevent abuse of these drugs.
Phenylephrine hydrochloride history :
Phenylephrine hydrochloride is a alpha adrenergic receptor to treat the hypotension they dilate the pupil , induce the local vasoconstriction , It is the used as the decongestant. It is narrowing the small the blood vessel. It is patented in the year of the 1933 and came in the use year 1938 . Phenylephrine taken orally in these doses is generally well tolerated. It may cause side effects such as headache, reflex bradycardia, irritability, restlessness, and arrhythmias.[12] At doses above these, phenylephrine may increase blood pressure and decrease heart rate.[11] 45 mg of phenylephrine may increase systolic blood pressure by 20 mmHg.Art[11]Possible side effects with intravenous administration of phenylephrine are dose-dependent and may include bradycardia and reactivity. The Central Drugs Standard Control Organisation (CDSCO), along with drug regulatory authorities in Madhya Pradesh, has ordered pharmaceutical company Riemann Labs to stop producing a cough syrup that has been linked to child deaths in Cameroon. The World Health Organization (WHO) issued a warning on July 19 about a cough syrup supplied to Cameroon, saying analysis had found the product to contain "unacceptable amounts of diethylene glycol as a contaminant." The WHO said that the makers of autacoids list paracetamol, phenylephrine hydrochloride, and chlorpheniramine maleate as active ingredients, and that the combination of these three is used to relieve symptoms associated with influenza, colds, and allergic rhinitis.
Epidermology Of the Toxicity of The Paracetamol
In Western countries, this may be a result of under-reporting. The United States stands out from all other countries by reporting a huge number of cases of acute liver failure (ALF). Between 1990 and 1998, there were 56,000 emergency department visits, 26,000 hospitalizations, and 458 deaths from acetaminophen overdose. [6] As of 2007, there were 1,600 cases registered in the United States. In most cases of AKI, the underlying pathophysiology was paracetamol. In the UK, Paracetamol accounts for around 50% of self-poisoning cases, resulting in around 200 deaths each year.[7] The toxicity caused by the drug is less in children, but not completely absent.
The drug does not discriminate against age. Morbidity and mortality are low in countries where sales of paracetamol are restricted Purchases are made one at a time. [8,9] In India, data on paracetamol autointoxication is rare and scarce compared to Western countries. A 10-year retrospective study conducted in a hospital setting reported 0.32?ses of acute paracetamol overdose due to accidental exposure. Key Points to remember: Survival from a paracetamol overdose is generally considered to be 100% in cases receiving NAC within 8 hours of exposure. Efficacy declines after this point. The threshold for potential liver damage from paracetamol in adults is > 10 g or > 200 mg/kg (whichever is lower) in 24 hours.
- Epidermology Of The Toxicity Of Mefenamic Acid
Overall, 11 % of mephenamic acid patients developed convulsions. This occurred more frequently in 15-19 years (23.9 %) than 20 years or more (6.0 %, P).
- Epidermology Of The Toxicity Of Phenylephrine Hcl
Etiology and pathophysiology
Toxicology of paracetamol
Paracetamol is metabolized in the liver by: Pathways - glucuronidation, sulfation, or Cytochrome P450 enzyme system in the liver.
The toxic effects of paracetamol are due to:
Alkylated metabolites N-acetyl-p-benzoquinone
imine (NAPQI). [10] In the case of acetaminophen
When taken orally in therapeutic doses, approximately 90% of the original compound is exposed to sulfate and glucuronide combinations
Conjugation. [10] These conjugates are released
as non-toxic metabolites. [11, 12, 13] More details
5% is excreted in the urine. A small portion (approximately 4%-5%) is metabolized by the mixed function cytochrome oxidase system.
P450, mainly by the CYP2E1 enzyme to produce the highly reactive and toxic metabolite N-acetyl-p-benzoquinoneimine (NAPQI). Endogenous Glutathione in the liver serves as a substrate for
NAPQI, which is a non-toxic metabolite, mercapturic acid, excreted in urine. However, if an overdose occurs, normal metabolic pathways become saturated and more paracetamol is produced. Derived from the P450 system. Thus, more NAPQI is produced, and when glutathione is depleted by 70%, the excess amount of NAPQI binds to hepatocytes and cytotoxicity manifested by. hepatic necrosis. [14]
Clinical course features of paracetamol toxicity due to hepatic necrosis is divided into four stages. [10] During the first few hours, patients may be relatively asymptomatic. However, the clinical course changes over 24 hours, gastrointestinal symptoms such as nausea, abdominal pain, and vomiting predominate. On second stage (12-48 hours after eating) gastrointestinal discomfort may disappear, but subclinical hepatotoxicity progresses and begins to express itself. Abdominal pain may recur and the patient may complain of tenderness in the right upper quadrant. Laboratory values ??begin to show signs of hepatotoxicity - liver function tests such as aspartate (AST) and alanine transaminases (ALT) rise sharply and an increase in the international normalized ratio is also observed (INR), which reflects the severity of the underlying pathology. During the latter part of stage 2, AST and ALT approach maximum values. In stage 3 (48 to 96 hours), as liver damage accelerates, the following signs of liver failure become apparent: bleeding, encephalopathy, jaundice, acidosis, renal failure.
If the patient survives the physiological damage of stage 3, he or she will progress to the final stage of recovery.[11.12.13]
Dosage
The recommended dose of oral or rectal paracetamol for symptomatic fever (temperature > 38.5°C) is 15 mg/kg every 6 hours
Toxicity Prevention Measures
by Regulatory Agencies
USFDA (USA) [17]
1999: Issued a regulation to all paracetamol manufacturers to include a label warning about alcohol 2002: Recommended distinctive labeling and a special warning about liver toxicity. 2004: Launched a patient education campaign. 2007-2009: A working group was established to identify the epidemiology of toxicity during the use of over-the-counter (OTC) drugs. 2011: Recommendation to manufacturers to reduce the concentration of paracetamol to 325 mg per combination. He also advised reducing the maximum daily intake from 4g to 3g, and recommended that manufacturers include a "black box" warning. [18,19] MHRA (UK) [20] 1999: Legislation was passed to reduce the number of paracetamol tablets with salicylates to a maximum of 16 in supermarkets and a maximum of 32 in supermarkets pharmacy. D.C.GI (India) 2003: DCGI required all manufacturers to include a mandatory warning on packs of paracetamol. [21]
2007: DCGI banned a series of paracetamol Combined drugs such as paracetamol + Alprazolam, paracetamol + analgin, Chloraxazone + ibuprofen + paracetamol + diclofenac + oxypenbutazone + magnesium Hydroxide. [22]
2011: DCGI did this mandatory for all Manufacturers of the combination of paracetamol
medicines to put the box on the composition Packing, warning the consumer of the patient Potential toxicity of the liver if the medicine is used More than the recommended daily dose. [twenty three] 2013: The DCGI office was addressed to the whole state Reduced drug administrator The contents of up to 325 mg of paracetamol are applied Combination of other paracetamol Pain /anti -inflammatory drug. [twenty four] Considering that there is the most overdose Normal fever and medication with pain decrease Possibility of liver damage, the United States Product and medication control control(FDA) In this case, the dose of 325 mg is limited.
Combination with non -steroidal anti -inflammatory drug (NSAID)
It has been known to have a negative effect on the liver. According to FDA regulations, paracetamol can only be used in combination with aspirin and opioids. However, there are some potentially dangerous combinations, including: Paracetamol at 500 mg or more with an NSAID, the experts pointed out. "For example, paracetamol can be used in combination with nimesulide, piroxicam, etodolac, lornoxicam, dexibuprofen. There are several products available in the market that are sold without prescription, which contain paracetamol 500 mg in the form of D Cold tablets, Vicks Action 500. Indian experts have called for a review of combination pain relief and fever medications sold in the country after US regulators recommended limiting the dosage of paracetamol when it is used in combination with other medicines.
CONCLUSION
Paracetamol is widely used to treat: Fever and pain relief. It is available over the counter In various combinations and doses. Lightweight Available without prescription, several Drugs in various doses, Used excessively to suppress fever and its symptoms Used unintentionally believing it is safe
Drugs contribute to therapeutic misfortune. Though there is epidemiology of liver failure, Use of this drug in India is low, manufacturers bear Responsibility for inclusion Proper labeling and effective use Product use. Recommendation Reducing risk is to educate Caregivers who is toxic possibility. Dose folder based on age and weight Doctors need to be examined between each
visit. The Indian drug regulator needs to respond immediately by taking steps to reduce the incidence of toxicity. A country-wide education campaign could be competent. As effective measures have been shown to significantly reduce morbidity and mortality, the government should immediately reverse them to avoid a further increase in the numbers of people at risk of liver failure.
Method of study of the toxicity of the Mefenamic acid
The national poisons information services commissioned by public health England . These department gives the information about clinical advice and acute & chronic poisoning to UK and other countries to the health professionals . The recent evaluate information from NIPS of the following ways : through the clinical data 2) through NIPS telephone history 3) And the official report . There are following data are included 1) patient age 2) patient gender 3) reported dose 4) route of administration The clinical features reported when the telephone enquiry. They are classified expose are following 1) International overdose 2) Accidental overdose 3) These reputation errors 4) Drug abuse then the establishment of the one enquiry can be single record CNS toxicity is known as the 1) convulsions 2) Altered the convulsions 3) Aggression 4) confusion Mefenamic acid is not licensed for use in children under 12 years of age, so there is no recommendation for this group. daily dose. We therefore used the maximum daily dose in mg kg 1 derived from the maximum adult dose and assuming an adult weight of 70 kg. For children, the maximum daily dose was expressed in mg kg 1 of BNF, and if the child's weight was not recorded, weight was estimated from the age
50th percentile of the male or female height chart
compiled by the World Health Organization and the Royal College of Pediatrics and Child Health [25]. Sensitivity analyses were performed to assess the robustness of the results using alternative approaches, which were used toxicity threshold doses for each NSAID, as indicated found in TOXBASE®, the online poisons database provided by NPIS to UK healthcare professionals (Table 1). When available, reported drug doses were standardized by recorded weight; when this was not documented, the weight of adults was assumed to be the average weight for a male (84 kg) or female (70 kg) from the National Statistics Health Survey for England 2012 and the Welsh Health Survey 2009 [26, 27]. For children, the weight was calculated as described above. For a 70 kg adult, these toxic threshold doses are 1.9 (Mefenamic acid) to 3.3 (diclofenac) times the maximum daily dose as recommended in the BNF. We then standardized for doses between drugs by calculating the ratio of reportedingested dose to the toxic dose, expressed in the present study as ingested-to-toxic dose ratio (Table 1). When analyzing clinical manifestations of toxicity patients who may have been exposed to other drugs were excluded. Logistic regression models were applied using IBM SPSS v.22 software to compare the probability of developing CNS toxicity between different NSAIDs. Likelihood ratio tests were used to compare models in which drug type was included as a covariate with a model in which drug type was excluded to determine whether drug type as a significant predictor of outcome. If significant differences were found, pairwise comparisons were performed between each drug pair and P values ??were adjusted using Bonferroni correction for multiple testing. This model was not applicable to the analysis of reported seizure cases because the number of patients who developed seizures was small and not all NSAIDs were associated with seizures. Instead, other models were constructed for CNS toxicity outcomes and seizures compared with Mefenamic acid and all other NSAIDs. Multivariate logistic regression models were used to test for drug differences after adjusting for age and sex and the ratio of ingested dose to toxic dose. The term "age squared" was added to the model to account for a possible quadratic relationship between age and odds of toxicity. Conditions considered Interactions between drug, age, sex and intake, were also tested but were not statistically significant and were therefore not included in the final model. Patients with missing data by age, sex or intake were excluded from this model. In the UK, surveillance studies of this type do not require ethical approval as they involve the analysis of anonymised and aggregated clinical information collected and regularly published as part of the NPIS clinical file. The British National Formulary recommends maximum daily doses of Mefenamic acid, ibuprofen, diclofenac and naproxen, as well as toxic doses for each drug identified in TOXBASE®. Note: The maximum daily dose for any given indication has been used. Maximum Daily Dose Toxic Dose (mg kg 1 Adults (mg) Children)
Mefenamic Acid 1500
• Under 12 years: not approved
• 12 to 18 years: 1500 mg
40
Ibuprofen 2400 • 1 to 3 months: 20 mg kg 1
• 3 months-12 years: 30 mg kg 1
• 12 to 18 years: 2400 mg
100
Diclofenac 150 • 6 months-18 years: 5 mg kg 1 7
Naproxen 1250 • 1 month to 2 years: 15 mg kg 1
• 2 to 18 years 10 mg kg 1
35
CNS Toxicity of Mefenamic Acid
Br J clinical pharmacy (2017) 83
CONCLUSION
Between January 2007 and December 2013, there were 23 144
NPIS telephone enquiries relating to 22 937 separate exposure’s to the four NSAIDs studied. Exposures were less common for Mefenamic acid (925) than for ibuprofen (17 302), diclofenac (3385) or naproxen (1325). The median age of Mefenamic acid patients was younger (17 years) than those involved in enquiries about ibuprofen (23 years), diclofenac (29 years) or naproxen (32 years) and there was a significantly higher proportion of female patients in the Mefenamic acid group compared with the other groups combined (P <0>
compared with the other NSAIDs studied. Acute intentional overdose was the most prevalent exposure type overall (Table 2).There were 10 398 exposures to one of the studied NSAIDs where co-exposure to other drugs was not reported. In these, CNS toxicity was recorded in 3% overall (Table 3) and, after adjustment for age, gender and reported dose ingested Table 1 British National Formulary-recommended maximum daily doses for Mefenamic acid, ibuprofen, diclofenac and naproxen, and the toxic doses for each drug as defined on TOXBASE®. Note: The maximum daily dose for any given indication for a drug was used Maximum daily dose Toxic dose (mg kg1 Adults (mg) Children )
Mefenamic acid 1500 • Less than 12 years: not licensed
• 12–18 years: 1500 mg
40
Ibuprofen 2400 • 1–3 months: 20 mg kg1
• 3 months-12 years: 30 mg kg1
• 12–18 years: 2400 mg
100
Diclofenac 150 • 6 months–18 years: 5 mg kg1 7
Naproxen 1250 • 1 month–2 years: 15 mg kg1
• 2–18 years 10 mg kg1
35
DISCUSSION
This study confirmed that excessive intake of Mefenamic acid was in collaboration with the toxicity of CNS, especially related to doses. Convention, and that this is greatly common After overdose with other frequently used NSAID. Al, intentional overdose formed a big share Request for Mefenamic acid, average registration (Share of maximum recommended dose) The same is the same for each NSAID studied and the difference
It was saved after adjusting by the invitation of the dose. These results match the previous research Animals and people. In mice, high single doses of Mefenamic acid caused central nervous system stimulation followed by loss of coordination, central nervous system depression, and seizures [28]. Clinical Signs of CNS toxicity reported in humans include: seizures, mild drowsiness and disorientation leading to coma, and respiratory arrest [15-19, 29, 30]. Seizures Usually occur 2–7 hours after overdose, but may occur up to 12 hours after ingestion [16, 31]. A retrospective study conducted by the Swiss Toxicology Information Centre reported that the proportion of acute single-drug overdoses up to 1997 in 2010 at least one seizure occurred. An overdose of mild acid was 16.3% of all cases. In general, in 11% of patients with crime acid developed more frequent convulsions in 15-19- Years (23.9%) than for 20 years and more (6.0%, P < 0>
dosing (1–10 mg l-1
) [16]. However, seizures can occur in patients with Mefenamic acid concentrations below this threshold line [16, 18, 19], and the lowest 4-h Mefenamic acid concentration at which convulsion has been documented was 21 mg l-1 in a 13-year-old girl [18]. Unpublished data from a retrospective study of 241 patients with Mefenamic acid overdose showed that the reported oral dose was directly related to the severity of toxicity, including CNS toxicity, and the lowest dose at which moderate or severe symptoms developed was 3.5 g [34]. The lowest reported Mefenamic acid overdose causing seizures was 2.5 g [31]. Furthermore, mephenamic acid is also linked. To seizures after treatment [17, 35]. On the other hand, these Previous studies provide evidence of risk -related risks of convulsion after overdose of mephenamic acid, and we will not compare them.
Risk of alternative NSAID.
Accurate mechanism that NSAID triggers seizures
Excessive intake is not clear. It has been postulated that NSAIDs reduce the convulsive threshold by inhibiting cerebral prostaglandin and/or thromboxane synthesis [36]. Modulation of
?-aminobutyric acid (GABA) receptors in the CNS has also been suggested as a possible cause for lowering seizure threshold in poisoned patients [37–39]. Slope Caproxis is more nervous than other NSAID Excessive intake is currently inexplicable. No information The relationship between age and central nervous system toxicity and convulsions. The line may be given age compared to a 20 -year -old patient
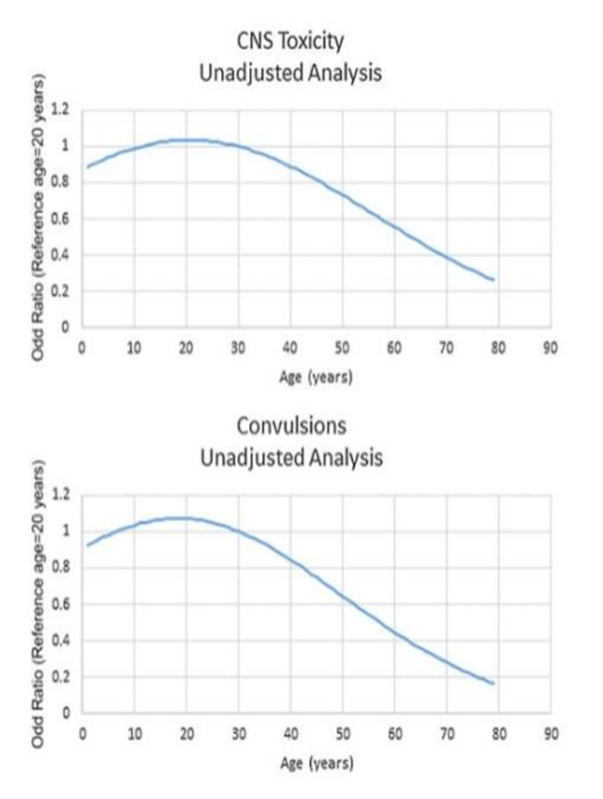
Relative activity of individual NSAIDs to reduce
convulsive threshold, and there are no comparative data As for the effectiveness of various NSAIDs in penetration.
Bloody barrier.
The results of this study are important from -s
Physical risk from seizures, including jury risk, aspiration and hypoxia. Sudden death may occur in this situation, although it is probably rare.Social impact The impact of a seizure on an individual is also important but differs from country to country. For example, patients who develop the disease may be unable to drive for a period of time, and employment in certain occupations may also be affected. This study had important limitations that should be considered: the number of requests to the NPIS for specific drugs or medications reported in this study does not correspond to the number of patients actually exposed. It is also possible that the NPIS may be contacted multiple times for the same patient, especially in cases with severe or prolonged clinical symptoms. Although attempts were made to identify and consolidate duplicate requests, this was not always possible and some duplicate requests may have been missed. Not all cases of overdose are referred to the NPIS because
the responsible physician may have confidence in the treatment, with or without referral to TOXBASE®. NPIS Requisition Numbers
There is unlikely to be a direct correlation with patient visits to hospital, as patients with mild or no clinical manifestations are less likely to present. Details of exposure
are as initially reported by the patient and then passed on by the enquirer, and this may sometimes be unreliable. Analytical confirmation of exposure and exclusion of other potentially toxins is not available as this is not performed as part of the routine care of patients with NSAID overdose. NPIS attempts to track severe intoxication symptoms, but this is often not possible, resulting in clinical impacts.Post-investigation symptoms (e.g. delayed onset seizures) may not be taken into account. NPIS data do not necessarily discern Important confounding factors such as history of epilepsy, alcohol abuse, and head trauma. However, these limitations applied to all NSAIDs studied, making it unlikely that bias in data collection would occur across NSAIDs.
Another limitation was caused by the difficulty of comparing doses across drugs. The dose declared according to the situation Overdose of the drug may be unreliable. In addition, Mefenamic acid is not permitted in children. Therefore, there are no recommended guidelines for this population. Daily dosage. Farther The difficulty was that the patient's weight is not always documented and had to be deduced in many cases. These LIMs, however, are very unlikely to have explained the
Substantial differences between Mefenamic acid and other Aims in terms of toxicity and would not have had an effect By studying the dose-toxicity relationship for a specific NSAID. The sensitivity analysis that we have carried out, standardizing the doses between drugs using toxic dose thresholds provided on Toxbase®, also gave similar results.
Despite the inherent limitations, this demon study committed the potential value of the data collected regularly by Poisons is centered to assess the safety of drugs when taken in overdose. It has been confirmed that the risk of CNS toxicity, particularly convulsions, is increased after Mefenamic acid overdose compared with other commonly used NSAIDs. In light of these findings, the balance of benefits and harms of mefenamic acid needs to be reevaluated. Although previously considered an appropriate treatment for menstrual pain and bleeding [8, 9], recent evidence does not show that mefenamic acid is more effective than other NSAIDs, or that NSAIDs are more effective than alternative interventions [10–14]. Target the drug in women with Dysmenorrhea or menorrhagia is anxious because adolescents and young people who usually affect be an increased risk of self-over-dose [40]. Given that mild acid does not depend on the proven clinical advantages, alternative drugs should attribute to controlling inflammatory conditions and Menstrual problems, especially in those who run a higher risk autoimmunization. Mefenamic acid should only be considered if alternatives are contraindicated or not tolerated, if used at all.
Regulatory authorities should reassess the benefit–risk profile of Mefenamic acid, taking into account the available information on CNS toxicity with normal use as well as overdose, and consider if further measures are needed to reduce the public health risk from Mefenamic acid toxicity.
Systematic Adverse Effect And Toxicity Of The Phenylephrine Hcl:
Results
Total Phenylephrine Dose Administered In the United Kingdom, MHRA-approved formulations
include phenylephrine 2.5% and 10% eyedrops, phenylephrine IC solution (Mydrane, a mixture of phenylephrine 0.31%, tropicamide 0.02%, and lidocaine 1%, Thea Pharma GmbH), and phenylephrine conjunctival insert (Mydriasert, a mixture of phenylephrine 5.4 mg and tropicamide 0.28 mg, Thea Pharma GmbH). Elsewhere, an ´ irrigation solution containing phenylephrine and ketorolac (Omidria, a mixture of phenylephrine 1% and ketorolac 0.3%, Omeros Corp.) is now marketed as an alternative to the standard balanced salt irrigation solution for the duration of cataract surgery, to maintain mydriasis after administration of standard preoperative mydriatic eyedrops.21–24 The recommended practice is to add one 4-mL vial of Omidria (containing phenylephrine 40 mg) to a 500-mL bottle of irrigation solution, which produces a final concentration of phenylephrine 0.008% and ketorolac 0.0024%. Use of more concentrated solutions of phenylephrine as intradermal phenylephrine (use of pure and diluted solutions eye drops) is an off-label practice25. We calculated the total dose of phenylephrine administered in each eye for different formulas in daily clinical practice. We made the following assumptions: local the regimen includes 3 phenylephrine eye drops in total, each drop is 37 ml, and the volume of intradermal solution administered is 0.25 ml.26,27 Thus, we calculated that the total dose of phenylephrine injected into the eye
ranges from 0.78 to 40 mg per eye (Table 1). Additional eye drops, bilateral administration or repeated rinses with intradermal phenylephrine
will mean that an even higher dose of phenylephrine will be given. Data from pRCT with IC phenylephrine.
Introduction
There have been 7 pRCTs comparing IC phenylephrine with local mydriatics (Table 2)3,10,28–34. It should be noted from Table 2 that the total dose of phenylephrine given in the control groups (as eye drops) was higher, than in the IC phenylephrine groups, in several of these studies3,28,29,33,34. A total of 491 eyes received IC. phenylephrine, at a dose of 0.62 to 7.5 mg - 6 studies reported no serious adverse events associated with CI phenylephrine.3,10,28–30,33,34. In one study, Labetoulle et al. Originally reported the frequency of SAE in 4.8% in patients in whom The resulting IC phenylephrine (total doe is 0.62 mg) and 6%in Table 1. Comparison of phenylephrine compositions and general doses of phenylephrine entered by various routes The path of implementation General phenylephrine The dose is entered For the eyes (mg) Phenylephrine Eye 2.5?tual 2.78 Phenylephrine eyes 10% relevant 11.1 Phenylephrine insert 5.4 mg (MyDriasert, also contains a tropicamide of 0.28 mg) insert of the conjunctival system 5.4 A solution of phenylephrine 0.31% (MyDrane also contains tropicomide 0.02% and Lidocaine 1%) Inner Bolus 0.78 Phenylephrine solution 1.25% intracameral pain 3.13 Phenylephrine solution 2.5% intra -chamber bolosb 6.25 Phenylephrine solution 10% intra -chamber bolosb 25 Phenylephrine 0.008% in an irrigation solution of 500 ml (Omidria, also contains ketorolac 0.0024%) In an irrigation solution (bottle 500 ml) 40C An even higher total dose will be introduced if additional eye cliffs, bilateral administration, large volumes and/or repeated intracamberry irrigation executed. A All local administrations are designed for 3 drops, the size of each drop is 37 ml b All intracammer bolus injections are designed for an injection volume of 0.25 ml
It is assumed that a full 500 ml bottle is used for irrigation.

Table 2. Randomized controlled trials comparing bolus IC phenylephrine with topical phenylephrine
Search method results Lundberg and , Behndig 2003 (n = 60)28 Control (n = 30): TOP phenylephrine 10% + cyclopentolate 1%, 3 drops (3.7 mg/drop) (total dose 11.1 mg) Intervention (n = 30): Phenylephrine IC 1.5%, 0.15 ml (total dose 2.25 mg)
Method: "Blood pressure and pulse were measured" Evaluation immediately before and after surgery Level of systemic side effects" Results: Statistically significant reduction in heart rate (8.2 ± 6.6 beats/min) in the control group (P 180 in 2/30 (6.6%) patients in the IC phenylephrine group, but no antihypertensive medication was required. PAS > 180 in 9/30 (30%) in control group A, including 8/30 (26.6%) requiring antihypertensive treatment. PAS > 180 in 3/30 (10%) in control group B, including 2/30 (6.6%) requiring antihypertensive treatment. No other French SAEs were mentioned. Backstrom et al., 2012 (n = 60)30 Controls (n = 30): Phenylephrine TOP 1.5%+ Cyclopentolate 0.85%, 3 drops (0.56 mg / drop) (total dose 1.67 mg) Interventions (n ??= 30): IC Phenylephrine 1.5% + Cyclopentolate 0.1% + Lidocaine 1%, 0.15 ml (total dose 2.25 mg) Methods: "Intraoperatively, the total duration of the operation… is recorded as well as all complications » Results: All reported complications were ocular diseases. No significant differences were found in complications, but there was no significant difference incomplicating factors, surgical performance, or postoperative response. No other SAEs mentioned. Limitations: No definition/parameters for SAEs Lay Suan et al., 2017 (n = 112)10 Control group (n = 56): TOP phenylephrine 2.5%+tropicamide 1%, 3-4 drops (0.93 mg/drop) (maximum total dose 3.7 mg) Intervention group (n = 56): IC phenylephrine 1.5%, 0.2 mL (total dose 3 mg) Methods: "Blood pressure and heart rate were recorded in all patients" Results: There was a statistically significant increase in mean SBP from pre- to post-op in the control group (+3.5 mm Hg) and a statistically significant decrease in mean SBP from pre- to post-op in the intervention group (3 mm Hg). The difference between the control and intervention groups was statistically significant. No other mention of SAE. Labetoulle et al., 2016 (n = 555)3 Guell et al., 2019 (n = 555)32 (analysis of Labetoulle et al.'s 2016 data) Labetoulle et al., 2020 (n = 90)31 (post-hoc analysis of Labetoulle et al.'s 2016 data) T2D patients) Control (n = 283): TOP phenylephrine 10% + tropicamide 0.5%, 3 drops (3.7 mg/drop) (total dose 11.1 mg) Intervention (n = 271): IC Midran 0.31% phenylephrine, 0.2 ml (total dose 0.62 mg)
In the original study (Labetoulle et al., 2016), SAEs The reported frequency of . 4.8% in Midran and 6% in the control group (P = 0.577). Midran
SAEs included systemic infections (3 patients, 1.1%) and neurological disorders (3 patients, 1.1%). No furtherdescription given. Post hoc analysis of SBP, DBP, and HR in same patients (Guell2019) showed that instances of hypertension (SBP >200 mm Hg, DBP >100 mm Hg) or tachycardia (HR >120 bpm) were statistically more common in the control group (11.2%) vs the
intervention group (6%, P = .033) Post hoc subgroup analysis on patients with T2DM (Labetoulle et al., 2020) showed 1 patient in the Mydrane group had a TIA on postop day 6, which was deemed unrelated to the study medication. Nazim-lipsky et al., 2020 (n = 64) 33 Control (n = 35): Upper phenylephrine 10rop + tropicomide 1%, 3 drops (total number dose 3.7 mg) Intervention (n = 29): ic mydrane containing phenylefrin 0.31%, 0.2 ml (total dose 0.62 mg)Results: "There were no intraoperative complications" Restrictions: no definition/parameters for complications Souki et al., 2021 (n = 50) 34 Control (n = 50): upper phenylefrin 10%+ tropicomide 1%, 3 drops (total dose11.1 mg) Intervention (n = 50): ic mydrane containing phenylefrin 0.31%, 0.2 ml (total dose 0.62 mg) Results: “19 AES reported, none of which was considered Be serious and not related to MyDrane or control treatment " Restriction: There is no definition/parameters for SAES AE = unfavorable effects; BPM = blows per minute; DBP = diastolic blood pressure; HR = heart rate; Htn = hypertension; IC = intra -chamber; Map = average arterial pressure; SAE = systemic adverse effect; SBP = systolic blood pressure; Tia = temporary ischemic attack; Top = local; T2DM = type 2 diabetes mellitus Review/Update: IC Phenylephrine system side effects and optimal dose 189 Volume 50 release February 2, 2024.
Evidence From Case Series and Nonrandomized
Comparative Studies
We found 4 comparative studies (not pRCTs) reviewing the SAEs of IC phenylephrine (Supplemental Table 3, available at http://links.lww.com/JRS/B7).38–41 A total of 393 eyes received IC phenylephrine; the total dose ranged from 0.62 to 7.5 mg; and compared with eyes that received topical phenylephrine, the total dose ranged from 1.67 to 11.1 mg.38,41 There were no significant SAEs associated with IC phenylephrine administration reported other than
local ocular complications in these studies.
We found 4 case series that assessed for SAEs with IC phenylephrine (Supplemental Table 4, available at http:// links.lww.com/JRS/B7). One large -scale future case series (n =421) It did not eliminate cardiovascular risk factors No contract period and statistically significant changes have been reported. Blood pressure of the patient who received the patient 0.25 ml IC phenylephrine 2.5 % (total amount 6.25 mg). 42 Small research to manage the IC mixture (n = 10) Phenylephrine (total amount 2.25 mg), cyclopen trate, and Li docorne for initial muscles for cataract surgery have also been reported. Heart rate, shrinkage, and minimal changes in expansion of blood Pressures.43 Two cases series regarding the use of IC phenylephrine Pediatric white vestige surgery did not discover a significant change Heart rate and blood pressure: One series (n = 13, age range 1 month to 10 years) 0.025 ml was administered Combination of 0.31 % (total amount 0.078 mg), phenylephrine Tropicamide 0.02 %, lidocaine 1 %, and another series (n = 53, age was in a month to four years).0.1 ml of the same IC (total dose 0.31 mg). 44,45
Evidence from the case report
I could hardly find the case report of SAE related to IC phenylephrine. I found two published reports of severe SAE (Supplementary table 4, http: //links.lwww.com/jrs/ available B7). Regarding your response One death was reported during treatment with IFIS due to systemic absorption phenylephrine, but the route of administration was not specified and no clinical details were provided46. One case of spontaneous ventricular fibrillation occurred during the period. Phenylephrine IC (0.25 ml, 1.25% concentration)47 Also, in one communication, sudden hypertension was reported when using Phenylephrine IC.48 MHRA Yellow Card Database, search held in May 2020, 50 suspected adverse drug reactions were identified.
All were related to phenylephrine, but all were related to phenylephrine
local application. No other reports of SAEs were found
Classified as phenylephrine IC. Data from the review
We found 1 review article on the safety of phenylephrine IC,
including only 3 of the references we discussed
previously: 1 RCT that found a statistically significant lower
systolic blood pressure in the phenylephrine IC group compared with topical application, 1 case series that found no statistically significant increase in blood pressure
with phenylephrine IC, and 1 case report of ventricular fibrillation.10,42,47,49
Optimal concentration of phenylephrine IC for the treatment of IFIS
We found no pRCTs comparing different concentrations/doses of phenylephrine IC in the treatment of IFIS. The case series used a CI with a different concentration There is a risk of IFI I have iodiet.
These standards and the definition of inclusion
Success varies. In other words, the next research means not.
Strictly equivalent. In a small case series of seven patients with cataracts taking tamsulosin, 0.5–1.0 mL of phenylephrine 0.5% IC was administered (total dose 2.5–5 mg). The authors reported that all patients had sustained pupillary dilation and improved iris relaxation6. No serious side effects were reported. A prospective randomized multipatient study examined signs of IFIS (miosis and iris prolapse) in 42 patients (84 eyes) treated with tamsulosin who had undergone cataract surgery36. No signs of IFIS were detected (0%, n = 0/42) from eyes treated with phenylephrine IC (1.5%, 0.6 ml = total 9 mg), compared with 88% (37/42) of patients receiving placebo IC. All eyes with signs of IFIS in the placebo group were
successfully reoriented with IC phenylephrine. No
significant differences were observed in blood pressure and heart rate
from preoperative baseline values ??in any
patient treated with IC phenylephrine.36
DISCUSSION
One of the major concerns regarding the use of
phenylephrine is its potential adverse cardiovascular effects. Local Phenylephrine can do that
It is absorbed by blood circulation via a nose Lacimal.
There are nasopharyngium and reports
Hypertension, heart julhitis, angina, acute myocardium
Inforce, pulmonary edema, and probably dead
With this management road.
Interior vein (iris). However, the roll of water -based humor
The rate (blood barrier-aqueux) is lower because it may be restricted.
Risk of whole body absorption compared to
The volume and excessive CI injection arrangement with the opthalmic viscosurgical device, phenylephrine solution IC shift in front
Rooms on the surface of the eyes, potentially cause
Absorption via nasal and nasopharyngium, Similar to local administration. Notably, the total amount of phenylephrine administered (0.78–25 mg) varies widely between the various MHRA-approved formulations and off-label dilutions, as shown in Table 1 For topical therapy, it is common to administer topical phenylephrine in three separate doses of 1 drop each (3 drops in total). If adequate pupil dilation is not achieved, additional drops are often administered at the surgeon's discretion. If a patient receives topical phenylephrine 10%, the cumulative dose can vary from 11.1 mg (3 drops) to 14.8 mg (4 drops) or more. 32 In different studies, the amount of drops varies from 25 ml to 70 ml. Thus, the actual dose can vary significantly when administering the same number of drops. 10, 26, 32, 52 To put this in perspective, Intravenous phenylephrine is recommended for the management of hypotension
Associated with spinal anesthesia during cesarean section is 0.025 to 0.05 mg depending on the degree of hypotension. 53 Small amounts of phenylephrine in the systemic circulation are sufficient to increase blood pressure. It is therefore not surprising that serious cardiovascular adverse events have been reported with topical phenylephrine. As mentioned above, phenylephrine was detectable in the bloodstream of 100% of patients after 3 drops of 10% phenylephrine , but only in 14.3% of patients who
received phenylephrine IC 0.31% (Mydrane) instead.32 Excessive dosage of phenylephrine IC could theoretically also lead to similar cardiovascular events due to absorption through the intraocular veins, nasolacrimal ducts, and nasopharynx. Most clinical research uses relatively low measurements.
Volume (0.15 to 0.30 ml) Phenylephrine IC, and even lower of pediatrics (0.025 to 0.1 ml)case. However, 29,32,35,44,45 has been introduced by other surgeons. Volume (0.5-1.0 ml) .6.36 Excessive volume, higher Front bedroom volume (center) 0.25 ml), the remaining part comes out of surgical injury Potentially increase risks on the surface of the eyes The risk of the whole body absorption, as a result, the risk of sae.27.54.55 is IS. Practice must be discouraged. Eye surface irrigation Immediately after the introduction of Phenylephrine IC Acceleration of nose Crymal Canal is required) Reduce the risk of whole body and SAE absorption. The actual introduction of Phenylpherine is less likely to obtain organic in the front room. As a result, a higher concentration solution is needed to reach the appropriate Metrian Sia.Comparison and comparison of IC.1 10 in Guell Study Others can use additional reconstruction The complete effect of the office and local group patients has changed from 10.2 to 34 mg of Phenylphrin. (10 % of the 3 to 10 eyes of the solution) 0.62 1.24 mg of Phenylphrin (0.31 % solution of 200-400 ml) In the IC group, PhenyleFrin.32 is not surprising In the same study, Phenylphrin was found in plasma. In the case of 100 % (n = 15/15), a patient who has been administered local Phenylephrine to 14.3 % (n = 2/15) suffering from Phenylephrine IC. It also explains why systemic hypertension was more commonly observed with topical phenylephrine 10% than with IC phenylephrine in other studies.13,15–18 Reassuringly, a systematic review on the cardiovascular effects of topical phenylephrine eyedrops found that 1 to 3 drops of phenylephrine 2.5% (but not 10%) eyedrops provide adequate mydriasis, but no clinically
meaningful change in blood pressure or heart rate, and concluded that 2.5% eyedrops are safe to use in routine clinical practice.19 A Cochrane review concluded that cardiovascular-related adverse events were rarely mentioned with IC phenylephrine.56 Both volume and concentration (= total dose) of IC phenylephrine administered play an important role in the risk of SAEs. Commercially available formulations have the advantage of a precise and accurate concentration. Using larger volumes of a lower concentration solution should be better tolerated and potentially carries less risk of SAEs in principle. The use of highly concentrated IR solutions (both pure and diluted) is an off-label practice and is currently Special way to nurture clinicians for IC preparation It has been shown to be inaccurate phenylphrin High energy dose 25. If there is no reliable evidence In the case of secure concentration phenyl phrine, general dose When an adult introduces it, it seems safe from 0.62 to 9 mg The patient had nothing to do with what was registered. PRCT SAE 35,36,41,42,48. However, there is no major change Frequency of heart contraction and blood pressure from IC Pediatric case phenylphrin (age was in the range) 1 to 10 years, total amount is 0.078-0.31 mg), WE I couldn't give a recommendation as a number Small (n = 66) among the researched patients. 44.45 The consistency and underestimation of complications
It is distributed in surgical literature. 57 We suspect that we are ther It could be an important underestimation of SAE using IC phenylephrine. Clinical doctors can reluctantly publish details Complications, especially when using drugs label. In addition, it may be genuinely difficult to know
whether an adverse event was caused by phenylephrine or other causes such as perioperative anxiety, underlying health problems, other medications given, or omitting usual medications on the day of surgery. Thus, we suspect that the small number of reported complications is the tip of the iceberg and advise continued caution. Others have already Patients receiving IC phenylephrine should receive appropriate monitoring of their cardiovascular status 47,48. We sought evidence regarding the maximum safe dose of IC phenylephrine. Previous studies have already concluded that For eye drops, the higher the dose of phenylephrine, the higher the possibility of serious SAEs. 13,19 It is logical to assume that this principle of dose-dependent side effects also applies Although there was no effect on IC phenylephrine, evidence supporting this hypothesis has been published. Because of this uncertainty and lack of reliable evidence, and therefore the weakest, but most likely effective, CI, phenylephrine, is used in a variety of clinical settings. We sought evidence for the "ideal phenylephrine IC dose for effective mydriasis" in previously undeveloped studies that could be used to determine the pupil. Studies of various concentrations of phenylephrine IC (0.15 ml) as a single mydriatic agent revealed: A non-linear mydriatic dose-response relationship. Concentrations between 0.015% and 0.5% produced similar mydriasis. Pupillary size was approximately 4.3 mm, which may not be ideal. Cataract surgery. Two higher concentrations 1.5% and 3% Received statistically significant size from the student 5.80 ±0.79 mm and 6.65 ± 0.57 mm, respectively. 58 for Madriase at Patients who have not received preoperative eyes, we It is assumed that the 0.31% phenylephrine solution of the mixture (synergenic effect with the addition of anticholinergics and lido-cain) may be sufficient. This mixture with a lower concentration has reached the student size ? 6 mm shortly before capsulorexis in 97.6% of patients compared to real mydriatics Diet (100%), although more patients in the local group Had a student size ? 7 mm (98.1% against 80.8%) in PRCT 555 Eyes. 3 This means that the lower doses (for example Suitable for original metris for fewer subjects to surgeons. We sought evidence regarding the "optimal efficacy"
strength of IC phenylephrine in treating IFIS. Unfortunately, we did not find a single study comparing different strength of IC phenylephrine in treating IFIS, it is difficult to compare published studies because of differences in inclusion and success criteria. Llorente et al. found that in their study, all cases responded to phenylephrine IC of 1.5%. 36 In the absence of evidence to the contrary, we suggest that CI concentrations of 2.5% or more may not be necessary for IFIS treatment. Most studies of IC phenylephrine included patients who were at increased risk of developing serious adverse events when taking phenylephrine (e.g., patients with cardiovascular risk factors), and there was very little evidence of serious adverse events. There were hypotheses that Phenylephrine can be associated with a more unfavorable car diovascular effect in high -risk populations (for example, hypertension Patients, elderly and children) and studies can therefore Exclude patients in these high -risk categories. 4.59 However, we found that most published studies included patients with cardiovascular risk factors, and there were very few Proof of messages (additional table 5, available on http://links.lww.com/jrs/b7). Elderly patients with several Concomitant diseases representing cataracts for surgery are common Clinical practice. These patients with high -risk phenylephrine In the case of early MyDriasis (not a conventional local group), it may be profitable. This is because it is associated with a small number of Ziphonia systems that can promote appropriate microscopy. There is little cancellation of the patient. Included in other potential advantages of PHENYLEFRINE IC Evaluation cost reduction for drugs and reductions Pre -surgery and personal time (pre -surgery) Can be improved Efficiency and more cases can be executed The list of 3,10,60.61 is an improvement in preoperative efficiency. The scene is balanced depending on the longer surgical time: It reached 20, which reached 95 % of the maximum measurement effect A few seconds after IC management, not all eyes The same speed, and some students occupy many Develop longer. 28 It may not be so for a long time
Many surgeons prefer. Our review has limitations. There have been very few published data on SAEs associated with phenylephrine in CPB. Research mainly focused on the effectiveness of CI Phenylephrine is often not her SAE, but often It is mentioned as a passing comment. There was no research About the effectiveness of IC-EFRIN phenyl of IC-EFRIN phenyl for initial muscle disease or IFI treatment The existence of other large cities (anticholin operability and)Lidocaine) According to general clinical care. We therefore cannot provide specific recommendations regarding optimal concentrations for these purposes. Future studies focusing on SAEs and optimal concentrations are needed. Phenylephrine IC is useful and effective for initial mydriasis and treatment of IFIS. The optimal concentration for each of these purposes has not been studied, and there are very few published data on SAEs associated with phenylephrine IC. Local infusions often administer phenylephrine at concentrations significantly higher than commercial IC solutions, but off-label IC . Phenylephrine may still mean a high dose. We believe that in the absence of reliable evidence, it is the least likely, but most likely, level. Effective concentration of phenylephrine IC according to intended use. Use wherever possible. Additionally, limiting volume to 0.25 mL and reapplication as needed minimizes leakage, thereby reducing the potential risk of systemic absorption and serious adverse events.
REFERENCES
- Paracetamol toxicity : a review U B GAFFAR Naser Ashraf Tadvi
- The Economic Times , date 4 Jun 2023
- The INDIA TODAY, date 8 December 2023
- India today, date 1 December 2023
- Crabstick N, Coghlan D. Paracetamol efficacy and safety in children: the first 40 years. Am J of Ther 2000;7(2):135-41. [PubMed]
- Gunnels D, Hawton K, Murray V, Garnier R, Bismuth C, Fagg J, Simkin S. Use of Paracetamol for suicide and non-fatal poisoning in the UK and France: are restrictions on availability justified? J Epidemiol Community Health 1997; 51(2):175–9. [PubMed]
- Bronstein AC, Spyker DA, Cantilena LR Jr, Green JL, Rumack BH, Giffin SL. 2009 Annual report of the American Association of Poison Control Centers‘ National Poison Data System (NPDS): 27th annual report. Clin Toxicol (Phila) 2010;48(10):979–1178. [PubMed]
- Larson AM, Polson J, Fontana RJ, Davern TJ, Lalani E, Hynan LS, et al. Acetaminophen induced acute liver failure: results of a United States Multicenter, prospective study. Hepatol 2005;42(6):1364-72. [PubMed]
- Vidhya Malar HL, S Mary MB. Beware of Paracetamol Toxicity. J Clinic Toxicol 2012;2(6):1-3.
- US FDA. Drug safety information [online]. 2011[updated 2011 September8]. Available from:http://www.fda.gov/Drugs/DrugS safety/InformationbyDrugClass/ucm165107.htm
- Kumar R, Singh B, Singh N, Singh J. Review of Management of Common Poisoning in India. Indmedica. 2005;5( 2):4-6.
- Hawton, K, Townsend E, Deeks J, Appleby L., Gunnell D., Benn with O, Cooper J. Effects of legislation restricting pack sizes of Paracetamol and salicylate on self poisoning in the United Kingdom: before and after study. British Medical Journal 2001;322(7296):1203.[PubMed
- Gunnell D, Eddleston M. Suicide by intentional ingestion of pesticides: a continuing tragedy in developing countries. International journal of epidemiology 2003;32(6):902-9. [PubMed]
- Borne, Ronald F. ?Nonsteroidal Anti-inflammatory Drugs? In: Foye, William O, Lemke, Thomas L, Williams, David A, editors. Principles of Medicinal Chemistry. 4th ed. Williams & Wilkins; 1995. pp. 544–5. Zed PJ, Krenzelok EP. Treatment of acetaminophen overdose. Am J Health-Sys Pharm. 1999;56(11):1081-93. [PubToxic Rumack BH. Acetaminophen hepatotoxicity: the first 35 years. Clin Toxicol. 2002;40(1): 3-20.[PubMed]
- Prescott L. Oral or intravenous N-acetylcysteine for acetaminophen poisoning? Ann Emerg Med.2005;45(4):409-413. [PubMed]
- Dart RC, Rumack BH. Acetaminophen (Paracetamol). In: Medical Toxicology (Dart RC, Editor). 2004; Lippincott Williams & Wilkins, Philadelphia, 721-738
- Paracetamol use. A Position Statement of the New South Wales (NSW) Therapeutic Advisory Group Inc. December. 2008. Available from: http://www.ciap.health.nsw.gov.au/nswtag/publications/prostates/Paracetamol231208.pdf [last accessedon2009Jan16]
- Rumack BH, Matthew M. Acetaminophen poisoning and toxicity. Pediatrics.1975;55(6):871–6. [PubMed]
- U.S Food and drug administration. Advisory committee meeting [online]. 2011 [updated 2009 July 7]. Available from: http://www.fda.gov/AdvisoryCommittees/Calendar/ucm143083.htm.
- U.S Food and drug administration. Drug safety information [online]. 2011 [updated 2011September 8].Available from: http://www.fda.gov/Drugs/Drug safety/InformationbyDrugClass/ucm165107.htm
- The Times of India. US FDA asks cos to cap Paracetamol dose [online]. 2011 [cited 2011 February 4]. Available from: http://articles.timesofindia.indiatimes.com/2011-02-04/India business/28365051_1_Paracetamol liver diseases-acetaminophen
- Essential Drugs. Public Citizen Testimony of Paracetamol [online]. 2011 [cited 2002 September 20]. Available from: http://www.essentialdrugs.org/indiadrug/archive/200209/msg00009.php
- The Economic Times. Paracetamol has to pack liver damage warning [online]. 2011 [cited 2003October 22]. Available from: http://articles.economictimes.indiatimes.com/2003-10-22/news/27527737_1_Paracetamol nimesulide-drugs-technical-advisory-board.
- Rational Drugs. Fixed dose combinations [online]. 2011 [cited 2008 June]. Available from:http://www.cmai.org/activities/communications/pdf/Rotation Drugs 31&32.pdf
- The Financial Express. DCGI to cap Paracetamol content in combo drugs [online]. 2011[cited 2011 September 1]. Available from:http://www.financialexpress.com/news/ DCGI-to-cap-Paracetamol-content-in-combo-drugs
- Report of the 46th meeting of the drugs consultative committee held on 12th and 13th November, 2013 at the hotel metropolitan New Central nervous system toxicity of Mefenamic acid and overdose compared other NSAID analysis of the case reporterted in the United Kingdom National Poison Information service
- Southern C, Sharman JL, Benson HE, Faccenda E, Pawson AJ,Alexander SP, et al. The IUPHAR/BPS Guide to PHARMACOLOGYin 2016: towards curated quantitative interactions between 1300protein targets and 6000 ligands. Nucl Acids Res 2016; 44(Database Issue): D1054–D1068.
- Alexander SPH, Fabbro D, Kelly E, Marrion N, Peters JA, Benson HE, et al. The Concise Guide to PHARMACOLOGY 2015/16: Enzymes. Br J Pharmacol 2015; 172: 6024–109.
- Alexander SPH, Peters JA, Kelly E, Marrion N, Benson HE, Faccenda E,et al. The Concise Guide to PHARMACOLOGY 2015/16: Ligand-gatedion channels. Br J Pharmacol 2015; 172: 5870–903.
- Krause D, Suh H-S, Cui QL, Durafourt BA, Choi N, Bauman A,et al. The tryptophan metabolite 3-hydroxyanthranilic acid plays anti-inflammatory and neuroprotective roles during inflammation: role of hemeoxygenase- Am J Pathol 2011;S179: 1360–72.
- Zhang WY, Li Wan Po A. Efficacy of minor analgesics in primary dysmenorrhoea: a systematic review. Br J Obstet Gynaecol 1998;105: 780–9.
- Fraser IS, McCarron G, Markham R, Robinson M, Smyth E. Longterm treatment of menorrhagia with mefenamic acid. Obstet Gynecol 1983; 61: 109–12.
- Bonnar J, Sheppard BL. Treatment of menorrhagia during menstruation: randomised controlled trial of ethamsylate,
- Centre for Reviews and Dissemination, University of York. The management of menorrhagia. Effect Health Care Bull 1995; 1:1–4.
- National Collaborating Centre for Women’s and Children’sHealth Heavy menstrual bleeding: clinical guideline 2007. Available at https://www.nice.org.uk/guidance/cg44/evidence/full-guideline-195071293 (last accessed 25 November 2016).
- Majoribanks J, Ayeleke RO, Farquhar C, Proctor M. Nonsteroidal anti-inflammatory drugs for dysmenorrhoea. Cochrane Database Syst Rev 2015; 7 [online]. Available at http://onlinelibrary.wiley.com/d/10.1002/14651858.CD001751.pub3/full (last accessed19 January 2016).
- Lethaby A, Duckitt K, Farquhar C. Non-steroidal antiinflammatory drugs for heavy menstrual bleeding. Cochrane Database Syst Rev 2013; 1: CD000400.
- Khajehei M, Abdali K, Tabatabaee H. The effect of mefenamic acid and naproxen on heavy menstrual bleeding: a placebo-controlled study. S Afr J Obstet Gynaecol 2013; 19: 31–4.
- National Institute for Health and Care Excellence. Clinical Knowledge Summaries – Dysmenorrhoea 2014 [online]. Available at http://cks.nice.org.uk/dysmenorrhoea (last accessed 19th January 2016).
- National Institute for Health and Care Excellence. Clinical Guideline 44. Heavy menstrual bleeding: assessment and management 2007 [online]. Available at http://www.nice.org.uk/guidance/CG44/chapter/1-Guidance (last accessed 19 January2016).
- Young R. Mefenamic acid poisoning and epilepsy. Br Med J 1979;2: 6.
- Mood M, Proudfoot A, Critchley J, Prescott L. Mefenamic acid over dosage. Lancet 1981; 317: 1354–6.
- Prescott L, Balali-Mood M, Critchley J, Proudfoot A. Avoidance of mefenamic acid in epilepsy. Lancet 1981; 318: 418.
- Gössinger H, Hruby K, Haubenstock A, Jung M, Zwerina N. Coma in mefenamic acid poisoning. Lancet 1982; 320: 384.
- Frank J, Wightkin W, Hubner J. Acute toxicity of nonsteroidal antiinflammatory agents: seizure following amefenamic acid overdose. Drug Intell Clin Pharm 1983; 17:204–5.
- Shipton E, Müller F. Severe mefenamic acid poisoning. A case report. S Afr Med J 1985; 67: 823–4.
- Hendrickse M. Mefenamic acid overdose mimicking brainstem stroke. Lancet 1988; 332: 1019.
- McKillop G, Canning G. A Case of intraveneous and oral mefenamic acid poisoning. Scott Med J 1987; 32: 81–2.
- European Parliament. Directive 2010/84/EU of the European Parliament and of the council. Available at http://ec.europa.eu/ health/files/eudralex/vol 1/dir_2010_84/dir_2010_84_en.pdf (last accessed 4 January 2016).
- Joint Formulary Committee. British National Formulary [Online]London: BMJ Group and Pharmaceutical Press. Available at http://www.medicinescomplete.com (last accessed 4 January 2016).
- Royal College of Paediatrics and Child Health. UK-WHO growth charts [online]. Available at www.rcpch.ac.uk/child-health/standards-care/nutrition-and-growth/uk-who-growth-charts/ukwho-growth-charts (last accessed 4 January 2016).
- Health and Social Care Information Centre. Health Survey for England 2012 [online]. Available at http://www.hscic.gov.uk/ catalogue/PUB13218 (last accessed 4th January 2016).
- Welsh Assembly Government. Welsh Health Survey 2009. Available at http://wales.gov.uk/docs/statistics/2010/100915healthsurvey09en.pdf (last accessed 4 January 2016).
- Winder CV, Kaump DH, Glazko AJ, Holmes EL. Experimental observations on flufenamic, mephenamic, and meclofenamic acids. Rheumatology 1966; 8 (Suppl. 1): 7–49.
- Smolinske S, Hall A, Vandenberg S, Spoerke D, McBride P. Toxic effects of nonsteroidal anti-inflammatory drugs in overdose. Drug Saf 1990; 5: 252–74.
- Reichert C, Reichert P, Monnet-Tschudi F, Kupferschmidt H,Ceschi A, Rauber-Lüthy C. Seizures after single-agent overdose with pharmaceutical drugs: analysis of cases reported to a poison center. Clin Toxicol 2014; 52: 629–34.
- Court H, Volans G. Poisoning after overdose with non-steroidal anti-inflammatory drugs. Adverse Drug React Acute PoisoningRev 1984; 3: 1–21.
- Thundiyil J, Kearney T, Olson K. Evolving epidemiology of druginduced seizures reported to a Poison Control Center System. J Med Toxicol 2007; 3: 15–9.
- Robson RH, Balali M, Critchley J, Proudfoot A, Prescott L. Mefenamic acid poisoning and epilepsy. Br Med J 1979; 2: 1438.
- Laredo PB. Die akute Intoxikation mit Mefenaminsäure. [dissertation]. University Of Zurich; 2007.
- Mines D, Novelli L. The risk of first seizure associated with Mefenamic acid in women of reproductive age. Pharmacoepidemiol Drug Saf 2004; 13: S333–S334.
- Stein Hauser H, Hertting G. Lowering of the convulsive threshold by non-steroidal anti-inflammatory drugs. Eur J Pharmacol 1981;69: 199–203.
- Woodward R, Polenzani L, Miledi R. Effects of fenamates and other nonsteroidal anti-inflammatory drugs on rat brain GABAA receptors expressed in Xenopus oocytes. J Pharmacol Exp Ther 1994; 268: 806–17.
- Yakushiji T, Shirasaki T, Akaike N. Non-competitive inhibition of GABAA responses by a new class of quinolones and non-steroidal anti-inflammatories in dissociated frog sensory neurones. Br J Pharmacol 1992; 105: 13–8.
- Halliwell R, Thomas P, Patten D, James CH, Martinez-Torres A, Miledi R, et al. Subunit-selective modulation of GABAA receptors by the non-steroidal anti-inflammatory agent, Mefenamic acid. Eur J Neurosci 1999; 11: 2897–905.
- Royal College of Psychiatrists. Self-harm, suicide, and risk: helping people who self-harm. Final report of a working group2010 [online]. Available at http://www.rcpsych.ac.uk/files/ pdfversion/cr158.pdf (last accessed 14 July 2016).
- Motta MM, Coblentz J, Fernandes BF, Burnier MN Jr. Mydriatic and cardiovascular effects of phenylephrine 2.5% versus phenylephrine 10%, both associated with tropicamide 1%. Ophthalmic Res. 2009;42(2):87-89.
- Fraunfelder FW, Fraunfelder FT, Jensvold B. Adverse systemic effects from pledgets of topical ocular phenylephrine 10%. Am J Ophthalmol. 2002;134(4):624-625.
- Brown MM, Brown GC, Spaeth GL. Lack of side effects from topically administered 10% phenylephrine eyedrops: a controlled study. Arch Ophthalmol. 1980;98(3):487-489.
- Hakim OJ, Orton RB, Cadera W. Topical 2.5% and phenylephrine: comparison of effects on heart rate and blood pressure. Can J Ophthalmol. 1990 25(7):336-339.
- Parati G, Ochoa JE, Lombardi C, Salvi P, Bilo G.Assessment and interpretation of blood pressure variability in a clinical setting. Blood Press. 2013;22(6):345-354.
- Tatasciore A, Renda G, Zimarino M, et al. Awake systolic blood pressure variability correlates with target-organ damage in hypertensive subjects. Hypertension. 2007;50(2):325-332.
- Kannel WB. Elevated systolic blood pressure as a cardiovascular risk factor. Am J Cardiol. 2000;85(2):251-255.
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557-560.
- Bhatia J, Varghese M, Bhatia A. Effect of 10% phenylephrine eye drops on systemic blood pressure in normotensive and hypertensive patient Oman Med J. 2009;24(1):30-32.
- Chawdhary S, Angra SK, Zutshi R, Sachdev MS. Mydriasis: use of phenylephrine (a dose-responseconcept). Indian J Ophthalmol. 1984;32(4):213-216.
- Kumar V, Schoenwald RD, Chien DS, Packer AJ,Choi WW. Systemic absorption and cardiovascular effects of phenylephrine eyedrops. Am J Ophthalmol.1985;99(2):180-184.
- Morgado G, Barros P, Martins J, Lima A, Martins N. Comparative study of mydriasis in cataract surgery: topical versus Mydriasert versus intracameral mydriasis in cataract surgery. Eur J Ophthalmol. 2010;20(6):989-993.
- Mouly S, Mahé I, Haouchine B, et al. Pharmacodynamics of a new ophthalmic mydriatic insert in healthy volunteers: potential alternative as drug delivery system prior to cataract surgery. Basic Clin Pharmacology Toxicol. 2006;98(6):547-554.
- Symons RC, Walland MJ, Kaufman DV. A comparative study of the efficacy of 2.5% phenylephrine and 10% phenylephrine in pre-operative mydriasis for routine cataract surgery. Eye (Lond). 1997;11(pt 6):946-948.
- Torrón C, Calvo P, Ruiz-Moreno O, Leciñena J, Pérez-Iñigo A. Use of a new ocular insert versus conventional mydriasis in cataract surgery. Biomed Res Int. 2013;2013:849349.
- Yospaiboon Y, Luanratanakorn P, Noppawinyoowong C. Randomized double-blind study of phenylephrine 2.5% vs 10% on pupillary dilation.J Med Assoc Thai. 2004;87(11):1380-1384.
- Chugh AR, Loughran JH, Slaughter MS.Circadian variations in blood pressure in health and disease: implications for patient management. Chronophysiol Ther. 2011;1:17-31. doi:10.2147/CPTS15597.
- Manios E, Stamatelopoulos K, Tsivgoulis G,et al. Time rate of blood pressure variation: a new factor associated with coronary atherosclerosis.J Hyperten. 2011;29(6):1109-1114.
- Sander D, Kukla C, Klingelhöfer J, Winbeck K, Conrad B. Relationship between circadian blood pressure patterns and progression of early carotid atherosclerosis: a 3-year follow-up study. Circulation.2000;102(13):1536-1541.
- Borst C, Wieling W, van Brederode JF, Hond A, de Rijk LG, Dunning AJ. Mechanisms of initial heart rate response to postural change. Am J Physiol. 1982;243(5):H676-H68


 Nilesh Borse *
Nilesh Borse *
 Dusane G.V.
Dusane G.V.


 10.5281/zenodo.14292795
10.5281/zenodo.14292795