Abstract
Carbon nanotubes, often known as rolled-up graphene sheets, are cylindrical nanostructures composed of carbon atoms organized in a hexagonal lattice. One of the most fascinating discoveries in the field of nanoscale sciences is carbon nanotubes, or CNTs. They can have graphene arranged in one or more layers (multi-walled carbon nanotubes, or MWCNTs) or in a single layer (single-walled carbon nanotubes, or SWCNTs). Because of their remarkable mechanical strength, electrical conductivity, and thermal characteristics, CNTs are useful in a variety of applications. Applications for carbon nanotubes in biomedical include tissue engineering, gene therapy, and targeted medication delivery, among others. Several methods, including as chemical vapor deposition, laser ablation, arc discharge, and laser ablation, have been devised to create nanotubes in significant quantities. The study's focus on carbon nanotubes is on their kinds, characteristics, production processes, benefits and drawbacks, and biomedical applications.
Keywords
Carbon Nanotubes, Arc Discharge, Laser Ablation, Chemical Vapour Deposition, Biomedical.
Introduction
Carbon nanotubes are among the most remarkable examples of novel nanostructures created by bottom-up chemical synthesis methods. Although they exhibit what may be the highest level of complexity and variety, nanotubes have the simplest chemical composition and atomic bonding arrangement.[1] Carbon nanotubes (CNTs) have attracted the interest of several researchers in a range of sectors, including chemical science, electronics, health, and so on, since their discovery in 1991 by Iijima. Carbon nanotubes (CNTs) are one-dimensional, nanostructured carbon compounds that have a cylindrical shape and resemble coiled graphitic sheets. On the other hand, each carbon atom is connected to three other atoms on the x~y plane.The two types of carbon nanotubes (CNTs) are single-walled carbon nanotubes (SWCNTs) and multiwalled carbon nanotubes (MWCNTs).[2-8] CNTs contain all types of carbon. They are tubular in form and made of graphite. Because of their various special properties, CNTs are advantageous for both medication development and nanotechnology research. They are spherical and a few millimeters long at the nanoscale. You possess a wide range of structural, thermal, electrical, and other properties. Depending on the type of nanotube—identified by its length, chirality, diameter, or ability to bend and survive environmental conditions—these characteristics vary. In the pharmaceutical sector, their unique surface area, stiffness, tensile strength, and durability have created a lot of excitement.[9]
The prospects for using carbon nanotubes have recently taken center stage in research. Because of their special characteristics, a variety of uses, such as biomaterials and biomedical devices, are feasible for composite materials. Research in the medical and pharmaceutical fields have shown that carbon nanotubes may be used in biosensors and as a component of medication and vaccine delivery systems.[10-16]
Advantages of carbon nanotubes
- They are very elastic, biocompatible, non-biodegradable, and immuneogenic. They may also be delivered intracellularly.
- It might show little cytotoxicity.
- 96% excreted through urine and the remaining 4% through feces.
- Extremely low in weight and do not degrade when executing.
- CNTs can enter cells through a spontaneous method because of its tiny, tubular needle form.
- Its exterior and inner surfaces are different, allowing for differently altered for functionalization in chemistry and biology.[17-18]
Types of carbon nanotubes
The carbon nanotubes are of two types namely:
- Single walled carbon nanotubes (SWCNTs)
- Multiple walled carbon nanotube (MWCNTs)
Single walled carbon nanotubes (SWCNTs)
Single cylindrical carbon layers that range in diameter from 0.4 to 2 nm make up SWCNTs, depending on the temperature at which they were created. It was discovered that the diameter of CNTs increases with increasing growing temperature. SWCNTs can have chiral, helical, arm chair, or zigzag configurations in their structure. With an ultrahigh surface area of up to 1300 m2/g, the SWCNTs provide ample room for drug loading and bioconjugation. SWCNTs are known to be more effective than MWCNTs in the delivery of drugs. This can be attributed to the ultrahigh surface area and effective drug-loading capacity of SWCNTs.[19-22] One way to think of single wall carbon nanotubes is as rolled-up graphene sheet stripes. By rolling up the sheet so that the origin and the top of each lattice vector coincide, each lattice vector of the sheet identifies a specific nanotube.[23] Single-walled carbon nanotubes (SWCNTs) must be synthesized under strict supervision in order to be used in nanotechnology. A nanoscale catalyst metal is often needed for the production of SWCNTs.. Iron, cobalt, and nickel are members of the iron family and are known to be excellent catalyst species in the chemical vapor deposition (CVD) of SWCNTs, yielding significant SWCNT yields.[24-27] The medication is progressively eliminated from the body through the biliary channel and ultimately ends up in the stools once the functionalized SWCNT releases the medicine into a particular location. This suggested that SWCNTs are a potential nanoplatform for cancer therapies and appropriate candidates for drug delivery.[15]
Multiple walled carbon nanotube (MWCNTs)
Multi-walled carbon nanotubes are made up of many rolled layers (concentric tubes) of graphene layers arranged in a one-dimensional configuration. Multiple coaxial cylinders, each composed of a single grapheme sheet around a hollow center, make up MWCNTs. MWCNTs have a length of one to several micrometers and an outside diameter of two to one hundred nm, with an interior diameter of one to three nm. The interactions between neighboring cylindrical layers in MWCNTs, which lead to a less flexible and more structural flaws, are caused by the sp2 hybridization in MWCNTs, which generates a delocalized electron cloud along the wall.[28-29] Based on how the graphite layers are arranged, MWCNTs structures can be divided into two groups: the Russian doll model, which has layers of graphene sheets arranged within a concentric structure, and the parchment-like structure, which has a graphene sheet rolled up around it (16). The Russian Doll model describes a situation in which a carbon nanotube has another nanotube inside of it, with the outside nanotube having a larger diameter than the inner one. Conversely, the Parchment model is created when a single graphene sheet is wrapped around itself several times, much like a scroll of paper that has been rolled up.[30] Multiwall carbon nanotubes (MWCNTs) may be decorated by depositing nanoparticles on their walls or ends. These nanoparticles are linked to the MWCNTs by physical contact, and they have potential uses in biosensors, electrical devices, biological applications, and catalysis. For this, a variety of techniques are employed, such as precipitation, high-temperature hydrolysis, or chemical breakdown of a metal precursor.[31]
Table 1- Comparison between SWCNTs and MWCNTs [32-33]
|
Sr. No.
|
SWCNTs
|
MWCNTs
|
|
1
|
Graphene in a single layer.
|
Graphene with many layers.
|
|
2
|
Synthesis requires the use of a catalyst.
|
possible can be made without a catalyst
|
|
3
|
Bulk synthesis is challenging as it necessitates careful management of growth and ambient conditions.
|
Synthesis in bulk is simple.
|
|
4
|
Purity is weak.
|
Purity is rather high.
|
|
5
|
Defects are more likely to occur during functionalization.
|
Defects are less likely, but if they do, they're hard to cure.
|
|
6
|
Less buildup throughout the body.
|
More buildup inside the body.
|
|
7
|
It's simple to characterize and evaluate.
|
Its structure is quite complicated.
|
|
8
|
They are more flexible and readily twisted.
|
It is difficult to twist.
|
Properties of carbon nanotube[34]
There are four different physical properties associated with carbon nanotubes
- Strength of nanotubes
One of the strongest materials is carbon nanotube, both in terms of elastic modulus and tensile strength. The covalent Sp2 bonds that form between the individual carbon atoms give this strength.
2) Kinetic property
MWNTs, which are made up of many concentric nanotubes that are exactly packed within one another, have an amazing telescoping feature that allows an inner nanotube core to move practically easily within its outer nanotube shell, automatically producing a perfect linear or rational bearing.
3) Electrical property
The shape of a nanotube has a significant impact on its electrical characteristics because of the symmetry and distinct electronic structure of graphene. In theory, the electrical current density of metallic nanotubes can be 1,000 times higher than that of metals like copper and silver.
4) Thermal property
It is anticipated that nanotubes would exhibit "ballistic conducting" behavior, or strong heat conductivity along the tube axis, and good lateral to tube axis insulation.
5. Methods of synthesis of carbon nanotube
- Arc Discharge Method
Due to its simplicity, the carbon arc discharge method—which was first employed to create C60 fullerenes—is the most widely utilized and possibly the simplest approach to create carbon nanotubes. To extract the CNTs from the soot and any remaining catalytic metals in the crude product, further purification is necessary due to the complex combination of components produced by this process. By arcvaporizing two carbon rods arranged end to end and spaced apart by around 1 mm in a container typically filled with inert gas at low pressure, this technique produces carbon nanotubes (CNTs).
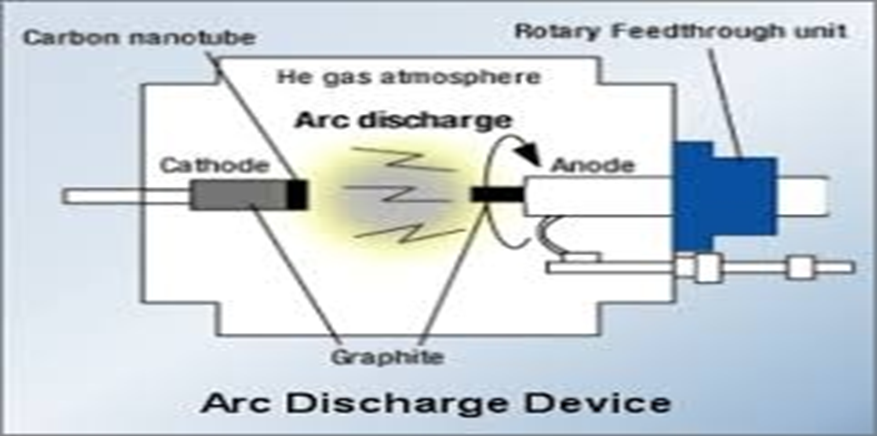
One of the carbon electrodes had its surface evaporated by the discharge, and the other electrode has a little rod-shaped deposit on it. The temperature of the deposit forming on the carbon electrode and the homogeneity of the plasma arc are critical factors in the high harvest production of carbon nanotubes.[35-36]
Advantages -
Easy, low-cost, high-grade nanotubes.
Disadvantages –
Tangled nanotubes, high temperature, and need for purification.
2) Laser Ablation
More recently, a technique called pulsed laser vaporization (PLV), also known as laser ablation, is being explored to produce nanotubes. This process vaporizes the carbon and then deposits it onto a substrate, much like the arc-discharge. The synthesis of CNTs by laser vaporization becomes an effective method for the synthesis of bundles of SWCNTs with a limited distribution, according to a 1995 study by Rice University's Smalley group. This technique involves using laser light at a high temperature in an inert environment to evaporate a piece of graphite target.[37]

When CNTs were first synthesized in 1996, a dual-pulsed laser was used to achieve yields of.70wt% purity. Graphite rods were laser-vaporized with a 50:50 catalyst mixture of cobalt and nickel at 1200 uC in flowing argon to create the samples. After that, the C60 and other fullerenes were eliminated using a vacuum-induced heat treatment at 1000 uC. A second laser vaporization pulse was transmitted after the first one in order to more equally evaporate the topic. When two successive laser pulses are employed, the amount of carbon deposited as soot is decreased. The second laser pulse breaks apart and feeds the larger particles that the previous pulse ablated into the growing nanotube structure. [35–36]
Advantages –
Relatively high purity, synthesized at room temperature.
Disadvantages –
Technique restricted to the lab scale, requires crude product purification.
3) Chemical Vapour Deposition
For almost two decades, the production of different carbon materials, including carbon fibers and filaments, has been achieved by the conventional process of chemical vapor deposition of hydrocarbons over a metal catalyst. Catalytic CVD of acetylene over cobalt and iron catalysts based on silica or zeolite may produce large volumes of CNTs. [35-36]
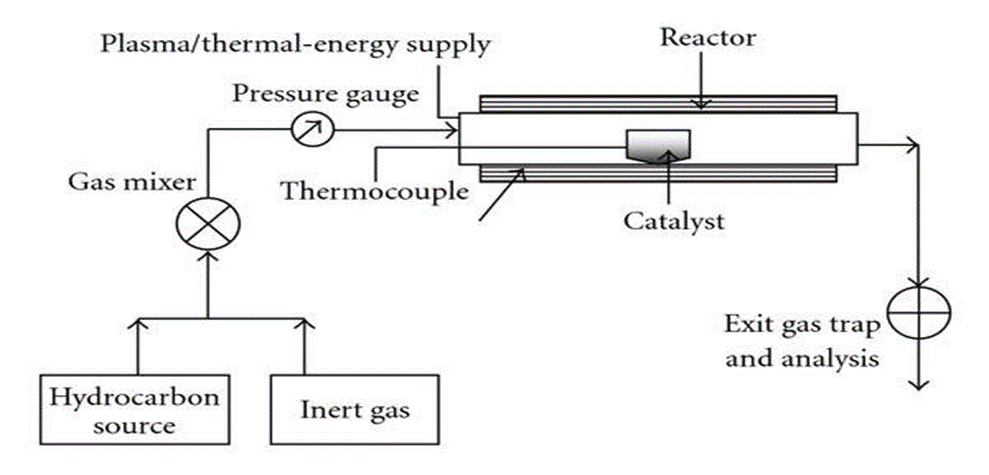
The process of catalytic CVD synthesis involves placing a carbon source in the gas phase and heating the gaseous carbon-containing molecules with either plasma or a resistively heated coil. The molecule is "crack" into reactive atomic carbon by the heat. Transition metals—Fe, Co, or Ni—are the most often utilized catalysts. The conventionally utilized catalysts are occasionally additionally doped with other metals, such Au. Hydrocarbons like methane, ethane, ethylene, acetylene, and xylene—and eventually their mixtures, isobutane or ethanol—are the most favored carbon sources in CVD. When a gaseous carbon source is used, the reactivity and concentration of gas phase intermediates that are created together with reactive species and free radicals as a result have a significant impact on the growth efficiency of carbon nanotubes.[38]
Advantages –
Simple, high purity, low temperature, large-scale manufacturing, and potential for aligned growth.
Disadvantages –
The majority of synthesized CNTs are MWNTs, or defects.
6. Biomedical Applications of Carbon Nanotubes
6.1) Targeted drug delivery
Active medicinal substances such as enzymes, antibodies, DNA, proteins, and others are coupled with carbon nanotubes (CNTs). High accessible intrinsic surface area, strong stability characteristics, and rich electron density with aromaticity are the primary causes of conjugation ability. These characteristics may allow the medicinal moiety to be delivered precisely. Additionally, CNTs have been combined with an anticancer moiety, and both in vitro and in vivo characteristics have been assessed. The results demonstrated their efficacy in treating different types of carcinomas. Certain medications have the ability to localize at greater quantities in certain tissues and are also connected to the magnetic interior of carbon nanotubes.[39-41]
6.2) Gene therapy
Baligelli and colleagues have assessed the targeting of intracellular organelles using CNT linked therapeutic moiety for the treatment of hereditary disorders. Effective gene therapy has been developed via research into various chemical bond types and their activities. The potential for treating and managing diseases has been demonstrated by studies examining the effective loading of siRNA, DNA, and other genes into the inner cavity of CNTs and the covalent and non-covalent bonding regions.[42-43]
6.3) Biosensors Based on CNT
CNT has been suggested as a sensing element in the biosensors area to monitor and identify a number of ailments, including bacterial infections and diabetes. In contrast to other approaches, Punbusayakul et al. employed electrochemical monitoring of immune complexes for salmonella detection, which shortened the detection time and made sample preparation easier. Grafting directed antibodies on the surface of DWCNTs to immobilize them also produced an immunosensor for adiponectin, a biomarker of obesity. Fast detection and quantification are made possible by the reaction between a second antibody and horse radish peroxidase (HRP)-streptavidin during cyclic voltammetry monitoring. The second antibody binds to adiponectin.[44-45]
6.5) CNT-Based Hydrogels for Diagnostic
Since glucose detection is well-documented, the majority of biosensors created with CNT-based hydrogel were concentrated on this area. A number of them are made up of electrodes that make it possible to track the electron exchanges that occur between glucose oxidase (GOx) and glucose. Kim et al. directly employed conductive bacterial cellulose (BC)-CNT-GOx sheets as electrodes, providing an easy-to-use and biocompatible material for cyclic voltammetry monitoring.[46]
6.6) Tissue engineering
These devices can function as a sprout and encapsulate cells or tissues for tissue engineering because of the excellent mechanical and electrical capabilities of CNT. These methods have been investigated for heart cells, bone tissues, and brain development. Tissue regeneration, myoblast and fibroblast proliferation, bone formation after the attraction of more calcium ions, and other processes have been demonstrated to benefit from CNT-based tissue engineering.[47-49]
6.7) Biohybrid tissue regeneration
Tissue actuators and bio-robotics are created using CNT-coupled novel technologies for medication screening and disease therapy.[47,50-52]
6.8) CNT Use for Diagnostic
Reducing detection times is especially important since timely diagnosis is essential to effective treatment. Thanks to immune complex detection, in vitro biomarker analysis is currently feasible with excellent accuracy; however, employing traditional dose methodologies can be time-consuming and need significant numbers of biological material. Many groups have explored employing carbon nanotubes (CNTs) as the primary component of electrochemical sensors because of their electrical characteristics, and many types of label-free CNT-based biosensors have been created.[53]
Limitations Of CNTs
• Insoluble in the majority of biologically acceptable solvents (aqueous based).
• The capacity to create quantities of CNTs with reproducible, uniform qualities in terms of both chemistry and architecture. • The challenge of preserving excellent quality and low impurities.[54]
Future prospect of CNTs in biomedical applications
At the moment, CNTs are mostly used in orthopedic and cancer therapies. Broad potential for CNTs in various medical therapies that are still in the early stages of development and might play a significant role in biomedical applications in the ensuing decades. Although several researchers have up to now proposed various methods for the potential application of carbon nanotubes (CNTs) in the biomedical field, one of the main obstacles to commercializing CNTs in the market remains the expansion of CNT-based applications from laboratory to industrial scale. Applications based on CNTs are a great substitute because of their many distinguishing qualities. But generating non-defective CNTs and manufacturing them at a reasonable cost provide a challenge. Therefore, more research may be done to create cost-effective novel production processes that yield high-quality, error-free CNTs, therefore minimizing waste. Because of their poisonous effects, carbon nanotubes (CNTs) have extraordinary characteristics, but biological studies are still pending. One of the main areas of study for CNT uses in the bioscience field is toxicity reduction.[55-57] Consequently, it is reasonable to state that there are still potential economic prospects for CNTs.
CONCLUSION
CNT materials show promising for use in the biomedical industry. Among the cutting edge, developing technologies that might be used for gene, vaccine, and medication delivery are carbon nanotubes. By functionalizing their surface, they can produce highly soluble polymers that are compatible with biological systems when further derivatized with active molecules. Consequently, a wide range of biological applications are possible. It was demonstrated that the mechanical and electrical characteristics of hydrogels could be significantly enhanced by the insertion of carbon nanotubes (CNTs), and that these nanocomposites could be used to drug delivery, tissue engineering, and biosensing without running the risk of CNT leakage into cells. In CNTs have shown to be beneficial in a variety of applications, including the creation of novel and enhanced composites, because of these fascinating features. Better understanding of biological and physical chemical processes might result from advancements in carbon nanotube technology. This will enable the discovery of substances that are more compatible with carbon nanotube technology and enable the more efficient use of nanotubes as delivery systems in applications related to therapeutic, preventative, and diagnostic nanomedicine.
REFERENCES
- Dresselhaus, M. S.; Dresselhaus, G.; Eklund, P. C. Science of Fullerenes and Carbon Nanotubes; Academic Press: San Diego, 1996; p 985
- S. Iijima, Helical microtubules of graphitic carbon, Nature 354 (1991) 56-58.
- J.P. Chinta, N. Waiskopf, G. Lubin, D. Rand, Y. Hanein, U. Banin, et al., Carbon nanotube and semiconductor nanorods hybrids: preparation, characterization, and evaluation of photocurrent generation, Langmuir (2017).
- P. Andricevi ? c, M. Kolla ´ ´r, X. Mettan, B. Na´fra´di, A. Sienkiewicz, D. Fejes, et al., 3-dimensionally enlarged photoelectrodes by a protogenetic inclusion of vertically aligned carbon nanotubes into CH 3 NH 3 PbBr 3 single crystals, J. Phys. Chem. C (2017).
- M. Batmunkh, C.J. Shearer, M. Bat-Erdene, M.J. Biggs, J.G. Shapter, Single-walled carbon nanotubes enhance the efficiency and stability of mesoscopic perovskite solar cells, ACS Appl. Mater. Interf. (2017).
- J. Budhathoki-Uprety, R.E. Langenbacher, P.V. Jena, D. Roxbury, D.A. Heller, A carbon nanotube optical sensor reports nuclear entry via a noncanonical pathway, ACS Nano 11 (2017) 3875-3882.
- O. Breuer, U. Sundararaj, Big returns from small fibers: a review of polymer/carbon nanotube composites, Polym. Compos 25 (2004) 630-645.
- I.V. Zaporotskova, N.P. Boroznina, Y.N. Parkhomenko, L.V. Kozhitov, Carbon nanotubes: sensor properties. A review, Mod. Electron. Mater. 2 (2016) 95-105.
- http://en.wikipedia.org/www/Carbon %nanotube.
- Popov, V.N. Carbon nanotubes: Properties and application. Mat. Sci. Eng. R: Reports 2004, 43, 61–102.
- Paradise, M.; Goswami, T. Carbon nanotubes-production and industrial application. Mat. Design 2007, 28, 1477–1489.
- Grunlum, J.C.; Mehrabi, A.R.; Bannon, M.V.; Bahr, J.L. Water-based single-walled-nanotubefilled polymer composite with an exceptionally low percolation threshold. Adv. Mat. 2004, 16, 150–153.
- Supronowicz, P.R.; Ajayan, P.M.; Ullman, K.R.; Arulanandam, B.P.; Metzger, D.W.; Bizios, R. Novel current-conducting composite substrates for exposing osteoblast to alternating current stimulation. J. Biomed. Mat. Res. A 2002, 59, 499–506.
- Koerner, H.; Price, G.; Pearce, N.A.; Alexander, M.; Varia, R.A. Remotely actuated polymer nanocomposite-stress- recovery of carbon nanotube filled thermoplastic elastomers. Nature Mater. 2004, 3, 115–120
- Sen, R.; Zhao, B.; Perea, D.; Itkis, M.E.; Hu, H.; Love, J.; Bekyarova, E.; Haddon, R.C. Preparation of single-walled carbon nanotube reinforced polystyrene and polyurethane nanofibers and membranes by electrospinning. NanoLett. 2004, 4, 45.
- Lin, Y.; Taylor, S.; Li, H.; Fernando, S.K.A.; Qu, L.; Wang, W.; Gu, L.; Zhou, B.; Sun, Y.P. Advances toward bioapplications of carbon nanotubes. J. Mater. Chem. 2004, 14, 527–541.
- Mehra NK, Jain AK, Lodhi N, Dubey V, Mishra D, Raj R et al. Challenges in the use of carbon nanotubes in biomedical applications. Crit Rev Ther Drug Carr Syst 2008; 25(2):169-176.
- He H, Pham-Huy LA, Dramou P, Xiao D, Zuo P, PhamHuy C. Carbon nanotubes: applications in pharmacy and medicine, BioMed Research International, 2013, 12.
- Klumpp C, Kostarelos K, Prato M, Bianco A. Functionalized carbon nanotubes as emerging nanovectors for the delivery of therapeutics, Biochem Biophys Acta 2006; 1758:404-412.
- Danailov D, Keblinski P, Nayak S, Ajayan PM. Bending properties of carbon nanotubes encapsulating solid nanowires, J Nano Sci Nanotechnol. 2002; 2:503-507.
- Zhang B, Chen Q, Tang H, Xie Q, Ma M, Tan L et al. Characterization and biomolecule immobilization on the biocompatible multi-walled carbon nanotubes generated by functionalization with polyamidoamine dendrimers. Colloids Surf B Biointerfaces 2010; 80:18-25.
- Saito N, Haniu H, Usui Y, Aoki K, Hara K, Takanashi S, et al. Safe clinical use of carbon nanotubes as innovative biomaterials Chem Rev 2014; 114(11):6040-6079.
- H. Kuzmany, W. Plank, M. Hulman, C. Kramberger, A. Grüneis, T. Pichler, H. Peterlik, H. Kataura, Y. Achiba, Eur. Phys. J. B 22 (2001) 307.
- Iijima, S.; Ichihashi, T. Nature 1993, 363, 603-605
- Radushkevich LV, Lukyanovich VM. Soviet Journal of Physical Chemistry 1952; 26: 88– 95.
- Oberlin A, Endo M, Koyana T. Filamentous growth of carbon through benzene decomposition. J Cryst Growth 1976; 32: 335- 349.
- Abrahamson J, Wiles PG, Rhodes B. Structure of carbon fibers found on carbon arc anodes. Carbon 1999; 37: 1873-1875.
- Rastogi V, Yadav P, Bhattacharya SS, Mishra AK, Verma N, Verma A et al. Carbon Nanotubes: An Emerging Drug Carrier for Targeting Cancer Cells, Journal of Drug Delivery. 2014, 23.
- Xia ZH, Guduru PR, Curtin WA. Enhancing mechanical properties of multiwall carbon nanotubes via sp3 interwall bridging, Physical Review Letters 2007; 98:245501- 245504.
- Madani SY, Naderi N, Dissanayake O, Tan A, Seifalian AM. A new era of cancer treatment: carbon nanotubes as drug delivery tools. Int J Nanomedicine. 2011; 6(1):2963- 2979.
- Uc-Cayetano EG, Aviles F, Cauich-Rodriguez JV, Schonfelder R, Bachmatiuk A, Rummeli MH et al. Influence of nanotube physicochemical properties on the decoration of multiwall carbon nanotubes with magnetic particles. Journal of Nanoparticle Research. 2014; 16:2192.
- Monthioux M, Kuznetsov V. Who should be given the credit for the discovery of carbon nanotubes.
- www.rdg.ac.uk/~scsharip/tubebooks. Peter Harris’s nanotube book.
- Endo M, M S Strano MS, Ajayan PM. Potential Applications of Carbon Nanotubes. Appl Physics. 2008; 111: 13–62.
- Iijima, S. (1991) Helical microtubules of graphitic carbon. Nature, 354: 56– 58.
- Ebbesen, T.W. and Ajayan, P.M. (1992) Large-scale synthesis of carbon nanotubes. Nature, 358: 220–222.
- T. Guo, P. Nikolaev, A. Thess, D.T. Colbert, R.E. Smalley, Chem. Phys. Lett. 243 (1995) 49–54.
- Varshney K. Carbon nanotubes: A review on synthesis, properties and applications, Int J Eng Res Gen Sci. 2014; 2(4):660-677.
- Tang L, Xiao Q, Mei Y, et al. Insights on functionalized carbon nanotubes for cancer theranostics. J Nanobiotechnology 2021; 19(1): 423.
- Aubert JD, Juillerat JL. Endothelin-receptor antagonists beyond pulmonary arterial hypertension: Cancer and fibrosis. J Med Chem 2016; 59(18): 8168-88.
- Chadar R, Afzal O, Alqahtani SM, Kesharwani P. Carbon nanotubes as an emerging nanocarrier for the delivery of doxorubicin for improved chemotherapy. Colloids Surf B Biointerfaces 2021; 208: 112044.
- Vander WRL, Berger GM, Ticich TM. Carbon nanotube synthesis in a flame using laser ablation for in situ catalyst generation. Appl Phy A 2003; 77(7): 885-9.
- Karimi M, Solati N, Ghasemi A, et al. Carbon nanotubes part II: A remarkable carrier for drug and gene delivery. Expert Opin Drug Deliv 2015; 12(7): 1089-105.
- Punbusayakul, N.; Talapatra, S.; Ajayan, P.M.; Surareungchai, W. Label-Free as-Grown Double Wall Carbon Nanotubes Bundles for Salmonella Typhimurium Immunoassay. Chem. Cent. J. 2013, 7, 102.
- Ojeda, I.; Barrejón, M.; Arellano, L.M.; González-Cortés, A.; Yáñez-Sedeño, P.; Langa, F.; Pingarrón, J.M. Grafted-Double Walled Carbon Nanotubes as Electrochemical Platforms for Immobilization of Antibodies Using a Metallic-Complex Chelating Polymer: Application to the Determination of Adiponectin Cytokine in Serum. Biosens. Bioelectron. 2015, 74, 24–29.
- Kim, Y.-H.; Park, S.; Won, K.; Kim, H.J.; Lee, S.H. Bacterial Cellulose-Carbon Nanotube Composite as a Biocompatible Electrode for the Direct Electron Transfer of Glucose Oxidase: Biocompatible Electrode for Direct Electron Transfer of Glucose Oxidase. J. Chem. Technol. Biotechnol. 2013, 88, 1067–1070.
- Singh R, Pantarotto D, Lacerda L, et al. Tissue biodistribution and blood clearance rates of intravenously administered carbon nanotube radiotracers. Proc Natl Acad Sci USA 2006; 103(9): 3357-62. http://dx.doi.org/10.1073/pnas.0509009103 PMID: 16492781.
- Singh A, Rai SK, Manisha M, Yadav SK. Immobilized L-ribose isomerase for the sustained synthesis of a rare sugar D-talose. Molecular Catalysis 2021; 511: 1117
- Harrison BS, Atala A. Carbon nanotube applications for tissue engineering. Biomaterials 2007; 28(2): 344-53.
- Gorain B, Choudhury H, Pandey M, et al. Carbon nanotube scaffolds as emerging nanoplatform for myocardial tissue regeneration: A review of recent developments and therapeutic implications. Biomed Pharmacother 2018; 104: 496.
- Fotouhi A, Maleki A, Dolati S, Aghebati MA, Aghebati ML. Platelet rich plasma, stromal vascular fraction and autologous conditioned serum in treatment of knee osteoarthritis. Biomed Pharmacother 2018; 104: 652.
- Saha S, Panigrahi DP, Patil S, Bhutia SK. Autophagy in health and disease: A comprehensive review. Biomed Pharmacother 2018; 104: 485-95.
- Gong, H.; Peng, R.; Liu, Z. Carbon Nanotubes for Biomedical Imaging: The Recent Advances. Adv. Drug Deliv. Rev. 2013, 65, 1951–1963.
- Lacerda L, Bianco A, Prato M, Kostarelos K. Carbon nanotubes as nanomedicines: From toxicology to pharmacology. Adv. Drug. Deli. Rev. 2006; 58:1460-1470.
- Rubin EH, Gilliland DG (2012) Drug development and clinical trials—the path to an approved cancer drug. Nat Rev Clin Oncol 9(4):215–222.
- Osherovich L (2011) Hedging against academic risk. ScienceBus eXchange 4(15)-416.
- Moher D, Glasziou P, Chalmers I et al (2016) Increasing value and reducing waste in biomedical research: who’s listening? The Lancet 387(10027):1573–1586.


 Prathamesh Mahadev Patil* 1
Prathamesh Mahadev Patil* 1
 Seema Shinde 2
Seema Shinde 2



 10.5281/zenodo.14028656
10.5281/zenodo.14028656