Abstract
Cosmetic is one of the fastest growing market and the regulation, the regulatory environments of three significant markets the US, Canada, and the Eu are examined and contrasted. Under the Federal Food, Drug, and Cosmetic Act, which places a greater emphasis on product safety and labeling standards while allowing for greater industry self-regulation, the Food and Drug Administration (FDA) is responsible for regulating cosmetics in the United States. Canada's strategy, overseen by Health Canada, is based on the Food and Drugs Act's Cosmetics Regulations, which place a stronger emphasis on ingredient limitations and safety as well as product disclosure.
Keywords
Cosmetic, Regulation, USA, CANADA, Europe
Introduction
Cosmetics are items that are used to improve or change how the face and body look. They include a broad spectrum of products, including skincare (like serums and moisturizers), hair care (like shampoos and conditioners), scents (like colognes and perfumes), cosmetics (including foundation, eye shadow, and lipstick), and nail care (like polishes and treatments). These products are made with both synthetic and natural substances, and their purpose is to preserve and enhance the health, look, and fragrance of the skin, hair, and nails. In terms of personal grooming and self-expression, cosmetics are crucial. The cosmetics business is one of the fastest-growing sectors of the economy, with a market valuation estimated at USD 262.21 billion in 2022 and projected to reach USD 272.43 billion in 2023. Cosmetics are subject to international regulation and oversight to guarantee product efficacy and safety. However, each nation's regulatory policies may differ greatly from one another and have an effect on the industry's capacity to compete and remain profitable. This paper presents a quick review of the structure and registration procedures for cosmetic regulations in one of the leading exporting countries.
Cosmetic regulation in USA:
The US FDA defines a cosmetic is a product, (except soap), intend to be applied to the human body for cleansing, beautifying, promoting attractiveness or altering the appearance. These cosmetic products are generally regulated by Federal Food, Drug, and Cosmetic Act (FD&C Act), Fair Packaging and Labeling Act External Link Disclaimer (FP&L Act).
Registration:
The Law does not require that cosmetic products or ingredients (with the exception of color additives) to have FDA approval prior to entering the market. It is the firm’s responsibility to ensure that its cosmetic products and ingredients are safe and properly labeled, in full compliance with the law. The FDA highly recommends voluntary registration of their cosmetics through the Voluntary Cosmetic Registration Program (VCRP). The VCRP applies only to cosmetic products being sold to consumers in the United States. It does not apply to cosmetic products for professional use only, such as products used in beauty salons, spas, or skin care clinics
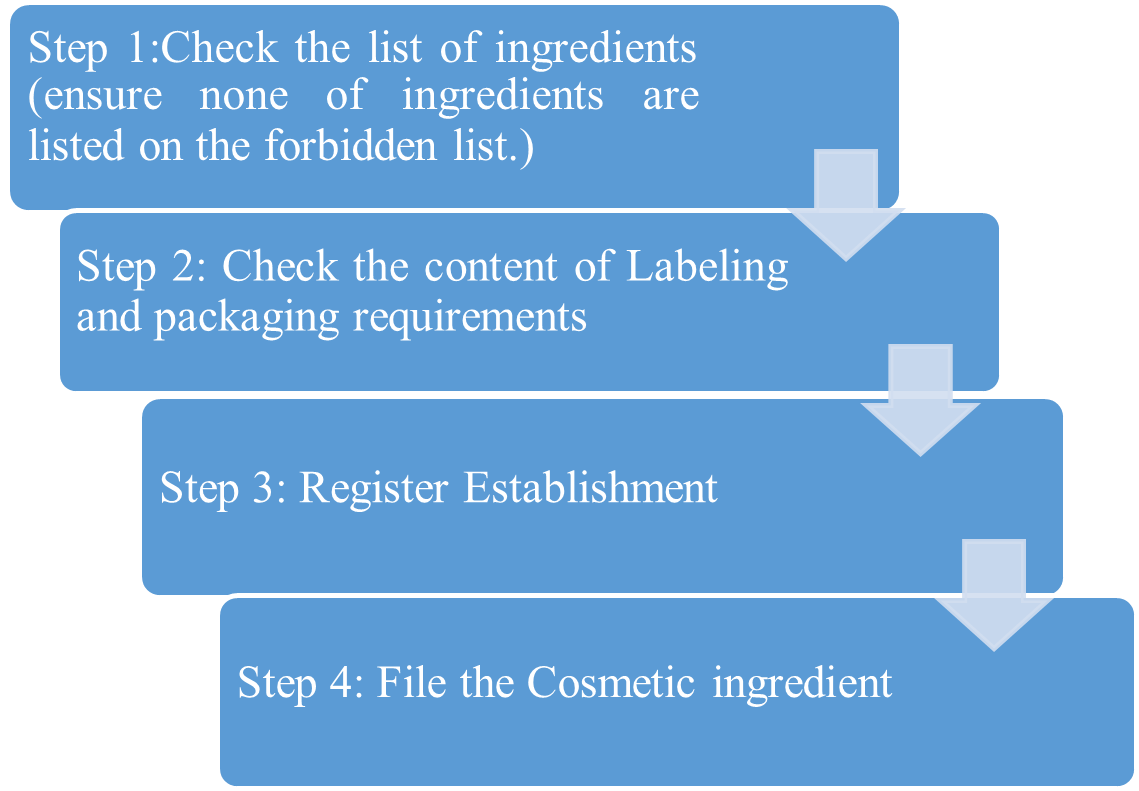
Chart 1: VCRP Registration process
Labeling requirements:
The Federal Food, Drug, and Cosmetic Act (FD&C Act) and the Fair Packaging and Labeling Act (FPLA) provide the FDA the jurisdiction to regulate cosmetic labeling. According to the statute, the FDA lacks the resources and power necessary to approve cosmetic product labels before they go on sale. Proper labeling of items is the duty of the maker and/or distributor. A product with an incorrect brand might arise from labeling rules not being followed. The language used on labels must be English. Products marketed only in a U.S. territory where a different language is the primary language, like Puerto Rico, are the only exception to this provision.
Establishment Registration with FDA:
In order to register an establishment with the FDA, a new account must be requested from the FDA. Owners or operators of facilities that manufacture or package cosmetics can fill out FDA Form 2511 and upload it to the VCRP platform after creating an account. It is noteworthy that distributors are not permitted to register an institution. To fill out Form 2511, you must have the following details:
• Parent Company name
• Address
• Owner or Operator of the Facility
• Other businesses Trade names
• Establishment Authorized individual
File Cosmetic Product Ingredient Statement An institution must first apply for a new account with the FDA in order to register it. Following account creation, owners or operators of facilities that manufacture or package cosmetics can fill out FDA Form 2511 and upload it to the VCRP platform. It is essential to remember that distributors are not allowed to register a business. The following details are needed in order to fill out Form 2511:
• Labeler information
• Manufacturer Information
• Packer information
• Indicate whether filed product is already distributed in the USA
• Product information
» Select product category
» Product code
» Brand name/ specific product name
» Product website
» Upload label images
• Ingredient Information
» Can enter either CAS/ VCRP code number or common, usual, chemical name.
• Authorized individual name
Requirements for Principal Display Panel Regulation
Table 1: Information requirements for both the principal display panel and information panel on cosmetics.
|
Requirements for Principal Display Panel
|
Regulation
|
|
Identity statement
|
indicating the nature and use of the product, by means of either the common or usual name, a descriptive name, a fanciful name understood by the public, or an illustration
|
[21 CFR 701.11].
|
|
Net quantity of contents
Statement
|
An accurate statement of the net quantity of contents, in terms of weight, measure, numerical count or a combination of numerical count and weight or measure.
|
[21 CFR 701.13].
|
|
Required Information on side Panel
|
Regulation
|
|
Name and place of business.
|
- This may be the manufacturer, packer, or distributor. This includes the street address, city, state, and ZIP Code.
- You may omit the street address if it is listed in a current phone directory or city directory
|
[21 CFR 701.12(a)].
|
|
Distributor Statement
|
- If the name and address are not those of the manufacturer, the label must say “Manufactured for...” or “Distributed by...,” or similar wording expressing the facts
|
[21 CFR 701.12(c)].
|
|
Required Information on side Panel
|
Regulation
|
|
Material facts
|
- Failure to reveal material facts is one form of misleading labeling and therefore makes a product misbranded
- An example is directions for safe use, if a product could be unsafe if used incorrectly.
|
[21 CFR 1.21].
|
|
Warning and caution statements
|
- The FD&C Act and related regulations specify warning and caution statements related to specific products
- Cosmetics that may be hazardous to consumers must bear appropriate label warnings
|
[21 CFR part 700].
[21 CFR 740.1].
|
|
Ingredients
|
- If the product is sold on a retail basis to consumers, even it is labeled “For professional use only” or words to that effect, the ingredients must appear on an information panel, in descending order of predominance
- If the product is also a drug, its labeling must comply with the regulations for both OTC drug and cosmetic ingredient labeling
|
[21 CFR 701.3].
|
The Canada:
Cosmetics must be made, maintained, packaged, and kept in a sanitary manner according to Canadian regulations. In order to guarantee adherence, producers and importers must inform Health Canada of their plan to market the product and provide an ingredient list. Cosmetics must also adhere to the guidelines set out in the Consumer Packaging and Labeling Act, which guarantees appropriate labeling and packaging procedures.
The three main tiers of Canada's legal and regulatory framework are Act, Regulation, and Guidance. The Act provides the fundamental guidelines for managing many facets of society. The second level is Regulation, which comes from the Act and offers more thorough and precise guidelines for enforcing the Act. Thirdly, the Guidance functions as an interpretative document that compiles the requirements specified in pertinent acts and regulations, although not being a legally binding act or law in Canada. To guarantee compliance, it is advised that businesses adhere to the Guidance. These three tiers work together to provide a thorough framework that guarantees adherence to the law and offers direction within Canada's regulatory system.
Regulations
Requirements for General Safety
Through the Food and Drugs Act and the Cosmetic Regulations, Health Canada establishes safety guidelines. Every cosmetic that is offered in Canada needs to guarantee:
Use of Compliant Ingredients
Cosmetics must be devoid of impurities and materials that, when applied correctly, can endanger users. The Cosmetic Ingredient Hotlist, which lists banned and restricted substances in cosmetics, is kept up to date by Health Canada. Businesses are required to report to Health Canada the ingredients in their cosmetics so that they may be tracked and validated against the Cosmetic Ingredient Hotlist. Should any safety issues surface, the cosmetic product could not be allowed to be sold.
Hygienic conditions for production and storage
To guarantee customer safety, cosmetics must be produced, prepared, preserved, packed, and stored in a sanitary manner. Although regulation does not specify precise procedures for demonstrating product safety, it is the duty of producers to ascertain and prove the safety of their goods. Companies are advised to follow ISO Cosmetics GMP Standard 22716, even though Canada does not rigorously enforce Good Manufacturing Practices (GMP) or require the acquisition of a manufacturing license. Setting up quality control procedures for contaminants and microbes is crucial, as is maintaining suitable storage and packing circumstances.
Conforming Label
The Food and Drugs Act, Cosmetic Regulations, Consumer Packaging and Labeling Act, and other legislation and regulations specify the criteria that cosmetic product labels must meet.
Cosmetic Notification
Manufacturers and importers must file a Cosmetic Notification Form (CNF) to Health Canada within ten days of the first cosmetic sale in Canada, according to Section 30 of the Cosmetic Regulations. It is best to let Health Canada know about imported goods before they are brought into the country. If notice is not given, admission to Canada may be refused or the item may be taken off the market. The producer, importer, distributor, or a third party with authorization may be the notifier. The notice must include the contact details of a Canadian manufacturer, importer, or distributor, even if the notifier does not have to be a Canadian business. There are no fees associated with submitting the notification. After the CNF has been completed, no publicly available platform will disclose information on the corresponding products. For comprehensive instructions on completing the CNF, please refer to the How to Complete a Cosmetic Notification Form.
|
Information Required for Notification
|
- Notification type
- Brand and product name
- First sale date (actual or expected)
- Other product names
- Product description
- Application area
- Product function
- Product dosage form
- The name and address of the manufacturer on the label and the actual manufacturer
- The name and address of the Canadian manufacturer or importer or distributor
- Name and position of the notifier
- Product labels (under certain circumstances, such as cosmetics with safety warning labeling requirements)
- Product formula (ingredient name and concentration/concentration range)
|
Notification Review
You can electronically submit the CNF to Health Canada. Following submission, the business can move forward with releasing the product onto the market. It's crucial to remember that filing the CNF does not mean that the product has received approval for sale, has been given the cosmetics classification, or has complied with all applicable laws. Health Canada will thereafter assess the CNF. Health Canada may have questions about the product notification information throughout the assessment process, or it may come across problems with the product itself, such as unidentified substances, information missing, safety issues, incorrect categorization, etc. In these situations, Health Canada will get in touch with the notifying person to request more details in order to guarantee the product's safety. If problems are identified health Canada will contact person. Health Canada will provide the notifier a Cosmetic Number (CN), distinct 7-digit identification, once the problems have been addressed and rectified. Companies are allowed to release the product into the market after completing the CNF filing, even though the review procedure can take many months. They are not required to wait for the CN to be given. Enterprises can accelerate processing by inputting the CN in the online system. The CN is generally used as a reference for future revisions of the CNF or termination of sale.
Notification Amendments and Discontinuations
It is important to keep the most accurate and up-to-date information in the CNF. Manufacturers and importers must amend and resubmit the CNF to Health Canada within 10 days when there are changes on the product that affect the information provided. Examples of such changes include
- Modifications to the cosmetic formulation;
- Changes in the product name;
- Discontinuation of sale;
- New company name, address, or contact information.
Cosmetic Labeling
Cosmetic labeling is regulated by two Acts and their associated Regulations:
- Food and Drugs Act and its subordinate Cosmetic Regulations:
- Consumer Packaging and Labeling Act and its subordinate Consumer Packaging and Labeling Regulations:
The following table outlines the five essential pieces of information that must be included on the label.
- The identity of the cosmetic in terms of its common or generic name or in terms of its function, unless the identity is obvious
- Net quantity
- The name of the manufacturer and the address of their principal place of business
- List of ingredients
- Avoidable hazards and cautions
Advance Notice of Importation (ANI)
Upon arrival at the Canadian border, the Canada Border Services Agency (CBSA) refers cosmetics to Health Canada, which assesses them to see if they are compliant with Canadian cosmetics regulations. If the labeling of a specific batch of imported cosmetics fails to meet regulatory requirements, the following actions should be taken by importers:
- Advance Notice of Importation (ANI) Submission: Importers must submit an ANI form to Health Canada before importing the cosmetics.
- Changes or Relabeling: Importers are required to make necessary changes or relabel the products within three months after importation.
However, submitting an ANI form does not guarantee cosmetics and drugs entry into Canada. Health Canada will recommend entry refusal of cosmetics that cannot achieve compliance through modification or relabeling.
Post-market Surveillance
1. Compliance & Enforcement (C&E) Activities: Working with Product Safety Inspectors
C&E activities encompass the measures undertaken by regulatory authorities to ensure that manufacturers, importers, distributors, and other stakeholders comply with the established rules, regulations, and requirements governing the cosmetic industry.
Product safety inspectors play a key role in performing C&E activities. According to the Food and Drugs Act and its subordinate Cosmetic Regulations, product safety inspectors are empowered to:
- Conduct inspections at premises where cosmetics are sold, manufactured, or stored, including collecting samples for testing or take photographs as evidence;
- Make recommendations regarding the refusal of imports and provide an opportunity for non-compliant products to be rectified to achieve compliance under their supervision;
- Seize non-compliant cosmetic products
The EU:
The European Union (EU) was formally founded, resulting in a single market for its Member States. The free flow of cash, people, products, and services throughout Europe is made possible by this single market, and it has throughout time fueled the expansion and prosperity of the region's economy. In July 1976, the European Union published Directive 76/768/EEC (Cosmetic Directive) with the goal of removing trade obstacles and facilitating the circulation of cosmetic items in the European market. However, the Cosmetic Directive's implementation did not successfully lower trade barriers inside the EU because it was merely a framework agreement that the Member States had to modify to fit their unique national conditions. In order to solve this problem, the European Union (EU) enacted the Cosmetic Regulation (EC) No 1223/2009 in 2013, completely replacing the Cosmetic Directive.
Rules and Regulation:
|
Regulation (EC) No 1223/2009
|
As the core regulation for cosmetics, it establishes rules that mainly concern cosmetic safety requirements, the responsible person system, safety assessment, product information file (PIF), product notification, restrictions on the use of ingredients, information exchange on post-market surveillance, etc.
|
|
Commission Regulation (EU) 655/2013
|
This regulation supplements the claim-related provisions in the Cosmetics Regulation by stipulating six common criteria that cosmetic claims shall meet.
|
|
SUE Reporting Guidelines
|
This guideline clearly stipulates the procedures for communicating information about the serious undesirable effect (SUE), the methods for assessing causality, and the precise measures that the responsible person and competent authorities should take after SUEs occur.
|
|
Glossary of Common Ingredient Names
|
This glossary provides cosmetic enterprises and competent authorities with a reference list of ingredient names that the labelling must use.
|
|
The SCCS Notes of Guidance for the Testing of Cosmetic Ingredients and Their Safety Evaluation (12th Revision)
|
This guidance provides details on the methods for cosmetic safety assessment and the special factors that need to be considered in the assessment process. It also timely supplements the latest toxicological research results on cosmetic ingredients, serving as an important criterion for cosmetic enterprises.
|
Product Notification
Cosmetic items do not need pre-market clearance in the EU. Rather, before to being put on the market, they must pass a centralized notification system known as the Cosmetic Products Notification Portal (CPNP). No additional national notice of the product is required after it has been reported by the CPNP. The responsible party, or the distributor in some cases, is required to report a product through the CPNP. The documentation that must be supplied differ according on who the notifier is. However, the notification must contain all pertinent product information in any scenario.
|
Notifier
|
Applicable conditions
|
Info Required
|
|
Responsible person
|
- If the responsible person places the product on the market;
- If a product was placed on the market before July 11, 2013, but was no longer available as of that date, and a distributor (who is not the responsible person) introduces that product into a Member State after that date.
|
- Category and name of the product;
- Name and address of the responsible person;
- Country of origin (only for imported cosmetics);
- Member State in which the product is to be placed on the market;
- Contact details of a physical person to contact in the case of necessity;
- Presence of nanomaterials, including identification and reasonably foreseeable exposure conditions;
- Identification of substances classified as carcinogenic, mutagenic or toxic for reproduction (CMR) of category 1A or 1B;
- Frame formulation;
- Original labelling and packaging (after the product is placed on the market).
|
|
Distributor
|
- If a distributor (who is not the responsible person) makes a product available in a Member State when it has already been placed on the market in other Member States, and translates any element of the product labelling to comply with the national law.
|
- Category and name of the product;
- Member State in which the product is made available;
- Name and address of the distributor;
- Name and address of the responsible person where the PIF is made readily accessible.
|
The Cosmetics Regulation does not stipulate when the product notice must be completed, but it must be done prior to the product being placed on the market. In the rare event that a product contains nonmaterial, notice must be finished six months prior to the product being sold. Following notification, the Commission will electronically make cosmetics available to all relevant authorities, as well as Member State poison centers and other comparable organizations. While the latter will utilize the information for essential medical care, the former will use it for customer information, market analysis, assessment, and surveillance.Moreover, it is important to note that a product may not always meet all of the standards of the Cosmetics Regulation just because it has been successfully notified through the CPNP. The notification data gathered by the CPNP is not examined or evaluated by the Commission. Rather, it is the duty of the relevant national authorities to verify whether a product conforms to the Cosmetics Regulation.
Production Information File (PIF)
For every cosmetic product that is put on the market, the responsible party must keep a PIF. This document must contain information about the product and its manufacturing process, a GMP compliance statement, a cosmetic product safety report, supporting documentation for any claims made about the product, and information about any animal testing that was done. The PIF's contents must be accessible in a language that the Member State's competent authorities may easily understand. After the last batch of the cosmetic product was put on the market, the responsible party is required to maintain the PIF at the location listed on the label, update the information as needed, and save it for ten years.
Safety Assessment
The person in charge must make sure a cosmetic product has passed a safety evaluation before putting it on the market. A person with a diploma or other proof of formal qualifications awarded upon completion of a university course combining theoretical and practical study in pharmacy, toxicology, medicine, or a related field, or a course recognized as equivalent by a Member State, shall, as required, conduct this assessment. A related safety report has to be put together once the safety evaluation is finished. Consists of two sections, each of which must at minimum have the data indicated below. Furthermore, the report has to be current with any new, pertinent information that comes to light after the product is released onto the market.
|
Part A: Cosmetic product Safety Information
|
Part B: Cosmetic product safety assessment
|
-
-
- Quantitative and qualitative composition of the cosmetic product;
- Physical and chemical characteristics and stability of the cosmetic product;
- Microbiological quality;
- Impurities, traces, information about the packaging material;
- Normal and reasonably foreseeable use;
- Data on the exposure to the cosmetic product;
- Data on the exposure to the ingredients contained in the cosmetic product;
- Toxicological profile of the ingredients;
- Undesirable effects and serious undesirable effects;
- Other relevant information on the cosmetic product.
|
- Assessment conclusion;
- Labelled warnings and instructions of use;
- Explanation of the scientific reasoning leading to the assessment conclusion, as well as the need to label any
- Assessor's credentials and approval of part
|
Labelling:
Consumers are the end users of cosmetics, while the vast majority of them lack professional knowledge about cosmetics. It is difficult for them to identify the performance and quality of cosmetics based solely on appearance. Considering this, the Commission strives to provide necessary and reliable information to consumers, protecting their right to know and choose through standardizing cosmetic labelling.
For cosmetics made available on the market, their container and packaging shall bear the following information in indelible, easily legible and visible lettering:
- Name/registered name and the address of the responsible person;
- Contents;
- Date of minimum durability, or period of opening (for products with a minimum durability of more than 30 months);
- Special precautions;
- Batch number, or the reference for identifying the cosmetic product;
- Function of the cosmetic product;
- A list of ingredients.
As per the Cosmetics Regulation, the ingredients shall be expressed by the common ingredient names in the Glossary of Common Ingredient Names. If a common ingredient name is not available, term from a generally accepted nomenclature shall be used. Regarding the language requirement, it shall be determined by the laws of the Member States in which the product is made available to the consumer. If it is impractical to label the precautions and ingredients of a product, the information shall be mentioned on an enclosed or attached leaflet, label, tape, tag or card. Additionally, abbreviated information or the symbol given below shall be included on the container or packaging as a hint for the precautions information, and on the packaging as a hint for the ingredients information.
Comparison between countries regulations
|
|
US
|
Canada
|
Eu
|
|
Regulatory Authorities
|
The Food and Drug Administration (FDA) oversees cosmetics, but regulations are relatively lax. Cosmetic products and ingredients (except for color additives) do not need FDA approval before going on the market.
|
Health Canada regulates cosmetics under the Food and Drugs Act and the Cosmetic Regulations. While Canada has stricter guidelines than the U.S., many products can still be sold without pre-market approval.
|
The European Commission regulates cosmetics under the EU Cosmetics Regulation (EC) No 1223/2009. The EU has some of the strictest cosmetic regulations, requiring pre-market safety assessments and prohibiting certain harmful substances.
|
|
Ingredient Restriction
|
The FDA bans or restricts only about 11 ingredients. Companies are responsible for ensuring their products' safety, but there are fewer restrictions compared to the EU and Canada.
|
Health Canada maintains a Hotlist of prohibited and restricted ingredients. The list contains hundreds of chemicals and substances that are considered unsafe.
|
The EU bans over 1,600 substances from cosmetics. These include carcinogens, mutagens, and reproductive toxicants. Any new ingredient must undergo rigorous testing before it can be used in cosmetic products.
|
|
Animal testing
|
There is no federal ban on animal testing for cosmetics. Companies are encouraged to use alternative methods, but animal testing is not prohibited.
|
While Canada does not currently ban animal testing for cosmetics, it encourages alternatives. There have been ongoing discussions about aligning regulations with the EU’s stance.
|
The EU has a comprehensive ban on animal testing for cosmetics. Since 2013, it has been illegal to sell any cosmetics in the EU that have been tested on animals, even if the testing occurred outside of Europe.
|
|
Product Labeling
|
Labeling must comply with FDA regulations, but companies are not required to disclose all ingredients on their labels, especially those used in fragrance formulations (considered trade secrets).
|
Canada mandates full ingredient disclosure on cosmetic labels, including fragrances, which must be listed in order of predominance.
|
The EU requires full transparency in labeling, with all ingredients listed in descending order by weight. Ingredients must comply with the INCI (International Nomenclature of Cosmetic Ingredients) system.
|
|
Safety Assessment
|
The FDA does not require pre-market safety assessments for most cosmetics. Companies are responsible for ensuring the safety of their products.
|
Health Canada requires manufacturers to submit a Cosmetic Notification Form before selling products, ensuring that all ingredients comply with regulations. While there is oversight, not all products are tested before they hit the market.
|
The EU requires a Cosmetic Product Safety Report (CPSR) before products are sold. This report includes a thorough safety assessment by a qualified professional and covers everything from ingredient safety to packaging.
|
REFERENCES
- https://www.fda.gov/cosmetics/cosmetics-laws-regulations/cosmetics-us-law
- Patel, Veer & Patel, Dasharath. (2021). A comprehensive review on registration requirements for Drug Approval in India, South Africa and US. International Journal of Drug Regulatory Affairs. 9. 62-71. 10.22270/ijdra.v9i1.456.
- Katz, Linda & Lewis, Kathleen & Spence, Susan & Sadrieh, Nakissa. (2022). Regulation of Cosmetics in the United States. Dermatologic Clinics. 40. 10.1016/j.det.2022.02.006.
- Gautam, Preeti & Ghanghas, Deepak & Mittal, Payal & Gupta, Vikas & Dhiman, Kanika. (2022). Regulatory framework of cosmetic in the European Union. 10.53388/20220202013.
- Eixarch, Helena & Wyness, Louis & Sibanda, Musa. (2019). The Regulation of Personalized Cosmetics in the EU. Cosmetics. 6. 29. 10.3390/cosmetics6020029.
- Ferreira, Mariana & Matos, Ana & Couras, Ana & Marto, Joana & Ribeiro, Helena. (2022). Overview of Cosmetic Regulatory Frameworks around the World. Cosmetics. 9. 72. 10.3390/cosmetics9040072.
- Morel, S.; Sapino, S.; Peira, E.; Chirio, D.; Gallarate, M. Regulatory Requirements for Exporting Cosmetic Products to Extra-EU Countries. Cosmetics 2023, 10, 62. https://doi.org/10.3390/cosmetics1002002


 Rupala Savan P *
Rupala Savan P *
 Dr. Devang Tandel
Dr. Devang Tandel

 10.5281/zenodo.14525192
10.5281/zenodo.14525192