Abstract
Among the various routes of administration, oral route is the most suitable and convenient. Drug actions can be improved by developing new oral drug delivery systems such as mucoadhesive buccal drug delivery system. The oral cavity is an ideal site for drug delivery, bypassing intestinal degradation and first pass hepatic metabolism. Mucoadhesion can be defined as a state in which two components, of which one is of biological origin are held together for extended periods of time by the help of interfacial forces mucoadhesion is the attachment of the drug along with a suitable carrier to the mucous membrane. Mucosal surfaces benefit to drug molecules not to amenable to the oral route. Mucoadhesive dosage forms provide prolonged retention at the site of application and providing a controlled drug relese for enhanced therapeutic effects. Mucoadhesion is a complex phenomenon which involves wetting, adsorption and interpenetration of polymer chains. Many researchers are working on the investigations of the interfacial phenomena of mucoadhesion with the mucus. Mucoadhesion is currently explained by six theories which are electronic, adsorption, wettability, diffusion and mechanical. Buccal mucosa’s accessibility and immobility make it ideal for retentive dosage forms. The aim of this review is the mechanisms and theories involved in mucoadhesion.
Keywords
Mucoadhesion, Buccal mucosa, Buccal drug delivery, Controlled drug release.
Introduction
Buccal drug administration is most common route for drug delivery. Buccal mucosal delivery offers several advantages, including: it is patient friendly, has the ease of self medication, allows for a flexible and controlled dosing schedule in comparison to most other drug delivery system. However, it has following demerits together with hepatic first pass metabolism and enzymatic degradation within the gastro intestinal tract, the restrict oral administration of certain classes of the drugs mainly peptides and proteins. Consequently, different absorptive mucosa is taken into consideration as potential sites for drug administration [1]. Buccal drug delivery involves administration drugs through the mucous membranes in mouth. The different transmucosal route, buccal mucosa has first- rate accessibility, an expanse of smotth muscle and generally stationary mucosa, hence appropriate for administration of retentive dosage forms [2]. Transmucosal drug delivey through rectal, vaginal, ocular nasal and oral cavities offers advantages over traditional systemic administration methods. These advantages includes rapid drug loss, avoidance of presystemic elimination within the GI tract and depending on the particular drug, a beneficial gut microbes for absorption [3]. Oral mucosal cavity drug delivery falls into two main categories: (a) sublingual delivery (b) buccal delivery. Mucoadhesion can be defined as a state in which two components, of which one is of biological origin, are held together for extended periods of time by the help of interfacial forces.
Bioadhesion And Mucoadhesion
Bioadhesion describe adhesion between any biological and synthetic surface. Adhesion occurring in a biological setting is termed “bioadhesion”. Bioadhesion is defined as the state in which two materials, at least one biological in nature, are held together for extended period of time by internal forces. In bioadhesive drug delivery the term bioadhesion refers to the bonding between biodegradable polymer and soft tissues, such as intestinal mucosa [4]. Mucoadhesion describe the particular interaction of a mucosal membrane with a synthetic surface. In general, bioadhesion is a term which includes adhesive interactions with any biological or biologically derived substance, and mucoadhesion is used when the bond is formed with a mucosal surface [5].
Mechanism Of Mucoadhesion
Mucoadhesion involves attaching drugs to mucous membranes using suitable carrier. Mucoadhesion has the following mechanism:
- Intimate contact between a bioadhesive and a membrane (wetting or swelling phenomenon)
- Penetration of the bioadhesive into the tissue or into the surface of the mucous membrane [6].
The Residence time for mucosal routes is typically in a minute or less than an hour [7].
Theories Of Mucoadhesion
- Wettability theory
- Electronic theory
- Fracture theory
- Adsorption theory
- Diffusion theory
Wetting Theory Of Mucoadhesion
The wetting theory applied to liquid or low viscosity bioadhesives. The wetting theory was developed predominantly in regard to liquid adhesives, uses interfacial tension to predict spreading and in turn adhesion. The wetting theory calculates contact angles and the thermodynamic work of adhesion [8].
The contact angle (Q) ideally zero for optimal spreading is linked to interfacial tensions(g) as per the Youngs eqation,
Gtg = gbt + gbg COS Q
Where t, g and b represent tissue, gastrointestinal contents and bioadhesive polymer.
The spreading coefficient, Sb/t can be given by,
Sb/t= gtg - gbt - gbg
For the bioadhesion to take place the spreading coefficient should be positive. It gives advantages to maximize the interfacial tension at the tissue GI- contents interface and minimizing the surface tension at the other two interfaces [9].
Electrostatic Theory Of Mucoadhesion
This theory is based on both mucoadhesive and biological materials posses opposite electrical charges. When these materials come into contact with each other, they transfer the electrons leading to formation of double electronic layer at the interface, where attractive forces within this layer determines the mucoadhesive strength [10].
Fracture Theory Of Adhesion This theory based on mechanical measurement of mucoadhesion [11]. It gives relation between the forces required for detachment of polymers from the mucus and strength of their adhesive bond. The work fracture is greater when the network strands are longer or the degree of cross – linking is reduced [12].
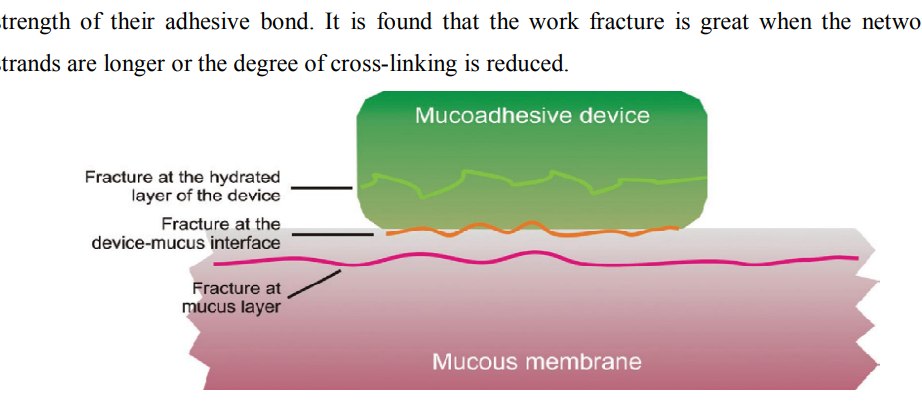
Figure 1: Regions where the mucoadhesive bond ruptures can occur.
Adsorption Theory Of Mucoadhesion
After initial contact, surface adhere due to surface forces between their chemical structure. When polar molecules or groups are present, they reorientate at the interface. The properties of polymer decide the formation of chemical bond [13].
Diffusion Theory
According to this theory mucin and polymer chain penetrates each other to sufficient depth to create a semi permanent adhesive bond [14,15]. The degree of penetrate depends on diffusion coefficient, flexibility and nature of mucoadhesive chains, mobility and contact time of polymer chain. The depth of interpenetration required to produce an efficient bioadhesive bond lies in range 0.2-0.5 µm [16].
Factor Affecting Mucoadhesion In The Oral Cavity
Mucoadhesion Depends On Bioadhesive Polymer And Medium In Which The Polymer Will Reside [17]. The Various Factor Affect Mucoadhesive Properties Of Polymers Such As Molecular Weight, Flexibility, Hydrogen Bonding capacity, cross linking density, charge concentration and swelling of polymer [18].
Polymer related factor
Molecular weight:
Maximum bioadhesion occurs at an optical molecular weight. Bioadhesion strength of polymer increase with molecular weight up to 100,000 and beyond this level there is no significant effect on bioadhesive strength [19]. Size and configuration of polymer are important.
Concentration of active polymer:
When the concentration of polymer is low, the interaction between polymer and mucus is unstable. More concentration of polymer would result in longer penetrating chain length and better adhesion [20,21].
Flexibility of polymer chains:
Bioadhesion starts with diffusion of polymer chains in the internal region. Polymer chains require substantial flexibility in order to achieve the desired entanglement with the mucus [22]. The mobility and flexibility of polymer are related to their viscosity and diffusion coefficients. Cross linking in water soluble polymer reduces the mobility of polymer chains [23].
pH:
pH was found to exert a significant effect on mucoadhesion [24]. pH of medium is determinant factor for degree of hydration of highly cross linked polyacrylic acid polymers and it will be increases between pH 4-5 and decreases more at alkaline pH [25].
Swelling:
Hydrogen is necessary for mucoadhesive polymer to expand and create a proper macromolecular mesh of sufficient size and also induce mobility in polymer chains to enhance interpenetration process between polymer and mucin [26]. Polymer swelling exposes the bioadhesive sites for hydrogen bonding, thus permits mechanical entanglement [27].
Physiological variables
Mucin turnover rate:
The natural turnover of mucin molecules from mucus layer is important because the mucin turnover is expected to limit the residence time of mucoadhesive on the mucus layer and the mucin turnover results in substantial amount of soluble mucin molecules. These molecules interact with mucoadhesive before they interact with mucus layer [28].
Disease state:
During the disease conditions such as common cold, gastric ulcer, bacterial and fungal infections of female reproductive tract the physiochemical properties of mucus changes [29]. Mucoadhesive properties of delivery system should be checked under these condition [30].
Advantages Of Oral Mucoadhesive Drug Delivery
- Prolongs the residence time of the dosage form at the site of absorption [31].
- Due to an increase residence time it enhances absorption
- Excellent accessibility [32].
- Rapid absorption because of enormous blood supply and good blood flow rates.
- Increase in drug bioavailability due to first pass metabolism avoidance [33].
- Improved patient compliance ease of drug administration.
- Faster onset of action is achieved due to mucosal surface.
- Drug is protected from degradation in the acidic environment in the GIT [34].
Limitation Of Buccal Drug Delivery:
- The drugs which irritate mucosa or have bitter or unpleasant taste or an obnoxious odour cannot be administered by this route.
- Drugs which are unstable at buccal pH can be administered.
- Only drugs with small dose requirement can be administered.
- There is possibility of swallowing of the tablet.
- Eating or drinking may become restricted [35].
Buccal Drug Delivery System:
Drug delivery via membranes of oral cavity can be subdivided as follows:
- Sublingual delivery: The administration is via sublingual mucosa to the systemic circulation.
- Buccal delivery: The drug administered through the lining of cheek to the systemic circulation.
- Local delivery: For treatment of conditions of oral cavity such as ulcer fungal conditions and periodontal disease by application of bioadhesive system either to the palate, gingival or cheek [36].
Basic Components Of Buccal Drug Delivery
Permeability:
The oral mucosae in general are somewhat leaky epithelia intermediate between that of the epidermis and intestinal mucosa. It is estimated that the permeability of buccal mucosa is 4-4000 times greater than that of the skin. Substances that facilitate the permeation through buccal mucosa are called permeation enhancer [37].
List of compounds used as oral mucosal permeation enhancers [37]
|
Permeation Enhancer(Siegel IA et al., 1981)
|
|
23- lauryl ether
|
|
Aprotinin
|
|
Azone
|
|
Benzalkonium chloride
|
|
Cetylpyridinium chloride
|
|
Cetyltrimethylammonium bromide
|
|
cyclodextrin
|
|
Dextran sulfate
|
|
Lauric acid
|
|
Lysophosphatidylcholine
|
|
Menthol
|
|
Oleic acid
|
|
Sodium EDTA
|
Drug substance:
Before formulating buccoadhesive drug delivery system, the intended release duration (rapid/prolonged) and effect (local/systemic) must be determined. The selection of suitable drug for the design of buccoadhesive drug delivery system should be based on pK properties.
Characteristics of drug substance:
- The conventional single dose of the drug should be small.
- The drug absorption should be passive when given orally.
- The drugs with biological half life of 2-8 hours are good candidates for controlled drug delivery.
- Through oral route drug may exhibit first pass effect or presystemic rug elimination.
Buccoadhesive polymer [38]
|
Criteria
|
Categories
|
Example
|
|
Source
|
Natural
Synthetic
|
Agarose, Chitosan, Gelatin, Gums(Guar, xanthan, pectin)
Cellulose derivatives: CMC, HEC, HPc, HPMC
Polyacrylic acid derivative: CP, PC, PAA, Polymethacrylate
Other: PVA, PVP, Thiolated polymer.
|
|
Aqueous solubility
|
Water soluble
Water Insoluble
|
CP, HEC, HPC, HPMC, PAA, Sodium CMC.
Chitosan, EC, PC
|
|
Charge
|
Cationic
Anionic
Non-ionic
|
Aminodextran, chitosan
EDTA, CP, CMC, PAA, Pectin
Hydroxyethyl starch, HPC, PVA.
|
|
Potential bioadhesive forces
|
Covalent
Hydrogen
Electrostatic interaction
|
Cyanoacrylate.
Acrylates, methacrylic acid, CO, PC, PVA
Chitosan
|
Characteristics of an ideal mucoadhesive polymer:
- It should be inert and compatible with environment.
- The polymer and its degradation products should be non toxic.
- It should adhere quickly to moist tissue surface.
- The polymer should be economic and easily available in the market.
Backing membrane [37]:
Backing membrane plays very important role in attachment of bioadhesive devices to the mucus membrane. The material used as backing membrane should be inert, and impermeable to the drug and penetration enhancer. Commonly used materials in backing membrane are carbopol, magnesium stearate, HPMC, HPC,CMC.
Buccal Route Of Drug Absorption:
For drug transport across oral mucosa there are 2 permeation pathways: paracellular and transcellular routes. Permeants can utilize both routes simultaneously, but one route is typically favored based on the diffusant’s physiochemical properties. Lipophilic compounds have poor solubility in hydrophilic environments like intercellular spaces and cytoplasm. The cell membrane’s lipophilic nature hinders hydrophilic compounds, while intercellular spaces impede lipophilic compound, due to their respective partition coefficient [39].
Buccal Formulation [40] :
- Delivery system sizes vary by formulation, ranging from 5-8 mm in diameter for tablets to 10-15 cm2 in area for flexible buccal patch.
- Mucoadhesive buccal patches, measuring 1-3 cm2 are the most acceptable size.
- The thickness of the delivery device is usually a few mm.
- The maximum duration of drug retention and absorption is 4-6 hr.
Types Of Buccal Formulation [41] :
- Buccal tablets.
- Buccal patches and films.
- Buccal semisolids( ointment and gels)
- Buccal powder.
Buccal Tablets:
- Tablets are held between gum and cheek
- Lozenges and troches are types of tablets used in oral cavity intended to exert a local effect in the mouth or throat.
- Buccoadhesive tablets may be monolithic or bilaminated.
- Backing layer may be of water insoluble material like ethyl cellulose or maybe polymeric coating layer.
- Backing layer avoids sticking of the tablet to the finger during application of the site.
Buccal Patches And Films:
Buccal patch made of two or multi layered of thin film round or oval as consisting of bioadhesive polymeric layer and impermeable backing layer to provide unidirectional flow of drug across bucccal ,mucosa.
Example:Isosorbide dinitrate in the form of unidirectional erodible buccal film are developed and characterized for improving bioavailability.
Buccal Semisolid Dosage Forms:
A semisolid dosage form consist of finely powdered natural or synthetis polymer dispersed in a polyethylene or in aqueous solution.
Example: gels, ointment
Gels are usually clear, transparent , semisolids containing solubilized active substances.
Buccal Powder Dosage Forms:
Buccal bioadhesive powder dosage forms are mixture of bioadhesive polymers and drug which are spread onto the buccal mucosa. A significance increase in residence time relative to oral solutions was observed.
CONCLUSION:
Mucoadhesive buccal drug delivery is a promising area with the aim of systemic delivery of orally inefficient drug as well as a feasible and attractive for non- invasive delivery of potent peptide and protein drug molecules [42]. The various advantages of oral mucoadhesive drug delivery system like prolongation of residence time of the drug which in turn increases the absorption of the drug are important factors in the oral bioavailability of many drugs [43]. The factors which are determinant in the success of the mucoadhesive drug delivery are polymer physiochemical properties and the in vivo factor such as mucin turnover rate, mucin flow [44]. The buccal mucosa is a promising delivery route for drugs that need to avoid the gastrointestinal tract due to degradation by the gastric pH, intestinal enzymes or due to a substantial hepatic first pass effect [45].
REFERENCES
-
-
-
- Bhatt DA, Pethe AM. Mucoadhesive drug delivery systems: an overview. J Pharm Res 2010;3:1743-47
- Sayani AP, Chien YW. Systemic delivery of peptides and proteins across absorptive mucosae. Critical reviews in therapeutic drug carrier systems. 1996;13(1-2):85-184.
- Harris D, Robinson JR. Drug delivery via the mucous membranes of the oral cavity. J Pharm Sci. 1992;81:1–10. doi: 10.1002/jps.2600810102
- Good WR. Transdermal nitro-controlled delivery of nitroglycerin via the transdermal route Drug Dev Ind Pharm. 1983;9:647–70
- Ahuja A, Khar RK, Ali J. Mucoadhesive drug delivery systems Drug Dev Ind Pharm. 1997;23:489–515
- Aungst BJ, Rogers NJ. Comparison of the effects of various transmucosal absorption promoters on buccal insulin delivery. Int. J. Pharm
- Andrews GP, Thomas P, Laverty TP, David S, Jones SD. Mucoadhesive polymeric platforms for controlled drug delivery. Eur. J. Pharm. Biopharm. 2009; 71: 505-518.
- Baszkin A, Proust JE, Monsengo P, Boissonnade MM. Wettability of polymers by mucion aqueous solutions. Biorheology. 1990; 27: 503-514
- Bateup BO. Surface chemistry and adhesion. Int J Adhes Adhesive. 1989; 7: 233-239
- P.A.Gandhi, Dr. M.R.Patel, Dr.K.R.Patel, Dr.N.M.Patel. A review article on mucoadhesive buccal drug delivery system. IJPRD, 2011; volume 3(5), July 2011, 159-173
- Izher Ahmed Syed et al. Buccal Mucoadhesive Based Drug Delivery Devices. WJPR 2012; 1(3): 548-575
- Yajaman Sudhakar, Ketousetuo Kuotsu, A.K.Bandopadhyay. Buccal bioadhesive drug delivery- A promising option for orally less efficient drugs. Journal of Controlled Release 114(2006) 15-40
- Lee JW et al. Bioadhesive based dosage forms: The next generation J. Pharm. Sci, 2000 89(7): 850-866
- Senel S, Kremer M, Katalin N, Squier C. Delivery of bioactive peptides and proteins across oral (buccal) mucosa. Current Pharmaceutical Biotechnology. 2001 Jun 1;2(2):175-86.
- Phanindra B, Moorthy BK, Muthukumaran M. Recent advances in mucoadhesive/bioadhesive drug delivery system: A review. International Journal of Pharma Medicine and Biological Sciences. 2013;2(1):68-84.
- Rao NR, Shravani B, Reddy MS. Overview on buccal drug delivery systems. Journal of Pharmaceutical Sciences and Research. 2013 Apr 1;5(4):80.
- Duch?ne D, Touchard F, Peppas NA. Pharmaceutical and medical aspects of bioadhesive systems for drug administration. Drug development and industrial pharmacy. 1988 Jan 1;14(2-3):283-318
- McBain JW, Hopkins DG. On adhesives and adhesive action J Phys Chem. 1925;29:188–204
- Jimenez-Castellanos MR, Zia H, Rhodes CT. Mucoadhesive drug delivery systems Drug Dev Ind Pharm. 1993;19:143–94
- Peppas NA, Buri PA. Surface, interfacial and molecular aspects of polymer bioadhesion on soft tissues J Control Release. 1985;2:257–75
- Reinhart CP, Peppas NA. Solute diffusion in swollen membranes ii. influence of crosslinking on diffusion properties J Memb Sci. 1984;18:227–39
- Gu JM, Robinson JR, Leung SH. Binding of acrylic polymers to mucin/epithelial surfaces: Structure–property relationships Crit Rev Ther Drug Carrier Syst. 1988;5:21–67
- Kumar V. Agrawal G. Zakir F. Choudhary A. buccal bioadhesive drug delivery- a novel technique. International journal of Pharmacy and Biological Sciences. Vol.1, Issue 3, July-Sept, 2011, 89-102
- Boddupalli et al. Mucoadhesive drug delivery- an overview. Journal of advance pharmaceutical technology and research 2010; 4(1): 381-387
- Vimal Kumar Yadav et al. Mucoadhesive Polymers: Means of Improving the Mucoadhesive Properties of Drug Delivery System. JCPR 2010; 2(5): 418-432.
- Gupta SK et al. Buccal adhesive Drug Delivery System: A Review. Asian Journal of Biochemical and Pharmaceutical Research 2011; 2(1): 105-114
- PathanSA, Iqbal Z, Sahani JK, Talegaonkar S, Khar RK, Ahmed FJ. Buccoadhesive drug delivery systems- extensive review on recent patents. Recent Patents on Drug Delivery and Formulations.2008; 72; 133-144.
- Smart JD, Nicholls TJ, Green KL, Rogers DJ, Cook JD. Lectins in Drug Delivery: a study of the acute local irritancy of the lectins from Solanum tuberosum and helix pomatia Eur J Pharm Sci. 1999;9:93–8
- Nicholls TJ, Green KL, Rogers DJ, Cook JD, Wolowacz S, Smart JD. Lectins in ocular drug delivery. An investigation of lectin binding sites on the corneal and conjunctival surfaces Int J Pharm. 1996;138:175–83
- Hornof M, Weyenberg W, Ludwig A, Bernkop-Schnurch A. A mucoadhesive ophthalmic insert based on thiolated poly(acrylic) acid: Development and in vivo evaluation in human volunteers J Control Release. 2003;89:419–28
- Albrecht K, Zirm EJ, Palmberger TF, Schlocker W, Bernkop-Schnurch A. Preparation of thiomer microparticles and in vitro evaluation of parameters influencing their mucoadhesive properties Drug Dev Ind Pharm. 2006;32:1149–57 th
- Cafaggi S, Leardi R, Parodi B, Caviglioli G, Russo E, Bignardi G. Preparation and evaluation of a chitosan salt-poloxamer 407 based matrix for buccal drug delivery. J. Control. Release. 2005; 102: 159-169.
- Derjaguin BV, Toporov YP, Muller VM, Aleinkova IN. On the relationship between the molecular component of the adhesion of elastic particles to a solid surface. J Colloid Interface Sci. 1966; 58: 528-533
- Guo JH. Investigating the surface properties and bioadhesion of buccal patches. J. Pharm. Pharmacol. 1994; 46: 647-650.
- Harris D, Robinson JR. Drug delivery via the mucous membranes of the oral cavity. J. Pharm. Sci. 1992; 81: 1-10
- Hill MW, Squier CA. The permeability of oral palatal mucosa maintained in organ culture, J. Anat. 128 (1979) 169-178
- Imam ME, Hornof M, Valenta, Reznicek G, Bernkop- Schnurch A. Evidence for the interpretation of mucoadhesive polymers into the mucus gel layer. STP Pharma. Sci. 2003; 13: 171-176
- Lele BS, Hoffman AS. Mucoadhesive Drug Carriers Based on Complexes of poly (acrylic acid) and PEGylated Drugs having Hydrolysable PEG-anhydride-drug Linkages. J. Control. Release. 2000; 69: 237-248.
- Li X, Shojaei AH. Novel copolymers of acrylic acid and poly ethylene glycol monomethylether monomethacrylate for buccoadhesion: In vitro assessment of buccoadhesion and analysis of surface free energy. Proceed. Int. Symp. Control. Rel. Bioact. Mater. 1996; 23: 165-166
- Gudeman L, Peppas NA. Preparation and characterisation of ph- sensitive, interpenetrating networks of poly(vinyl alcohol) and poly(acrylic acid) J Appl Polym Sci. 1995;55:919–28
- McCarron PA, Woolfson AD, Donnelly RF, Andrews GP, Zawislak A, Price JH. Influence of plasticiser type and storage conditions on the properties of poly(methyl vinyl ether-co-maleic anhydride) bioadhesive films J Appl Polym Sci. 2004;91:1576–89
- Donnelly RF, McCarron PA, Tunney MM, Woolfson AD. Potential of photodynamic therapy in treatment of fungal infections of the mouth.design and characterisation of a mucoadhesive patch containing toluidine Blue O J Photochem Photobiol B. 2007;86:59–69
- Kamath KR, Park KSwarbrick J, Boylan JC. Mucosal Adhesive Preparations Encyclopedia of Pharmaceutical Technology. 1992 New York Marcel Dekker:133
- McCarron PA, Donnelly RF, Zawislak A, Woolfson AD, Price JH, McClelland R. Evaluation of a Water-soluble Bioadhesive patch for photodynamic therapy of vulval lesions Int J Pharm. 2005;293:11–23
- Mikos AG, Peppas NA. Measurement of the surface tension of mucin solutions. Int J Pharm. 1989; 3: 1-5.


 Anandamoy Rudra*
Anandamoy Rudra*
 Archita Sadhukhan
Archita Sadhukhan
 Arindam Dutta
Arindam Dutta

 10.5281/zenodo.14305298
10.5281/zenodo.14305298