Abstract
The quantitative identification of medications and their metabolites in biological fluids is known as bioanalysis. This method is applied very early in the medication development phase to support programs that investigate the pharmacokinetics and metabolic metabolism of chemicals in living cells and animals. A number of excellent review articles addressing different scientific and technological facets of bioanalysis have been added to the literature in recent years. Nowadays, it is generally acknowledged that bioanalysis plays a crucial role in the pharmacokinetic and pharmacodynamic characterisation of a new chemical entity from the moment of discovery through the many phases of drug development that culminate in its approval for sale. The function of bioanalysis in the creation of pharmaceutical drugs is examined, with an emphasis on the specific tasks carried out at each phase of the process and the range of sample preparation matrices that are encountered. Examples of how these high throughput requirements are being satisfied in bioanalysis are shown, along with recent advancements and industry trends for quick sample throughput and data creation.
Keywords
Bioanalysis, Method validation, Metabolism, Pharmacokinetics, Toxicokinetics.
Introduction
A new medicine's development and discovery can cost up to $1 billion, and it may take ten years for the drug to be available for purchase.[1] The process of creating compounds and assessing each one's characteristics in order to ascertain whether only one new chemical entity (NCE) may be chosen to become a safe and effective medication is known as drug discovery and development [2]. It has also been proven that toxicokinetics is a crucial component of toxicity testing [3]. A sensitive and targeted bioanalytical method is crucial given the emphasis on PK/toxicokinetics and the higher potencies of the newer medications [4]. It is commonly known and acknowledged throughout the world that bioanalysis has emerged as a crucial tool in the drug discovery and development process [5]. Additionally, there are numerous analytical techniques accessible for both generic and prescription medications [6-7].A useful guidance for early clinical programs is the bioanalytical data produced in discovery and preclinical studies [8–9]. These comparisons are especially helpful in the initial dose escalation phase one investigation. In order to optimize this, we routinely generate PK data in between dose increases [10].
Bioanalysis:
The quantitative assessment of a substance or its metabolite in biological fluids, mainly blood, plasma, serum, urine, or tissue extract, is commonly referred to as bioanalysis [11]. Drug concentration in the biological matrix should be accurately measured by bioanalytical techniques that are sensitive, specific, repeatable, and accurate [12]. The complicated biological sample composition, low drug concentration, small sample volume, high variability among some patients at different timepoints, and having different subjects in the same group are some of the challenges related to bioanalytical assays [13]. Usually, chromatographic assays and ligand binding assays are used to detect and quantify the medicines in biological samples [14].
3.1-Chromatographic Assay:
Although a variety of detectors, such as ultraviolet, fluorescence, and chargrd aerosol detection, can be coupled with high performance liquid chromatography (HPLC) and ultra high performance liquid chromatography (UPLC), the gold standard is currently thought to be HPLC & UPLC coupled with liquid chromatography and mass spectrometric detection (LC-MS/MS). The standard analytical technique for measuring medications, peptides, and oligonucliotides in biological materials is LC-MS/MS [13]. Protein precipitation, solid phase extraction, and liquid-liquid extraction are frequently employed pretreatment techniques. During sample analysis, sample cleanup might also be the most labor-intensive and time-consuming phase. Therefore, would be a crucial factor to take into account while choosing a sample cleansing technique. [15]
- Physiochemical properties of the analyte,
- Sample matrix,
- Quantitation range,
- Lower limit of detection required,
- Number of sample to be analysed,
- Volume of sample to be available, &
- Analyte stability to extraction/ pretreatment.
This approach does, however, have several disadvantages. For precise detection and quantification of every analyte, the LC-MS/MS method employs an internal standard. An isotope-labeled version of the target analyte, known as a stable isotopes-labelled internal standard (SILIS), is the ideal internal standard [16].
Unfortunately, it can take a lot of time and money to synthesize the SILIS. Isobaric substances that co-elute at the same mass-to-charge ratio (m/z, the unit in which a mass spectrometer detects analytes) and fragment exactly like the analyte in the MS/MS may also be present in the sample. If so, these contaminants need to be separated chromatographically before being detected by MS/MS. Examples of such separations are size-exclusion chromatography or reverse phase separation combined with ion exchange chromatography [17].
3.2-Ligand Binding Assay:
An LBA assesses the binding interaction between the analyte and the binding target indirectly rather than directly. One of the most used plateforms for LBAs is the enzyme linked immunosorbent assay (ELISA). The four primary ELISA types vary in how the antigen or antibody is deposited onto the plate and how the signal is picked up [18–19].
3.2.1- Direct ELISA: A primary antibody that is specific to the antigen is incubated with the antigen after it has been directly adhered to the microtiter plate's wells. An enzyme for detection is pre-labeled onto the main antibody.
3.2.2- Indirect ELISA: A primary antibody that is specific to the antigen is incubated with the antigen after it has been adhered to the microtiter plate's wells. For detection, a secondary antibody that has been labeled against the primary antibody's host species attaches itself to the primary antibody.
3.2.3- Sandwich ELISA: Two antibodies that are specific to distinct antigen epitopes are needed for this format. The plate surface is coated with one of the antibodies, which then binds to the antigen. The antigen can be detected more easily thanks to the other antibody.
3.2.4- Competitive ELISA: This format involves an unlabeled sample antigen or antibody and a reference antigen or antibody competing for the same binding site. This format is compatible with all of the previous ELISA formats.The reference and sample antigens compete with one another to attach to the labeled antibody.
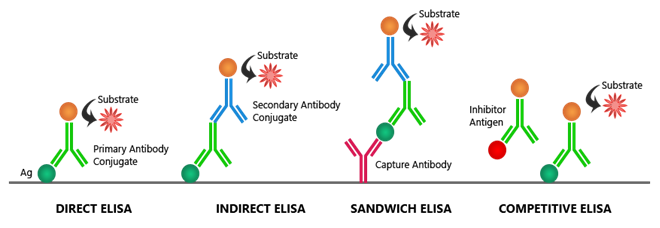
Fig:1- Types of ELISA Assay
The concentration of antigen in the sample is inferred using a calibration curve with a known concentration of reference antigen. Both colorimetric and fluorimetric detection signals are possible for each type of ELISA [18–19].
Validation Parametrs:
4.1-Specificity/Selectively -The terms "specificity" and "selectively" are used interchangeably in bioanalytical validation. The ability of the bioanalytical technology to yield a single analyte is referred to as specificity. On the other hand, selectivity refers to a method's capacity to distinguish an analyte of interest from another analyte [20].
4.2- Accuracy: An analytical method's accuracy indicates the actual value or concentration of an analyte.
4.3- Precision: When the process is repeated on several aliquots of a single homogenous volume, precision is the proximity of individual analyte measurements [21–22].
Quantiation limit:The quantification limit is the lowest concentration of analyte in the sample as determined by certain analytical techniques.
4.4- Detection Limit: The smallest quantity of an analyte that can be detected but not measured is known as the detection limit [23].
4.5- Ruggedness: The degree of reproducibility of the test result achieved following sample analysis under a range of typical test settings is known as the "ruggedness" of an analytical or bioanalytical process [24].
4.6- Stability: This refers to an analyte's chemical stability under particular circumstances. The purpose of the stability test is to identify any analyte degradation that may occur during the sample collection, processing, storage, preparation, and analysis phases [25].
Drug Discovery/ Design:
The goal of bioanalysis during the drug discovery phase is to supply acceptable concentrations of compounds that will be utilized for lead component identification and discrimination. As a result, the analyst's goal at this point should be to provide a quick, easy approach for identification with a high throughput [26]. Finding and characterizing new targets as well as synthesizing and screening novel lead molecules are all part of the drug discovery process [27]. Once the compound has the necessary biological activity and is appropriate for drug development, several analogs or chemically similar compounds will be produced and tested. Physical-chemical characteristics including solubility, lipophilia, and stability are assessed in the subsequent screening phase. Predicting protein binding, tissue distribution, and absorption in the gastrointestinal tract depends on these measurements [28]. ADME characteristics are the collective term for these tests. Information regarding overall drug deposition and progress is provided by these research [29].
Drug Development:
The process of introducing a new pharmaceutical medication to the market when a lead ingredient is found is known as drug development. Preclinical studies on microbes and animals are included. For an investigational novel medicine to start human clinical trials, it is crucial that the application for regulatory status be filed. The assessment of novel drug molecules' safety, toxicity, and effectiveness as well as the medication's pharmacokinetic characteristics are the main goals of drug development [30].
6.1-Preclinical Phase: Drug discovery produces molecules that are lead candidates for novel chemical entities. They show promise in combating a specific biological target, which is crucial in the fight against a given illness. First, the lead component's safety, toxicology, pharmacokinetics, and metabolism in humans are examined. The physical characteristics of the lead component, such as its chemical composition, stability, and solubility, must be taken into account throughout drug development. To meet the regulatory requirements of drug licensing authorities, drug development must be completed satisfactorily. The data gathered during preclinical testing was submitted as a new drug application to the drug regulatory body. Development proceeds to the clinical stage following this approval [31–32].
6.2- Clinical trials: It involves mainly 4 phases.
6.2.1- Phase-I: Healthy volunteers are used in Phase I to determine safety and dosage.
6.2.2-Phase-II: Small numbers of patients with a specific condition and an initial readout of a new medication entity are used in phase-II.
6.2.3-Phase-III: Although it is much larger, phase III is comparable to phases I and II. At this point, it is crucial to assess the safety and effectiveness of the treatment on a significant number of patients. Testing may end at this point and the lead component may be prepared for the new drug application stage if safety and efficacy are established by the regulatory agency.
6.2.4- Phase-IV: A post-marketing monitoring study known as phase-IV is conducted under restrictions set by the Food and Drug Administration.
During medication development, the majority of novel drugs fail because they either have unacceptable toxicity or do not exhibit the desired effect in clinical trials against the targeted disease [33–34].
Abbreviations:
ADME- Absorption, Distribution, Metabolism, Excretion. ELISA- Enzyme Linked Immuno Sorbent Assay. FDA- Food and Drug Administration. HPLC- High Performance Liquid Chromatography. SILIS- Stable Isotopes Labeled Internal Standard. UPLC- Ultra-High-Performance Liquid Chromatography. PD- Pharmacodynamics. PK- Pharmacokinetics. LBA- Ligand Binding Assay.
CONCLUSION:
Bioanalytical techniques are essential for drug research and discovery as well as for evaluating PK, PD, and toxicity data. This helps with regulatory decision-making and facilitates the assessment of a drug's safety and effectiveness. Before the validation research begins, a validation plan should explicitly define the acceptance criteria. The developed assay should be robust enough to allow for minor modifications and easy adoption to meet other bioanalytical needs, such as characterization of the metabolites' plasma levels, acceptability to a toxicokinetic study, and acceptability to a drug-drug interaction study. It was discussed what regulations apply to the development and validation of bioanalytical methods.
REFERENCES
- Hop CE, Prakash C. Application in drug discovery & development. In: Chowdhury SK, editor Chapter 6: Metabolic identification by LC-MS. Netherlands: Elsevier; 2005. p. 123.
- Humphrey MJ. Application of metabolism and pharmacokinetic studies to the drug discovery process. Drug Metab Rev. 1996; 28:473-89.
- D’Arcy PF, Harron DW, editors. Proceedings of the Third International Conference on Harmonizarion. Yokohama: 1995. ICH: Note for guidance on “toxicokinetics: The assessment of systemic exposure in toxicity studies; p. 721-34.
- Campbell B, Bode G. Proceedings of the DIA Workshop “Toxicokinetics: The way forward. Drug Inf J. 1994;28: 143- 295.
- Kantharaj E, Tuytelaars A, Proost PE, Ongel Z, Van AH, Gillissen AH. Simultaneous HPLC measurement of drug metabolites using ion-trap mass spectrometry. Rapid Commun Mass Spectrom. 2003;17:2661.
- Mercolini L, Mandrioli R, Finizio G, Boncompagni G, Raggi MA. Simultaneous HPLC determination of 14 tricyclic antidepressant and metabolites in human plasma. J Sep Sci. 2010;33: 23-30.
- Singh RP, Sabarinath S, Singh SK, Gupta RC. A sensitive & selective liquid chromatographic tandem mass spectrometric assay for simultaneous qualification of novel trioxane antimalerials in different biomatrices using sample- pooling approach for high throughput pharmacokinetic studies. J Chromatagr B Analyt Technol Biomed Life Sci. 2008;864 52-60.
- Navin G, Amin EL, Eugene G, Guenther H. Simultaneous Determination of Dexamethasone, Dexamethasone 21-Acetate, and Palcitaxel in a Simulated Biological Matrix by RP-HPLC: Assay Development and Validation. J Liq Chromatogr Relat Technol. 2008;31:1478-91.
- Podilsky G, Gryllaki MB, Testa B, Pannatier A. Development and Validation of an HPLC Method for the Simultaneous Monitoring of Bromazepam and Omeprazolam. J Liq Chromatography Relat Technol. 2008;31:1478-91.
- Smith DA,Beaumont K, Cussans NJ, Humphrey MJ, Jezequel SG, Rance DJ, et al. Bioanalytical data in decision making: Discovery and development Xenobiotica. 1992;22:1195-205.
- James CA, Breda M, Baratte S, Casati M,Grassi S,Pellegatta B, et al. analysis of Drug and Metabolites in tissues and other solid matrices. Chromatogr supple. 2004;59:149-56.
- Food and Drug Administration. Bioanalytical method validation [guidance]. Dated May 2018. Accessed 21 Jan 2023. https://www.fda.gov/files/drugs/published/Bioanalytical-Method-Validation-Guidance-for-Industry.pdf .
- Hayes RN. Bioanalytical methods for sample cleanup. Biopharm International. Published online 1 December 2012. Accessed 20 february 2023. https://www.biopharminternational.com/view/bioanalytical-methods-sample cleanup
- International Council for Harmonization. Bioanalytical method validation and study samples analysis-M10. Dated 24 May 2022. Accessed 10 February 2023. https://database.ich.org/sites/default/files/M10_Guideline_Step4_2022_0524.pdf
- Medvedovici A, et al. Sample preparation for large scale bioanalytical studies based on liquid chromatographic techniques. Biomedical Chromatogr. Published 16 December 2017. Accessed 10 february 2023. https://analyticalsciencejournals.onlinelibrary.wiley.com/doi/10.1002/bmc.4137
- Korfmacher WA, ed. Mass Spectroscopy for Drug Discovery and Drug Development. John Wiley, 2012
- Gorityala S, et al. Bioanalysis of small- molecule drugs and metabolites in physiological samples by LC-MS, Part 1 An overview. LCGC North America. Published 1 June 2021. Accessed 1 february 2023 https://www.chromatographyonline.com/view/bioanalysis-of-small-molecule-drugs-and-metabolites-in physiological-samples-by-lc-ms-part-1-an-overview
- Promega. Guide to immunoassays. Not dated. Accessed 15 February 2023 https://www.promega.co.uk/resources/pubhub/immunoassay-guide/
- Abcem. ELISA principles and types. Not dated. Accessed 23 february 2023. https://www.abcam.com/kits/elisa principle
- Kallner A. Quality specification based on the uncertainty of measurement. Scand J Lab Invest, 2005;59:513-6
- Trullols E, Ruisanchez I, Rius FX. Trends in Analytical Chemistry. J Lab Invest 2003;23:137-45.
- Nowatzke W, Woolf E. Best Practices during Bioanalytical Method Validation for the Characterization of Assay Reagents and the Evaluation of Analyte Stability in Assay Standards, Quality Controls,and Study Samples. AAPS J, 2007; 9:E117-22.
- Braggio S, Barnaby RJ, Grossi P, Cugola M. A strategy for validation of bioanalytical methods. J Pharm Biomed Anal, 1996; 14:375-88.
- Hartmann C, Massart D, McDowall RD. An analysis of the Washington Conference Report on bioanalytical method validation. J Pharm Biomed Anal, 2005; 12:1337-43.
- Peng GW, Chiou WL. Analysis of drugs and other toxic substances in biological samples for pharmacokinetic studies. J Chromatogr, 1990; 531:3-50.
- Srinivas NR. Applicability of bioanalysis of multiple analytes in drug discovery and development: review of select case studies including assay development considerations. Biomed Chromatogr, 2006; 20:383-414.
- Panchagnula R, Thomas NS. Biopharmaceutics and pharmacokinetics in drug research. Int J Pharm, 2000; 201:131-50.
- Watari N, Sugiyama Y, Kaneniwa N, Hiura M. Prediction of hepatic first-pass metabolism and plasma levels following intravenous and oral administration of barbiturates in the rabbit based on quantitative structure pharmacokinetic relationships. J Pharmacokinet Biopharm, 1988; 16:279-301.
- Wells DA. Fundamental strategies for bioanalytical sample preparation. High throughput bioanalytical sample preparation methods and automation strategies. Amsterdam: Elsevier, 2002, p. 41
- White RE, Manitpisitkul P. Pharmacokinetic theory of cassett e dosing in drug discovery Screening. Drug Metab Dispos, 2001; 29:957-66.
- Kostiainen R, Kotiaho T, Kuuranne T, Auriola S. Liquid chromatography/atmospheric pressure ionization-mass spectrometry in drug metabolism studies; J Mass Spectrom, 2003; 38:357-72.
- Joseph TD. Encyclopedia of clinical pharmacy. London, Newyork: Taylor and Francis Group, Marcel Dekker, 2003.
- De Bruijn P, Moghaddam-Helmantel IM, de Jonge MJ, Meyer T, Lam MH, Verweij J, et al. Validated bioanalytical method for the quantification of RGB 286638, a novel multi-targeted protein kinase inhibitor, in human plasma and urine by liquid chromatography/tandem triple- quadrupole mass spectrometry; J Pharm Biomed Ana, 2009, 50:977-82.
- Oswald S, Scheuch E, Cascorbi I, Siegmund W. LCMS/MS method to quantify the novel cholesterol lowering drug ezetimibe in human serum, urine and feces in healthy subjects genotyped for SLCO1B1; J Chromatogr B Analyt Technol Biomed Life Sci, 2006, 830:143-50


 Vivek Vishnoi*
Vivek Vishnoi*
 Dr. Abhinav Mishra
Dr. Abhinav Mishra

 10.5281/zenodo.14584659
10.5281/zenodo.14584659