Abstract
The formulating floating beads of Saxagliptin increase the gastric residence time so that beads retain in stomach for extended period of time, increases duration of drug release and improvesbioavailability. Floating drug delivery system helps to reduce the dosing frequency and total dose of drug, improves patient’s compliance and convenience, maintains plasma drug level andreduces gastrointestinal side effects.
Keywords
Formulation And Evaluatin, Stomach Specific Drug Delivery, System Of Saxagliptin.
Introduction
Oral dosage forms known as gastroretentive dosage forms have the capacity to be retained in the GI system and to withstand rapid stomach emptying. These systems are ideal for medications with narrow absorption window. They are created as formulations of modified release drug delivery systems that can be site-constrained in the GIT (stomach) and have the capacity to adjust the release rate. The efficiency of gastroretentive drug delivery systems is influenced by a number of variables, including dietary effects, stomach transit duration, and the medication's location of absorption. asy storage and transport of the drug, controlled delivery, flexibility in formulation and generally reduced pricing when compared to other dosage forms. The common objective of drug delivery systems is to achieve a systematic drug that could be taken as a single dosage form. Especially when the drug in question is to be taken periodically throughout the patient's life. An incorporated single unit dosage form would also reduce the frequency of medical administration. It should also be noted that even though it is the easiest form of drug delivery, in case of emergencies oral dosage forms are not applicable due to their slow absorption rate.
Anatomical and physiological barrier for gastrointestinal drug delivery system:
Stomach:
The majority of gastric retention occurs here. The formulation of GRDDS must take into account its anatomy and physiology. The stomach is situated in the upper region of the abdomen, just below the diaphragm. Its volume varies depending on how much it is distended after meals and can reach up to 1500 ml; however, once food has been emptied, it collapses to a resting volume of 25 to 50 ml. [1]
Anatomically, there are three parts of the stomach:
1. Fundus
2. Body
3. Antrum (pylorus).

Following a meal, a stomach typically has a volume of 1.5 liters, varying between 250 and 500 ml during the inter-digestive stages. The body and fundus, which make up the proximal portion, serve as a storage area for undigested materials, whereas the antrum serves as the primary location for mixing motions and serves as a pump for gastric emptying by accelerating the actions. [1]
Gastric emptying and gastric motility:
In both the fed and fasted states, the stomach empties. However, there are two states where the motility patent is unique. A series of electrical events known as the inter-digestive cycle pass through the stomach and intestines every two to three hours while someone is fasting. The migrating myoelectric cycle (MMC), also known as the inter-digestive myoelectric cycle, is the mechanism that causes this. [1]
The four phases of the migrating myoelectric cycle (MMC), as described by Whilson and Washington, are as follows.
- Phase I (basal phase), which includes contractions, lasts for 40 to 60 minutes.
- Phase II (the pre-burst phase) lasts 40 to 60 minutes and is characterized by sporadic contractions and action potentials. The intensity and frequency gradually increase as the phase progresses.
- Phase III (the burst phase) lasts 4 to 6 minutes. It consists of brief but incredibly strong and frequent contractions. This walve is responsible for sweeping all undigested matter from the stomach into the small intestine. The housekeeper wave is an alternative term for it.
- Phase IV, which happens between phases III and I of two successive cycles, lasts 0 to 5 minutes. The pattern of contractions switches from that of a fasted state to that of a fed state following the consumption of a mixed meal. In phase II of the fasted state, continuous contractions are present in this pattern, which is also known as the digestive motility pattern. These contractions cause the size of the food particles that are propelled toward the pylorus in suspension form to decrease (to less than 1mm) as a result. The gastric emptying rate slows down when MMC doesn't start acting right away in the fed state. [9]
Gastric Motility: A complex network of neuronal and hormonal cues controls the movement of the stomach. The vagus nerve, the sympathetic nervous system, the enteric nervous system, and the parasympathetic nervous system all participate in neural regulation. Numerous hormones have an impact on gastric motility; for instance,cholecystokinin and gastrin both calm the proximal stomach while increasing the distal stomach contractions. The final result of smooth muscle cells integrating a wide range of inhibitory and stimulatory impulses is most likely the pattern of stomach motility. [1]Gastric emptying rates were determined using scintinographic studies, which showed that controlled-release dosage forms taken orally are primarily affected by two issues:
1. The Short GRT (Gastric Residence Time)
2. Variable Gastric Emptying Time (Gastric Emptying Time)
Methods for dealing with gastric retention or the mechanistic elements of GRDFS:
To extend the gastric residence time, a variety of methods are currently being used, including bioadhesive systems, floating systems, high-density systems, swelling or expandable system.
Method of Preparation of floating beads:
Ionotropic Gelation Method:
The floating beads of Saxagliptin were prepared using the sodium alginate as gelling agent, calcium chloride as crosslinking agent, calcium carbonate as gas forming.
Saxagliptin alginate beads were made using the ionotropic gelation technique. Sodium alginate was dissolved in 20 ml of distilled water and stirred continuously. Saxagliptin and calciumcarbonate were added to the above solution. The mixture was set aside for 25 minutes to allow air bubbles to escape. This mixture was extruded into a solution of calcium chloride using a syringe. The beads were gathered, washed with distilled water, and then set to dry. Sodium alginate (gelling agent) was selected as an factor.

Table no 01: Formulation Table
In vitro evaluation of formulated floating beads of Saxagliptin.
Uniformity of content:
The drug content of the beads was determined by taking 50 mg beads of each formulation. 50 mg from each formulation were accurately weighed and triturated in a mortar and pestle. In the 100
ml of 0.1N HCL, the beads powder was added. It was stirred for 2hours using a magnetic stirrer. From this solution, 1 ml was withdrawn and added to a 10 ml volumetric flask, and finally the volume was made to 10 ml with 0.1 N HCL (10?g/ml), then the solution was filtered with Whatman filter paper, and the absorbance of the resulting solution was measured at 272nm using a UV spectrophotometer.
Drug content = Actual drug content / Total Weight of beads ×100
formula. Drug entrapment = (Practical drug content / Theoretical drug content) x100
In Vitro buoyancy study:
50 individual floating beads were introduced to 100 ml of simulated stomach fluid (pH 1.2), which was kept at 37.50C and swirled at 100 rpm with a magnetic stirrer. The quantity of floating beads was visually counted every hour for the next 12 hours. The number of beads floated were calculated by the following equation: % Floating beads = Floating beads / Total beads × 10
Characterization of formulated floating beads of saxagliptin.
Partical size:

Table No.2 Partical Size Analysis
All formulations were round in particle shape with smooth surface. The mean particle size of beads of saxagliptin ranged from 81.23to 95.24µm. The mean diameter of prepared beads was increased with an increase in sodium alginate concentration (F1-F6) due to increased viscosity of solution which influence the interaction between disperse metphase and dispersion medium that affect the size distribution of particle.Decreasing the sodium alginate concentration to 1% W/V resulted in clumping of the beads after drying whereas high concentration alginate (2% to 3%W/V) resulted free flowing beads.This could be attributed to an increase in relative viscosity at higher concentration of polymer and formation of larger particle. [38,69]
Percentage Yield
The studies were conducted, and the maximum percentage yield was found to be 92.24%
with F6 batch and minimum of 80.76% with batch F1. [68]

Table No.3 Formulations Percentage Yield
The studies were conducted, and the minimum percentage yield was found to be 80.23% with F1 batch and maximum of 92.24% with batch F6. [68]
Drug Content The data for drug content of floating beads of Saxagliptin is as shown below
Drug Content
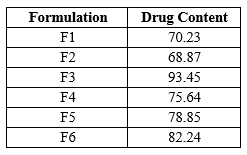
Table No.4 Drug Content
All values are expressed as mean . Determination of drug content was carried out to quantify the amount saxagliptin in prepared beads, it was found to be in range 68.87% to 93.45%. The all six batches’ results are shown in Table indicating drug content improved with an increase in sodium alginate concentrations. Enhanced drug content with an increase in sodium alginate concentration could be due to enhanced availability of the calcium binding site in anionic liner polysaccharide chain and consequently. [69]
Entrapment Efficiency

Table No. 5 Entrapment Efficiency
The determined encapsulation efficiency of prepared Saxagliptin beads is elaborated in Table. On examination of the results, an increase in percent entrapment efficiency from78.23 to 96.87 entrapment efficiency also increased. Additionally, the increased viscosity, which is directly related to polymer concentration, hindered drug mobility, which had an impact on entrapment efficiency. [67, 68]
In Vitro Buoyancy Study

Table No.6 Invitro Buoyancy Study
The shows the in vitro buoyancy data of Saxagliptin beads in simulated gastric fluid pH 1.2. all formulation had a lower density than simulated gastric fluid. The in vitro floating study revealed that all formulation had excellent floating ability. due to the formation of air bubbles during preparation, the beads containing gas producing agent floated for longer than 12 hours. [12]
In vitro Dissolution Study:
In vitro dissolution profile of formulation F1, F2,F3

Table No.7 Dissolution of F1, F2, F3

TableNo.8 In vitro dissolution profile of formulation F4, F5, F6
The floating beads were subjected to in-vitro release using paddle type 2 dissolution apparatus in 900ml of 0.1 N HCl medium. The dissolution profile of pure Saxagliptin alginate beads given in table The release of all formulation observed was between 74.87% to 90.36% The polymer concentration (1%W/V sodium alginate) shows release , 74.64 to 90.36 respectively
CONCLUSIONS
The objectives of the study were to develop sodium alginate beads for intragastric delivery of Saxagliptin.The beads were prepared by the gelation technique using calcium chloride as a crosslinker and calcium carbonate as a gas-forming floating inducer. The entrapment efficiency of alginate beads was significantly improved after the inclusion of sodium alginate in the matrices,and we compared formulated beads by using sodium alginate with beads formulated by pectin as natural polymer. Various aspects of the formulation, its characterization are particle size, percentyeild, drug content, drug entrapment efficiencyand dissolutionstudies. . The best formulation from batches F1-F6, found to be excellent practical size 81.23-95.24 Percentage yield,from 80.23-92.24% entrapment efficiency 78.23-96.87 and Invitro Dissolutin 76.64-90.36 and release of drug, was batch F2 with drug release of 74.36% hence It shows the microspheres formulated by using sodium alginate shows excellent results.
REFERENCE
- Chaudhary Sachin, Dua J.S., Prasad D.N. (2022) Recent Development in Floating Drug Delivery System: An Overview. Journal of Drug Delivery and Therapeutics, 12(01), 185-193
- Anuradha A. Birajdar, Madhuri T. Deshmukh, Rajkumar, V. Shete (2021) A Review on Gastro-Retentive Floating Microspheres. Journal of Drug Delivery and Therapeutics, 1(11), 131-138
- Krishna, Abhishek Kumar, Rajat Srivastava. (2021) In Vivo In Vitro Studies on floating microsphere for Gastroretentive Drug Delivery System. Asian Journal of Pharmaceutical and Clinical Research, 14.1, 13-26.
- Nur Sena Basarr, Burcu Mesut, Yildiz Ozsoy. (2020) A Review On Current Perspective Of Gastroretentive Drug Delivery Syetem Prioritising Floating Dosage Forms, Journal of Advanced Research in Health Science, 43- 64
- Mohamed Ibrahim, Youssef W Naguib, Hatem A Sarhan, Hamdy Abdelkader (2019) Gastro-retentive oral drug delivery systems: a promising approach for narrow absorption window drugs. Journal of Advanced Biomedical and Pharmaceutical Sciences, 02, 98-110.
- Beena Kumari (2018) recent development in floating drug delivery system: a review. Asian Journal of Pharmacy and Pharmacology. 14(01), 131-139.
- Uttam Kumar, Mandal, Bappaditya Chatterjee, Faria Gias Senjotis (2016) Gastroretentive drug delivery systems and their in vivo success: A recent update. Asian Journal of Pharmaceutical Sciences,02, 575-584
- Shivram Shinde, Imran Tadwee, Sadhana Shahi (2011) Gastro retentive Drug Delivery System: A Review. International Journal of Pharmaceutical Research & Allied Science, 01(01), 01-13.
- Ahmed N. Allam, Mohammed M. Mehanna. (2015) Formulation, Physicochemical Characterization and in-vivo evaluation of ion sensitive metformin loaded biopolymeric beads. Drug development and Industrial Pharmacy, 1-9.
- Eng-Seng Chan, Hui-Peng Lim, Chien-Wei Ooi, Beng-Ti Tey (2017). Controlled delivery of oral insulin aspart using pH-responsive alginate / k-carrageenan composite hydrogel beads. Reactive and Functional Polymer, 120, 20-29.
- Jitendra Naik, Gokul Khairnar, Vinod Mokale, Arun Mujumdar (2019). Development of nanoparticulate Sustained release Oral drug delivery system for the antihyperglycemic with antihypertensive drug. Material Technology Advanced Performance material, 1-13.
- Nikhil Bhiswas, Rajan Kumar Sahu (2015), Tropica Starch blended alginate mucoadhesive-floating beads for intragastric delivery of Metaprolol Tartrate. International journal of biological macromolecule, 83, 61-70.
- Nitin Rajendra Shirsath, Ajaygiri kamalgiri Goswami (2020). Vildagliptin-loaded gellan gum mucoadhesive beads for sustained drug delivery: design, optimisation and evaluation. Material Technology, 1-12.
- Pankaj Dangre, Swapnil Dudhkohar, Shailesh chalikwar (2019). Development of Alginate Neusilin US2 (Magnesium almino-metasilicate) micro composite hydrogel beads for oralsustained release of cilnidipine: a statistical optimization. Polymer Plastic Technology and Material, 1-9
- Yan Hendrika, Julia Reveny, Sumaiyah Sumaiyah (2017). Formulation and In vitro evaluation of gastroretentive floating beads of amoxicillin using pectin from banana peel (Musa Balbisiana ABB). Asian Journal of Pharmaceutical and Clinical Research, 11(4), 72-77.
- Zang Tong, Zang Yue, Zang Xi-Tong, Zang Qi, Wang Bing (2018). Formulation development and evaluation of gastroretentive floating beads with Brucea Javanica oil using ionotropic gelation technology. Chinese Journal of Natural Medicine, 16 (4), 293-302.
- Raymond C Rowe, Paul J Sheskey, Marian E Quinn, (2009) Handbook of Pharmaceutical Excipients Pharmacist Press and American Pharmacist association Royal Pharmacist Association of Great Britain, SIX edition, 1-917
- Vinay Wamorkar, Manjunath S. Y, M.Mohan Varma. (2011). Development and Validation of UV Spectroscopic method for determination of Saxagliptin in bulk and tablet formation. International Journal of pharmacy and pharmaceutical science, 3(3), 171 – 174.
- Huimin Yao, Huijuan Yao, Junyi Zhu, Junlin Yu, Lifan Zhang, (2012). Preparation and evaluation of a novel gastric floating alginate / poloxamer inner porous beads using foam solution. International Journal of Pharmaceutics, 422(1-2), 211-219
- Md.Lutful Amin, Tajnin Ahmed, Md Abdul Mannan, (2016). Development of floating mucoadhesive microsphere for site specific release of metronidazole. Advance Pharmaceutical Bulletin, 6 (2), 195-200.
- Shweta Agarwal, Abhilasha Thakur, Abhishek Sharma. (2022). Development and evaluation of ketoprofen loaded floating microsphere for sustained deliver. Materials Today: Proceedings, 68, 647-652. (httpts://doi.org/10.1016/j.matpr.2022.05.299.)
- www.hospira.com. Active ingredient Metoclopramide. This medication guide approved by the U. S. Food and Drug Administration. Distributed by Hospira. (2018).
- A.G. Stosik, H.E. Junginger, S. Kopp, K.K. Midha, V.P. Shah, S. Stavchansky, J.B. Dressman, D.M. Barends. (2007). Biowaiver monographs for immediate release solid oral dosage forms: Saxagliptin. Journal Of Pharmaceutical Sciences, 97 (9), 3700-3708. DOI 10.1002/jps.2127.
- Dili Bhai Gajapathy, U Ubaidulla, Priyanka Sinha, Grace Rathnam. (2022). Gastroretentive Floating Beads – An Emerging Trend In Drug Delivery. International Journal Of Pharmaceutical Research And Application, 7(2), 1510-1520
- British Pharmacopoeia, Volume 3, 1485- 1489. 26. Indian Pharmacopoeia, (2014), volume1, M130- M135 27. Mohd Abdul Hadi, A. Srinivasa Rao, Srinivas Martha, Y. Sirisha, P. Udaya Chandrika (2013). Development of a floating multiple unit-controlled release beads of zidovudine for the treatment of AIDS. Journal of pharmacy research, 6, 78 -8 3. 28. Sumeet Dwivedi1, Hemant Swami, Ashish M. Rathi, Manish G. Baheti , Amit Gangwal and Meenu Shukla, (2013). Formulation and Evaluation of Piperine Alginate Beads for StomachSpecific Delivery. Latin American Journal of Pharmacy (formerly Acta Farmacéutica Bonaerense),73-75.
- Gayathridevi M, J. Adlin Jino Nesalin and T. Tamizh Man, floating microsphere: a review floating microsphere: a review. International journal of research in pharmacy and chemistry, 6(3), 501-510.
- Hinal Prajapati, Keyur Patel, Arun Kumar Gupta, formulation and evaluation of floating microspheres of baclofen. International journal of pharmaceutical science and research, Vol. 12(3): 1482-1494
- Abdul Sayeed, Dr. Pawan Kumar, Dr. Syed Areefulla Hussainy, (2021). Formulation and evaluation of mouth dissolving tablets of metoclopramide hydrochloride using natural and synthetic super disintegrants. Indo American journal of pharmaceutical sciences, 08 (10), 126-141.
- B. Nagamani, J. Hindu Manognya, K. Naga Raju, B. Deepika, T. Regupathi, K. N. Vrao, K. Rajeswar Dutt, (2017). Formulation and characterisation of floating microspheres containing cinnarizine. Innovate International Journal of Medical & Pharmaceutical Sciences, 2(s1), 2456-8694.
- Deepti, Kapil Kumar, Gurleen Kaur and Deepak Teotia, (2020) Preparation and characterization of floating alginate beads of lafutidine as a gastroretentive dosage form. International journal of Pharmaceutical and Research, 11(6), 2752-2760.
- Sunita Adhikari, Kul Prasad Gharti and Shiva Pandeya (2019), formulation and invitro evaluation of metoclopramide hydrochloride orodispersible tablets, 10(4), 1916- 1921.
- T. S. Shinde, a. N. Barhate, (2019), A review on floating microspheres. International Journal of Pharmaceutical and Biological Science Archive, 7(3), 87-92 36. Wajid Ahmad, Rihan Jawed (2022), Design and Characterization of Sustained Released Alginate Beads of Meclizine Hydrochloride. Research Journal of Pharmaceutical Dosage Forms and Technology, 14(03), 199-


 Ms Rutuja S Nikam *
Ms Rutuja S Nikam *









 10.5281/zenodo.12787955
10.5281/zenodo.12787955