Abstract
These days, emulgels are a common drug delivery technology that are being developed specifically for the administration of hydrophobic medicines. This formulation, which combines gel and emulsion, is thought to be a novel kind of drug delivery method. This review aims to shed light on the emulgel preparation process and their evaluation, ultimately determining the significance of these dosage forms. It will be the most widely utilized in the upcoming years due to its ease of use and ability to improve patient compliance. Emulgels have a long shelf life, are translucent, emollient, easily removable, spreadable, thixotropic, and greaseless. These days, emulgels are utilized to deliver a wide range of medications, including antifungal, anti-inflammatory, analgesic, and anti-acne treatments. As a result, it has significant pharmacological value and is comparatively side effect-free.
Keywords
Emulgel, Hydrophobic medicine, Translucent, Patient compliance.
Introduction
The application of the necessary medication to the skin, or topical delivery, is the preferred method for treating cutaneous disorders such as psoriasis, acne, eczema, and others. There is a long history of this administrative route, but to improve patient compliance, new techniques and technologies are being researched and developed [1]. The current path of the ideal choice for cutaneous purposes is administration on skin, because the the most accessible organ is the skin, which makes it easier to administer medications that are more effective than alternative methods of management [2,3]. The most often used preparations are topical ones locally for confined effects at the application site [4]. Topical delivery can be applied vaginally, nasally, rectally, or ocularly to minimize side effects and improve bioavailability [5]. Adhesive goods are separated into two categories: internal and external topical. An external topical product covers the body's diseased areas by spreading into the tissues. Conversely, topical medications used internally are applied topically to the vaginal mucosa, rectal tissues, or oral cavity mucosa to produce a localized effect [6]. Patient compliance, simplicity of use, and increased bioavailability of the medication, minimal toxicity, improved physiological response, and pharmacologically speaking, it works best with medications with limited therapeutic windows . Lastly, but just as importantly, it prevents first-pass. Choosing the right vehicles and active ingredients are just two of the difficulties that need to be taken into account when developing topical drug delivery[7]. Among them are ointments, creams, lotions, gels, etc. Topical application forms have a number of drawbacks, including stickiness, instability issues, and reduced spreadability. It also results in allergic responses, inadequate absorption, permeability, and irritability [8]. When alternative drug delivery methods are ineffective, then the majority of drug delivery is topical [9]. Gels are created when a network of colloidal solid particles traps a significant volume of aqueous or hydro alcoholic liquid [10]. Despite they have many benefits, gels have a significant problem when it comes to the delivery of hydrophobic medications [11]. Emulgels are designed to get around these restrictions [12].
Emulgel
The two types of emulgels, water in oil or oil in water [13] are prepared by adding the gelling agent. In the pharmaceutical industry, both kinds of emulgels are frequently used as a means of delivering different medications to the skin [14]. They own a defining characteristic of their easy skin penetration [15]. The presence of a gelling agent in water phase causes the formation of a classical emulsion. They have a high level of patient acceptability since they have the characteristics of both gels and emulsions [16]. Their two fold qualities make them frequently utilized for the topical delivery of different medications[17]. Emulgels are sometimes referred to as creamed or gelled emulsions [18]. Being long-lasting, emollient, thixotropic, and easily removable shelf-life, biodegradable, aesthetically acceptable, and transparent, greaseless are the main advantages of emulgels [19]. The dispersed phase and the continuous phase are the two immiscible phases in emulsions [20, 21]. Emulsifying agents are used to improve stability [21]. Emulgels are O/W or W/O formulations in which the internal phase traps the drug particles and traverse through the external phase and slowly gets absorbed by the skin, producing a regulated result [22]. Gels offer a number of benefits, but there are still a lot of restrictions when it comes to the delivery of hydrophobic medications. To get around these restrictions and appreciate how medications are distributed in the gel formula, consider the emulgel theory. It shown the combination of hydrophobic medications in emulsion after which into the gel emulsion . A methodology by which both the benefits of gel and emulsion is obtained called emulgel.
Adding hydrophobic medications
Generally, o/w emulsions can be used to effectively incorporate hydrophobic drugs into gels. However, the majority of them are unable to be easily merged into a gel foundation. The explanation for this could mean that solvency acts as a barrier and the problem is elevated during the medication's release [16]. To address this Problem emulgels are applied. It facilitates the introduction of hydrophobic medications into the oil phase after oil globules are distributed in the aqueous phase, which produces an o/w emulsion. Better stability can be further achieved by mixing the prepared emulsion into the gel phase. Second, they're regarded as superior in terms of formation, use, and patient acceptability compared to alternative drug delivery methods [17].
Components necessary to prepare an emulgel
The oil:
The process of creating emulgels involves the use of different oils. For the oil phase, the most common oils are mineral oil, vegetable oil, or fish liver oil. Non-biodegradable minerals and castor oil are common oils used in oral preparations because they have a local laxative effect. Additional vegetable oils, including cottonseed, maize oil, and arachis serve as dietary supplements .The subsequent are distinct different kinds of oils, such as isopropyl palmitate, isopropyl myristate, and are liquid paraffin, and stearate .
Water-based substance
Water and alcohol are the two most common aqueous materials used in the aqueous phase of emulgels.
The Emulsifier
To encourage emulsification and manage stability during the manufacturing process. Stearic acid, sodium, PEG-40, tween-20, 40, 60, and 80 and sodium stearate is frequently employed .
Aggressive agent
These serve to encourage uniformity and can be employed as agents for gelling . Sodium alginate, carbomer 934,940, and sodium Gellan gum and CMC are frequently used . Different gelling agents have been employed, including Carbopol 940, HPMC 2910, Carbopol-934, HPMC, Sodium C.M.C., and others .
The penetration enhancer
The purpose of these agents is to increase transient skin permeability. They penetrate into and engage with skin constituents. Lecithin [5%], palmitate, sodium lauryl sulfate, clove oil, and olive oil and 1% oleic acid is frequently utilized . Various penetration Improvers are applied. Examples are : Oleic and Lecithin acid, urea, eucalyptus oil, menthol, and iso-propyl myristate. Penetration ehancers shouldn't be pharmacologically active within the body. It suggests that they shouldn't attach themselves to any of the recipient locations They should not irritate or disturb the skin in any way, and they should be worthy in terms of appearance . They should demonstrate good compatibility with drug and excipients. Barrier qualities should remain intact after removal from the skin and come back quickly .
The way penetration enhancers work:
The disruption of the stratum corneum initiates the penetration enhancer mechanism. Either through intracellular interactions with proteins or structure . The enhancement of co enhancers, cosolvents, or drug partitioning into the stratum corneum of the skin is another method by which penetration enhancers work .
Procedure for Preparation:
Emulgel preparation involves three fundamental steps, which are also shown in Figure 1.
Phase 1
Emulsion formulation, which can be W/O or O/W.
Phase 2
Combining water and gelling agents to create a gel base by continuous stirring and pH optimization.
Phase 3
Emulsion incorporation into gel base while heating and stirring continuously [2]. Emulgel preparation is a fairly easy and economical process approach. It comprises the key actions that are described in below , Figure 1 [23]. It incorporates the medication as needed[24]. After that, the gel base is formulated and followed by continuous stirring by the addition of emulsion.
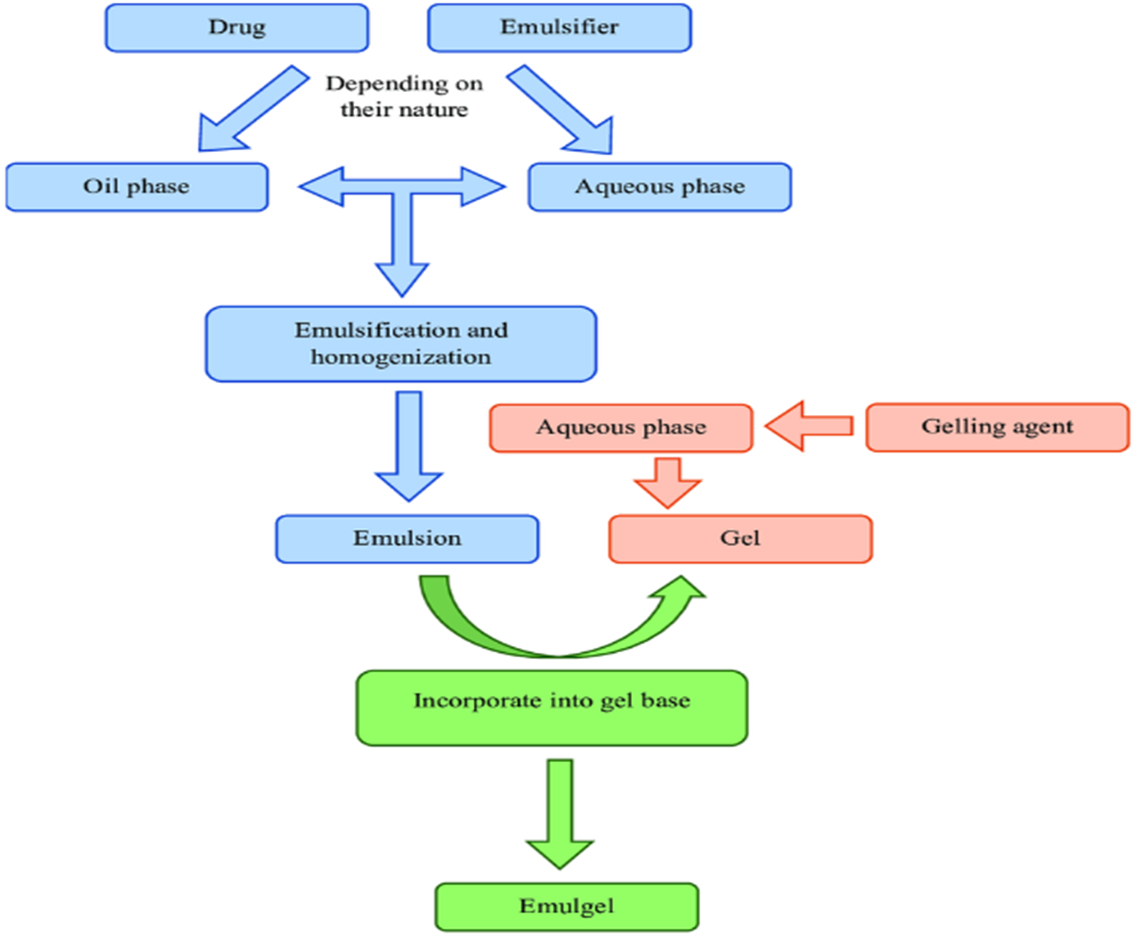
Figure 1: Emulgel preparation process in its simplest form.
Forming the gel base comes next, and then the emulsion is added to it while it is constantly stirred [25]. Developing or preparing emulsions requires the creation of the aqueous phase by mixing a solvent-fixing substance—that is, soluble—with filtered water and it's heated to 70°C. Emulsifying agents such as tween are also included in it . Following the preparation of the aqueous phase, the oil phase is retained in thinking about. In order to prepare it, surfactants like span is dissolved . It is heated after a hydrophobic medication is added at an identical temperature. Currently, the gel is made by continuously blending the polymer in filtered water at a reasonable speed. At this point, the pH is balanced between 6 and 6.5. Preservatives were added in the aqueous phase as the final step . Heat was applied to the aqueous phase and oil at 70–80 0C respectively; both phases were heated before the oily phase was added to aqueous phase and constant churning . Be sure that it is lowered to the surrounding temperature. The gel is mixed with the emulsion in the proportion of 1:1 for the emulgel formation.
An assessment of emulgel:
Following emulgel preparation, evaluation is required. Here are a few methods for evaluating Emulgel.
Rheological studies:
Using a Brookfield viscometer with a spindle (i.e., no. 96) spinning at 1.5 rpm, the viscosity of the emulgel formulation is measured at 25 0C . The assembly was attached to a water bath with a circulating thermostat that was kept at 25 0C . The beaker is filled with the emulgel whose viscosity is to be measured, the spindle is free to move, and the reading is recorded .
Patch test:
Another name for a patch test is a skin sensitivity test. For this particular test, a group of rats is typically selected. Emulgel is properly applied to the rat's skin. Unwanted skin alterations, such as changes in Skin morphology and colour were examined following the duration of One day [33].
Uniformity:
When emulgel is applied thinly to a slide, the homogeneity of the formulation is evaluated visually.
Measurement of pH:
The process involved dipping the glass electrode into a emulgel using a digital pH meter.
Distributability:
It is one of the requirements for an emulgel to fulfill its ideal properties. Spreadability is a term used to describe the extent the area to which the medication easily spreads across the skin's surface when applied. It is determined by a device known as the Mutimer. It includes glass on one end of a wood. An excessive amount of emulgel was put on the ground slide, followed by the preparation of the emulgel, positioned itself between the two sides. The duration needed for the top slide would cover a distance of 5 cm was measured. A better spreadability coefficient is indicated by a shorter interval . Wooden block that is connected to a pulley, slide was set on it.
Choosing one's physical characteristics:
The prepared emulgels are reportedly examined and verified. Their phase separation, consistency, homogeneity, and colour are verified .
Calculating the drug content:
One gram of emulgel is taken and mixed with an appropriate solvent, then strain it to get a solution that is clear. Calculate its absorbance via UV spectrophotometer application. The same solvent is used to prepare a drug's standard plot. It's Concentration and medicinal ingredient can be chosen using the identical standard plot by entering the absorbance values .
Swelling metric:
On permeable aluminium foil, one gram of emulgel is taken, and it is then placed in a separate 50 ml container that contains 10 ml of NaOH . Additionally, the samples were removed from the containers at various times and placed in an unhydrated surface. It is reweighed at a designated time.
Microbiological assay:
A microbiological assay is made using the "ditch plate method”. Bacteriostatic or fungi-static activities are quantified and assessed for a compound using this method. This method is primarily employed regarding semi-solid compositions. Sabouraud’s agar dried plates are used for the assay. The mixture is removed and put inside a ditch plate. Additionally, recently made loops of culture are evenly distributed at a precise right angle across the agar. In this manner, that is, from the ditch to the opposite edge of the dish .
Drug release investigation, i. e. in vitro:
Drug release research is conducted using an in vitro Franz diffusion cell, which has a dispersion region of 3 cm2 and a cell volume of 30 ml. A quantity of 500 milligrams of emulgel was taken and uniformly applied to the cellophane film. Clamping the cellophane film between the Diffusion cell donor and receptor chamber. Afterwards, In order to solubilize the drug, a freshly prepared buffer with a pH of 5.8 was poured into the receptor chamber . Then it is stirred at 50 rpm using a magnetic stirrer, and the temperature was kept at 37 ± 0.2 0C. At proper time interval, the samples were taken out. After that, they were examined with a UV visible spectrophotometer roughly at 260 nm .
Available emulgels:
These are the commercially prepared and marketed emulgels. Emulgels such as Diclomax, Voltarol, and Derma feet emulgels such as Isofen, Cataflam, etc. that have anti-inflammatory, anti-fungal, and anti-acne properties as well as exfoliating action.
CONCLUSION
This essay effectively reviewed emulgels since it addressed all of the main concerns and points while emphasizing how important they are. The majority of medications are available in hydrophobic form, and there has consistently been a difficult task for the creation of these medicines. Considering topical delivery of these medications, it implies applying traditional dosage forms, such as lotions, ointments, emulsions, lotions, etc. Because the hydrophobic properties of the medications, the question of bioavailability and stability comes up in a variety of dosage forms . Emulgel, a contemporary formulation concept, is offered as a solution, where in the medication is combined in the oily phase of the mixture. It achieves the controlled release effect , by mixing the aqueous phase with the oil. Additionally, it also increases the medication's bioavailability. Emulgel is a really practical topical dosage form, which enhances dermatological drug therapy. Since emulgel has a number of characteristics that adhesion, viscosity, spreadability, and other properties that improve patient compliance; applied to hydrophobic medications, it will boost its effectiveness and lessen any negative effects. The benefits outlined in this article provide emulgels, enormous significance for the delivery of hydrophobic medications, due to its higher adequacy and lower production cost.
REFERENCES
- Naga Sravan Kumar Varma V, Maheshwari PV, Navya M, et al. “Calcipotriol delivery into the skin as emulgel for effective permeation”. Saudi Pharm J. 2014;22(6):591–599.
- Rathbone M, Hadgraft J, Roberts M, et al. “Dermal and transdermal drug delivery”. Modified–release drug delivery technology. London: Informa Healthcare; 2002.
- Pednekar A, Dandagi P, Gadad A, et al. “Formulation and characterization of meloxicam loaded emulgel for topical application”. Int J Pharm Pharm Sci. 2015;7:216–222.
- Schreier H, Bouwstra JA. “Liposomes and niosomes as topical drug carriers: dermal and transdermal delivery”. J Control Rel. 1985;30:863–868.
- Bhowmik D, Gopinath H, Kumar P. “Recent advances in novel topical drug delivery system”. Pharma Innovation. 2012;1:12–31.
- Singh M, Mital N, Kaur G. “Topical drug delivery systems: a patent review”. Expert Opin Ther Pat. 2016;26(2):213–228
- Lachman L, Lieberman H, Kang J. “Theory and practice of industrial pharmacy”. Bombay: Verghese Publishing House; 1991.
- Baibhav J, Vikas S, Gurpreet S. Emulgel: “a comprehensive review on the recent advances in topical drug delivery”. Int Res J Pharmacy. 2011;2(11):66–70.
- Mendapara V, Purohit P, Kea A. “SUPAC of immediate release solid oral dosage form-eplerenone”. Invent Rapid Pharm Tech. 2013;3:1–7.
- Aher S, Banerjee S, Gadhave M. Emulgel: “a new dosage form for topical drug delivery”. IJIPLS. 2013;3:1–10.
- Ansel H, Allen J, Popovich NG. “Pharmaceutical dosage forms and drug delivery systems”. New York, NY: Lippincott; 1999.
- Panwar AS, Upadhyay N. “Emulgel: a review”. Asian J Pharmacy Life Sci. 2011;1(3):333–343.
- Manli W, Liang F. “Percutaneous absorption of diclofenac acid and its salts from emulgel”. Asian J Pharm Sci. 2008; 3(3):131–141.
- Naito S-I, Tominaga H. “Percutaneous absorption of diclofenac sodium ointment”. Int J Pharm. 1985;24(1):115–124.
- Choi HG, Yong CS, Sah H, et al. “Physicochemical characterization of diclofenac sodium-loaded poloxamer gel as a rectal delivery system with fast absorption”. Drug Dev Ind Pharm. 2003;29(5):545–553.
- N’Da D. “Prodrug strategies for enhancing the percutaneous absorption of drugs. Molecules”. 2014;19:20780–20807.
- Rieger M, Lachman L. The theory and practice of industrial pharmacy; 1986.
- Kakkar AP, Gupta A. “Gelatin based transdermal therapeutic system”. Ind Drugs. 1992;29:308–312.
- Charoenrein S, Tatirat O, Rengsutthi K, et al. Effect of konjac glucomannan on syneresis, textural properties and the microstructure of frozen rice starch gels. Carbohydr Polym. 2011;83(1):291–296
- Eswaraiah S, Swetha K, Lohita MM. “ Emulgel: review on novel approach to topical drug delivery”. Asian J Pharma Res. 2014;4(1):4–11.
- Ashara K, Soniwala M. “Emulgel: a novel drug delivery system”. J Pak Assoc Dermatol. 2016;26(3):244–249.
- Gibson M. “Pharmaceutical formulation and Preformulation”. Boca Raton, FL: Interpharm; 2004.
- Panchal B, Rathi S. “Topical emulgel: a review on state of art”. Int J Pharma Sci. 2018;9(1):253–264.
- Mori N, Ashara K, Sheth N. “Topical antifungal film forming transdermal spray composition and method of preparation thereof ”. Indian Patents; 2014.
- Gul R, Ahmed N, Ullah N, et al. “Biodegradable ingredientbased emulgel loaded with ketoprofen nanoparticles”. AAPS PharmSciTech. 208;19(4):1869–1881.
Maria Talata , Muhammad Zamana, et al. “Emulgel: an effective drug delivery system”. Drug development and industrial pharmacy. 25 Oct 2021


 SHILPA KD*
SHILPA KD*
 ASWATHY C
ASWATHY C
 FARHANA M K
FARHANA M K
 HASNA E K
HASNA E K
 BELNA AUGUSTINE
BELNA AUGUSTINE

 10.5281/zenodo.12739709
10.5281/zenodo.12739709