Abstract
Incidence of diabetes mellitus has increased over the past few years, mainly due to our eating habits and physical inactivity. This also includes the use of artificial sweetening agents which have broadly replaced other forms of sugars and have shown a paradoxical, negative effect on blood glucose. Ingestion of these artificial sweeteners (AS) results in the release of insulin from pancreas which is mistaken for glucose (due to their sweet taste). This increases the levels of insulin in blood eventually leading to decreased receptor activity due to insulin resistance. Over a century ago, Artificial Sweeteners (AS) were developed as food additives to provide sweetness without the associated high caloric content of sugar. The United States Food and Drug Administration(FDA) have approved five artificial sweeteners: aspartame, saccharin, acesulfame potassium, neotame and sucralose. These sweeteners have also However, despite the widespread consumption of artificial sweeteners by lean, overweight and obese individuals alike, obesity and diabetes continue to dramatically rise. This review examines the relationship between artificial sweeteners and diabetes and the need for continued investigation into the consumption of artificial sweeteners.
Keywords
Diabetes mellitus, Artificial sweeteners, Aspartame, Saccharin, Formulations, Guidelines of sweeteners.
Introduction
Diabetes mellitus is a long-term metabolic disorder that is characterized by high blood sugar, insulin resistance, and relative lack of insulin. Common symptoms include increased thirst, frequent urination, and unexplained weight loss. Insulin, a pancreatic hormone, helps glucose from food get into the cells to be used for energy.[1] Diabetes, characterized by hyperglycaemia is a chronic lifestyle disorder. The metabolic disorder results in elevated blood glucose due insufficient insulin secretion or action. Insulin is a hormone produced by the beta cells of pancreas responsible for regulation of the blood glucose.[2] Type 1 diabetes is also known insulin independent diabetes mellitus. In type 1 diabetes there is no insulin secretion due to destruction of beta cells in the pancreas. It is also known as juvenile diabetes and is less prominent as compared to type 2 diabetes. The genetic component responsible for autoimmune reaction is HLA complex. However, some environmental triggers are also responsible for altering the immune function and thereby resulting in the destruction of ? cells. [3] Type 1 diabetes was considered more prevalent in children and adolescents however this opinion has changed with progress in studies. The classic symptoms include polyuria, polyphagia and polydipsia along with hyperglycaemia. [4] Type 2 diabetes is known as non-insulin dependent diabetes mellitus is a progressive disease. The major factors responsible for the disease are insulin resistance or the ? cell dysfunction. Corresponding Author: A defect in insulin signalling leads to insulin resistance whereas due to amyloid deposition in the islets cells, excessive fatty acids, oxidative stress leads to ? cell dysfunction. [5] The use of oral hypoglycaemic drugs and lifestyle modifications is very important in the management of type 2 diabetes mellitus. The macro complications of diabetes include diabetic retinopathy, diabetic neuropathy and diabetic nephropathy. In diabetic retinopathy, the small retinal blood vessels are damaged resulting in blindness. It is observed that patients with long term diabetes often develop severe visual impairment. Up to 50% of the diabetes patients suffer from diabetic neuropathy causing nerve damage with the common symptoms of pain, tingling, weakness, numbness in the feet and hands. There are chances of increased foot ulcers eventually leading to limb amputation. A diabetes patient is also often prone to kidney failure and cardiovascular diseases primarily stroke. [WHO]
OBJECTIVES :
outline metabolic aspects of sugarsubstitutes.
- Evaluate health implications of various sugar substitute
- Explain indication potentials of sugars substitutes in some metabolic diseases.
SWEETENING AGENTS :
Sweetening agents are the substance which are added to a drug formulation to mask its better taste.
- Sugar is the most widely used natural sweeting agents
- Its imparts viscosity to drug and also even act as preservative for liquid dosage form
- Sugar having lot of disadvantages like dental caries ,high blood sugar ,calories etc .
Among this are various substitute available
These are 2 types:
- Natural sweeteners
- Artificial sweeteners

Ideal properties
- They are required to be effective when used in small concentration
- They must be stable at a wide range of temperature to which the formulations are likely to be exposed.
- Prolonged use of these agents containing preparations should not produce any carcinogenic effects.
- They should have very low or non calorific value.
- They should be compatible with other ingredients in formulations.
- They should not show batch to batch variations.
- They should be readily available and inexpensive.
CLASSIFICATION OF SWEETENERS:-
Classification of Natural sweetening agents;
The search for sugar substitutes from natural sources has led to the discovery of several substances that possess an intensely sweet taste or taste-modifying properties. About 150 plant materials have been found to taste sweet because they contain large amounts of sugars and/or Polyols or constituents.[6]. A schematic representation of the types of sweeteners and their origin is given below.

The sweeteners are classified on their nutritive values.
- Nutritive sweeteners
- Non-nutritive sweeteners.
NUTRITIVE SWEETNERS:-
Alternative nutritive sweeteners are sugar alcohol such as sorbitol, mannitol ,lactitol etc.
- It having properties like less sweet and less calories.
- 1.5 times sweetener than sugar and cost effective for food industry.
- Nutritive sweeteners such as sugars and sugar alcohols add carbohydrates to food and calories to your diet that contain few vitamins and minerals.
- Sugars are considered fine and do not cause special problems for people with carbohydrates in tolerance such as Diabetes mellitus or insulin resistance.
NON-NUTRITIVE SWEETENERS:-
Non-nutritive sweeteners are also called as artificial sweeteners .Examples include aspartame, saccharin , cyclamate , alitame etc.-More sweeteners and thus only small quantity is required for sweetening food preparation.-these sweeteners has been approved for use in a number of dietetic or low calorie foods and beverages .artificial sweeteners are many times sweetener than sugar. in terms of potential health risk they are deemed safe for all age groups although highly intakes of some have been linked to increased cancer risk in the past.
Natural sweeteners;
Natural sweeteners are sugar substitutes which provide sweet taste as an alternative to sugar. They are obtained naturally from plants and hence shows very less or no side effects. Natural sweeteners can be used in the pharmaceutical and food industry. In pharmaceutical industries natural sweeteners can be used in oral and liquid preparations to prepare the syrup base which maintains the consistency of the preparation. They are also used in lozenges, tablets and pills. These liquid preparations also help to mask the bitter taste of the drug. Sugar is helpful in coating of the tablet. In food industry, sweeteners are used for the preparation of jams, ice- creams and chocolates etc. They are effective when used in small concentrations and are stable at a wide range of temperatures. [7]
- Stevia;
Stevia also known as sweat leaf, sweat herb; is obtained from Stevia rebaudiana plant, from the chrysanthemum family, subgroup of Asteraceae family is a semi-humid subtropical plant. It is used since ancient times throughout the world. It grows 60-80cm tall and has oppositelyarranged leaves. Stevia has different species and each contains potent sweetening constituents. Stevia can be grown at home with pH 6.5-7.5. It is cultivated in many Indian states. [9]
- Honey;
Humans began using honey nearly 10,000 years ago and the evident for it is a cave painting revealed in the early 1900’s in Valencia, Spain in the Cave of the Spi- der (Cueve de la Arana) situated on the river Cazu- nta. Archaeologists have discovered honey comb in Egypt that were been buried in tombs at the pharaohs, the honey was still eatable. Honey is frequently used as a talisman and also as a symbol of sweetness (White and Doner 1980). In Ancient times, people of Egypt use honey for making of sweeten cakes and biscuits, and were used in many other dishes.
- Sucrose;
Sucrose is a that is efficiently hydrolysed (by sucrase) in the intestinal mucosa to its constituent monosaccharides. It has been established that glucose stimulates fructose uptake in a dose dependent manner and that monosaccharides derived from sucrose are essentially absorbed at a similar rate to glucose fructose mixtures . However, whereas glucose elicits a glycaemic and insulinemic response that stimulates its uptake into cells, fructose is mainly metabolized in the liver via an insulin independent pathway not regulated by energy supply. There it may be converted into trioses that can be used for the de novo synthesis of triglycerides (TG) and cholesterol . Thus, sucrose has the potential to influence both insulin sensitivity
4. Maple syrup;
-Maple syrup is produced from the sap of maple trees which is collected , filtered and boiled down to extremely sweet syrup with a distinctive flavour . it contains fewer calories and high concentration of minerals like calcium , zinc, potassium, magnesium. It also contains up to 54 types of antioxidants , some of which may have anticancer properties and is said to the 1.4 times sweetener than white sugar. Maple syrups glycemic index is slightly lower than of table sugar or sucrose. So it maybe less likely to cause quick blood sugar spikes
5. Black-strap molasses;
Blackstrap molasses is a thick liquid during cane sugar processing after the maximum amount of sugar crystals are removed. In comparison to other sweeteners , blackstrap molasses is higher in vitamins and minerals may contain up to 20% of daily value of iron , 10% of daily of vitamin B6 , and other nutrients including magnesium , potassium and calcium . it also has a lower of GI than table sugar and regular molasses. The back drop being that is not easily available in the Indian market
6. Erythritol;
Erythritol is the sugar alcohol derived from the fermentation of corn starch or wheat. It has very few calories and has no impact on blood sugar. while erythritol is less likely than others sugar alcohols can upset your stomach it is safe even in relatively large quantities. It is found naturally some in foods it also made thin like wine ,beer, and cheese ferment . it does not have a chance to metabolize turn into energy in our body
Side effects :
- Increasing your risk of heart attack andstoke.
- It can cause mild to severe digest issues.
- It includes bloating ,cramping ,excess gas and diarrhea.
Health effects of Natural sweeteners;
Alternate to sucrose, Stevia has potential health benefits and the sweetness occurs naturally. Stevia is considered as safe and effective for diabetic patients as it shows no effect on blood glucose. However, the raw form of the herb might harm kidney, reproductive and cardiovascular system. It may also interact with medicines that help in lowering the blood sugar as it drops blood pressure. The powdered leaf lowers cholesterol and triglycerides and also decreases calorie by providing food and beverages that contains Stevia. Hence, it is helpful in weight control and obesity. In pregnancy, glycoside Reb-A can be used in moderation. Sativoside prevents cancer by boosting death of cancer cells in breast cancer. Also, it decreases mitochondrial pathways. Stevia glycoside does not cause allergic reactions. Stevia can be used instead of table sugar; its pinch is equal to one teaspoon of table sugar. Raw Stevia replaces half of the total amount of sugar. There is no calorie in the pure form of stevia. Reb-A is considered safe for all patients. [8,9] As it does not affect the blood sugar levels, stevia is considered safe for diabetes. It has no neurological side effect and also possesses anti-fungal and anti-bacterial properties. [9]
Dis-advantages:
- They may be a sugar alternative, but natural sweeteners can leave a sour, and pleasant after
- test
- Small amount of sweeteners often produce a sweeter taste than sugar product, ironically living a bad taste in your mouth.
- Most people are able to tolerate natural sweeteners quite well, some may experience an initial period of gastro intestinal in the form of blotting or gas.
- Potential solution to this type of problems is use a variety of natural sugars or even combined then into the same dish.
- If you start to notice any stomach issues , begin
- Lowering the chances of experiencing gas or blotting.
ARTIFICIAL SWEETENERS :
The development of artificial sweeteners began in the late 19th century. The first one to be synthesized was saccharin having a sweetening property of about 300- 600 times than sugar .Aspartame (AS), acesulfame-K and sucralose are other artificial sweeteners. Recent studies have indicated that artificial sweeteners contribute to weight gain despite their lack of calories. [10,11] The different types of artificial sweeteners are discussed in the next sections. Artificial sweeteners used in processed foods are aspartame, acesulfame potassium, sucralose, saccharin, cyclamate, neotame, alitame, rare sugars, xylitol and D-allose (Whitehouse, Boullata and McCauley, 2008).
- Aspartame (AS);
Discovery of AS took place in the year 1965. Since 1974 it has been used as a novel sweetener in the U.S. There were many studies which proved that there was a relationship between A Sand brain toxicity and was also responsible for causing cancer. Further studies failed to prove the same and it was marketed for solid food. [12] Phenylalanine is an amino acid which is essential and it should be obtained from the diet as our body cannot metabolize it. Excess of it is broken and forms fumarate and acetoacetate, both are a part of energy metabolism. There are people who cannot convert phenylalanine to tyrosine due to enzyme inactivity or absence and are unable to metabolize phenylalanine normally. There is a name for such a condition, phenylketonuria. In this disease phenylalanine appears in the urine and hence the name. If this condition is not taken care of in time, then it can lead to problems in the brain. There are concerns related to brain toxicity
Health effects;
The individuals who are suffering from a rare phenylketonuria genetic disorder should avoid intake of aspartame. This caution of intake should be clearly mentioned on packaging of processed foods containing aspartame (FDA). The end products (methanol, aspartic acid and phenylalanine) after metabolism of aspartame causes the following health disorders: severe headache, improper vision, tumours in brain, sight problems, loss of memory and nausea sensation (Stegink, Filer and Baker, 1977).
- Acesulfame K;
It was first discovered in the year 1967. Acesulfame K is sweeter than normal table sugar
(almost 200 times sweeter) and is very low in calories. [13] It is a potassium salt of 6-methyl1,2,3-oxathiazin-4(3H)-one-dioxide. As it is not metabolized in the body it does not affect the potassium level even though it contains potassium. In the year 1988 USFDA approved the use of Acesulfame K in food and non-caloric beverages. A product formed due to the breakdown of ace-k is acetoacetamide which is known to be toxic if taken in large amount. [14] Exposure to the sweetener has caused irritation of the eye in animals. A test conducted in New Zealand has proved that the eye turns red on exposure to acesulfame-K. [15] Many animal studies have proved the fact that AS causes increase in weight. A taste which is sweet causes insulin response but AS does not increase blood sugar level. [16,17]
Health effects;
Methylene chloride which is present in Ace-k is carcinogenic which leads to cancer in human body. Excess intake of this may cause severe headache, depression, nausea sensation, mental disturbance, liver and renal problems. Ace-k forms acetoacetamide in human body upon metabolism, which is highly toxic and causes tumours in thyroid glands of rats, dogs and rabbits. Few studies suggest that only 1% of acetoacetamide is collected in human body for three months (Zeynep and Sifa, 2014).
- Saccharin;
Saccharin was the first artificial sweetener discovered as serendipity by Constantine Fahlberg in 1997. It was discovered during clinical trials that saccharin is linked to bladder cancer in rats.
Saccharin was the first artificial sweetener discovered as serendipity by Constantine Fahlberg in 1997. It was discovered during clinical trials that saccharin is linked to bladder cancer in rats. Saccharin is also known to cause allergic reactions and symptoms such as headache, skin problem, and diarrhoea. [18] The exposure study on saccharin proved its potential to induce cancer in animals (rats and dogs) and also in humans. The study included two generation bioassay and such bioassays are useful for finding the potential effects of substances. The study was done on a group of rats by exposing them to saccharin during their development. [19] Due to the above results saccharin was banned in Canada and a ban was proposed in the U.S. [20] [21]
Health effects;
Excess intake or ingestion of saccharin causes severe headache, difficulty in breathing, eruptions on skin and diarrhoea (Whitehouse, Boullata and McCauley, 2008).
- Sucralose;
It was discovered in the year 1976 and came to be known in the markets as Splenda, Sukrana, Candys, Novella. Sucralose is a molecule containing sucrose where chlorine atoms replace three of its hydroxyl groups. [11] shrunken thymus gland (40%), lymph follicles in thymus and spleen atrophy, decreased RBC count, enlarged liver and kidney, reduced growth rate and migraines etc. [21] The chemical structure of the sucralose contains 6-chloro-6-deoxyglucose which is responsible for causing infertility in rats. It was claimed in Japan that ingested sucralose leads to induction of DNA damage in gastrointestinal organs. [22].
Health effects;
Toxic effects of sucralose include shrinking of thymus glands, diarrhoea and giddiness (Sims, Roberts, Daniel and Renwick, 2000).
- Neotame;
;Neotame was approved by the FDA as a general-purpose sweetener in 2002 [20]. It is not available commercially and itis only used in food manufacturing. It is over seven thousand times sweeter than sucrose. No studies were found that met thecriteria with neotame.
Health effects:
Excess consumption of neotame causes toxicity in liver. few other toxic effects of neotame are change in human body weight, mild, headache, and loss of appetite.
Toxic effects of artificial sweeteners;
. Artificial sweeteners like saccharin, ace-K and aspartame involves in genetic change , especially in DNA of lymphatic cells. Few byproducts produced from sugar substitutes ,cause breakage in DNA strands. They can change the metabolic properties of human body . toxic effects of artificial sweeteners are given in table below.
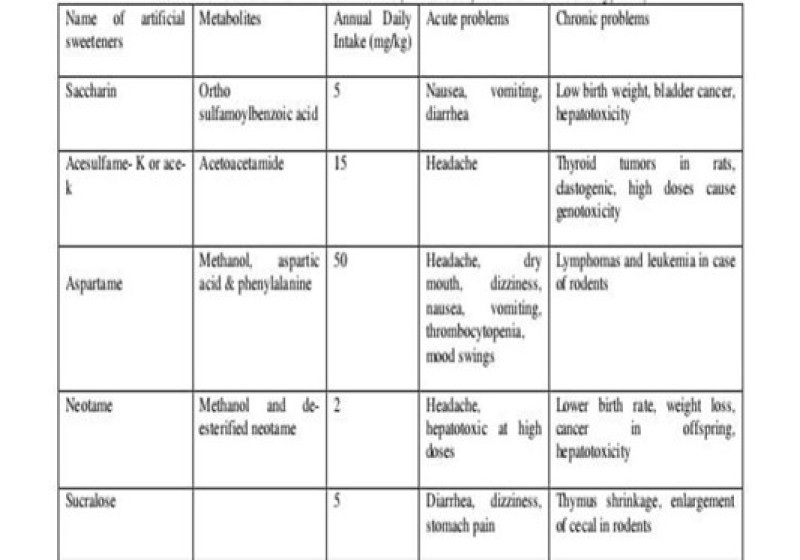
Disadvantages:
- Artificial sweeteners induced DNA damage in human peripheral lymphocytes.
- To cause a decrease in beneficial microorganisms . these degradation products may cause DNA strand breaks.
- They increase of components considered a possibility of gastro intestinal problems
- Aspartame has been thought to cause brain damage because one of its hydrolysed components in phenylalanine.
- Phenylalanine places an important role in a neurotransmitter regulation.

Symptoms of diabetes in sweetening agents;
Polyuria;
- Increased urination due to excess loss of fluid caused by osmotic diuresis.
Polydipsia;
- Increased thirst as a result of fluid loss and dehydration.
Polyphagia;
- Increased appetite resulting from the catabolic state caused by insulin deficiency and breakdown of proteins and fats.
- Fatigue and weakness;
- Feeling tired and lacking energy.
- Sudden vision changes;
- Rapid alterations in visual acuity.
- Tingling or numbness in hands or feet .sensations of pins and needles or loss of sensation in extremities.
Dry skin;
- Lacking moisture and becoming rough or flaky.
- Slow healing skin lesions or wounds. Delayed wound healing and impaired skin regeneration.
- Recurrent infections ;Frequent and persistent infections due to compromised immune infection.
- Nausea, vomiting, or abdominal pain
FORMULATIONS;
Purified water
Sugar (sucrose) or sugar substitutes (artificial sweeteners). Traditionally syrups are composed of sucrose (usually between 60 and 80%) and purified water. Due to the inherent sweetness and moderately high viscosity of these systems, the addition of other sweetening agents and viscosity modifying agents are not required. As the concentration of sucrose is reduced from the upper limit (e.g. through dilution), the addition of preservatives may be required. In some formulations, other non-sucrose bases may replace traditional syrup. One of the most popular is Sorbitol Solution, which contains 64% w/w Sorbitol although other alternatives are available that are based on mixtures of Sorbitol and glycerine. These non sucrose bases may be mixed with traditional syrup. If required, in the formulation of oral syrups that possess a low concentration of sucrose in comparison to traditional syrups. More recently, many products have been formulated as medicated sugar-free syrups due to the glycogenotic and cariogenic properties of sucrose. For the afore-mentioned reasons, all medicinal products designed for administration to children and to diabetic patients must be sugar-free. Syrup substitutes must therefore provide an equivalent sweetness, viscosity and preservation to the original syrups. To achieve these properties artificial sweeteners (typically saccharin sodium, aspartame), non glycogenotic viscosity modifiers (e.g. methylcellulose, hydroxyethyl cellulose) and preservatives (e.g. sodium benzoate, benzoic acid and Para hydroxybenzoate esters) are included. An important class of sugar substitutes is known as high-intensity sweeteners. These are compounds with many times the sweetness of sucrose, common table sugar. As a result, much less sweetener is required and energy contribution is often negligible. The sensation of sweetness caused by these compounds (the "sweetness profile") is sometimes notably different from sucrose, so they are often used in complex mixtures that achieve the most natural sweet sensation.[ 23] In the United States, seven intensely sweet sugar substitutes have been approved for use. They are stevia, aspartame, sucralose, neotame, acesulfame potassium (Ace-K), saccharin, and advantame. Cyclamates are used outside the U.S., but have been prohibited in the U.S. since 1969. There is some ongoing controversy over whether artificial sweetener usage poses health risks [24]. The majority of sugar substitutes approved for use are artificially synthesized compounds. However, some bulk natural sugar substitutes are known, including Sorbitol and xylitol, which are found in berries, fruit, vegetables, and mushrooms. It is not commercially viable to extract these products from fruits and vegetables, so they are produced by catalytic hydrogenation of the appropriate reducing sugar. For example, Xylose is converted to xylitol, lactose to lactitol, and glucose to Sorbitol. Other natural substitutes are known but are yet to gain official approval for food uses. Some non-sugar sweeteners are polyols, also known as "sugar alcohols". These are, in general, less sweet than sucrose but have similar bulk properties and can be used in a wide range of medicinal products. Sometimes the sweetness profile is 'fine-tuned' by mixing with highintensity sweeteners. As with all food products, the development of a formulation to replace sucrose is a complex proprietary process[25] Liquid formulations include solutions, syrups, suspensions and emulsions and are most appropriate for younger diabetic patients (e.g. birth to 8 years) who are unable to swallow capsules or tablets. The dose volume is a major consideration for the acceptability of a liquid formulation. Typical target dose volumes for diabetic liquid formulations are < 5>
Oral Effervescent Dosage Forms ;
Oral effervescent dosage forms include tablets, granules and powders that are dissolved in water prior to administration. Effervescent products are alternatives to liquid dosage forms for substances with insufficient stability in aqueous media. They are also more portable than conventional liquid formulations. The following points should be considered when formulating effervescent dosage forms Effervescent products should always be fully dissolved prior to administration, and large volumes of diluent may be required to do so which can be problematic for children. Therefore, it may be helpful to indicate the minimum volume an effervescent product can be dissolved/dispersed in as well as the solubility of the drug so that fractional doses can be given, if necessary.
- To minimise the possibility of ingesting bicarbonate, children should be instructed not to drink the solution until the effervescence has subsided.
- As effervescent tablets normally contain high sodium and/or potassium ion concentrations, they may not be suitable for all patients, e.g. those with renal insufficiency.
The organoleptic characteristics of chewable tablets that are important to consider include taste, after-taste, odor, flavour, texture, mouthfeel, and visual aesthetics of the product. The oral processing of chewable tablets makes taste-masking a necessity for most formulations. Sweeteners are almost always used and represent the simplest means by which to address taste concerns. Combinations of bulk sweeteners (e.g., sugars or polyols) with high-intensity sweeteners (e.g., aspartame) are common. The relative sweetness of some sweeteners used in chewable tablets . In addition to relative sweetness, the sweetness-response time profile should be considered. For example, monoammonium glycyrrhizinate, while having a slow onset, has a prolonged sweetness. Blends of sweeteners may therefore be used to provide synergistic effects. Some high-intensity sweeteners exhibit a bitter taste or aftertaste with increasing concentration (26), an effect which can also be mitigated by combining two or more sweeteners (27). Flavors are commonly used in chewable tablets. Mint and peppermint flavors are popular in antacid tablets. diabetic products often use fruit-based and bubblegum flavors .Dry powder forms of flavors are preferable as they avoid the loss of volatile aromatic components during drying. For the same reason, direct compression is often preferable for chewable tablet manufacturing. In cases where wet granulation is used, flavors should be added extragranularly. Flavors can be further modified by the addition of agents such as citric acid. When the addition of sweeteners is insufficient to address taste issues, either due to the high dose of the active or its inherent bitterness, other approaches may be used, such as coating, ion- exchange resins, or chemical modification of the drug molecule. Coating and microencapsulation techniques are based on using polymers or lipids to form a physical barrier around particles that prevents unpleasant tasting molecules from coming into direct contact with taste receptors in the mouth. Coated particles are blended with fillers, disintegrants, and lubricants prior to tableting .Polymers that are insoluble in saliva but dissolve in gastric acid (e.g., amino methacrylate copolymer) may be used if an immediate release dissolution profile.
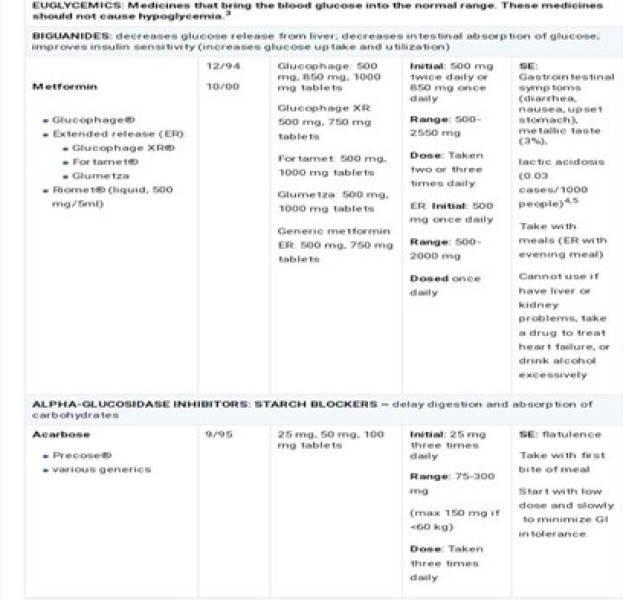
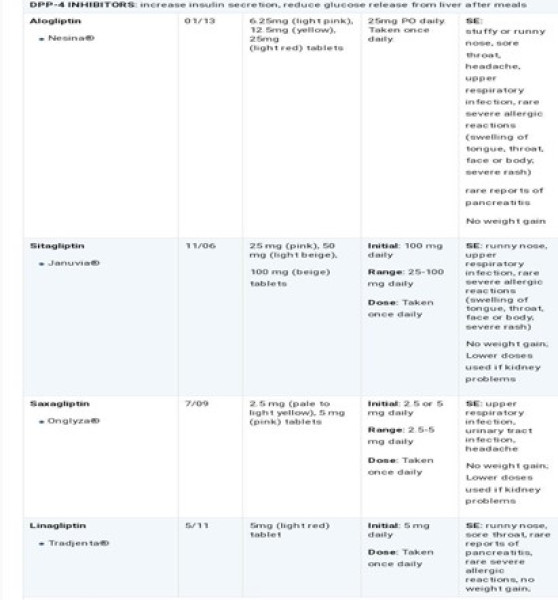
WHO Regulatory Guidelines in sweetening agents;
The World Health Organization (WHO) has released a new guideline on non-sugar sweeteners (NSS), which recommends against the use of NSS to control body weight or reduce the risk of noncommunicable diseases (NCDs). The recommendation is based on the findings of a systematic review of the available evidence which suggests that use of NSS does not confer any long-term suggest that there may be potential undesirable effects from long-term use of NSS, such as an increased risk of type 2 diabetes, cardiovascular diseases, and mortality in adults. “Replacing free sugars with NSS does not help with weight control in the long term. People need to consider other ways to reduce free sugars intake, such as consuming food with naturally occurring sugars, like fruit, or unsweetened food and beverabenefit in reducing body fat in adults or children. Results of the review also ges,” says Francesco Branca, WHO Director for Nutrition and Food Safety. "NSS are not essential dietary factors and have no nutritional value. People should reduce the sweetness of the diet altogether, starting early in life, to improve their health." The recommendation applies to all people except individuals with pre-existing diabetes and includes all synthetic and naturally occurring or modified non-nutritive sweeteners that are not classified as sugars found in manufactured foods and beverages, or sold on their own to be added to foods and beverages by consumers. Common NSS include acesulfame K, aspartame, advantame, cyclamates, neotame, saccharin, sucralose, stevia and stevia derivatives.
FDA Regulatory Guidelines in sweetening agents :
The FDA is aware of the International Agency for Research on Cancer (IARC) and Joint FAO/WHO Expert Committee on Food Additives (JECFA) conclusions about aspartame issued July 14, 2023. Aspartame being labelled by IARC as “possibly carcinogenic to humans” does not mean that aspartame is actually linked to cancer. The FDA disagrees with IARC’s conclusion that these studies support classifying aspartame as a possible carcinogen to humans. FDA scientists reviewed the scientific information included in IARC’s review in 2021 when it was first made available and identified significant shortcomings in the studies on which IARC relied. We note that JECFA did not raise safety concerns for aspartame under the current levels of use and did not change the Acceptable Daily Intake (ADI). Aspartame is one of the most studied food additives in the human food supply. FDA scientists do not have safety concerns when aspartame is used under the approved conditions. The sweetener is approved in many countries. Regulatory and scientific authorities, such as Health CanadaExternal Link Disclaimer and the European Food Safety AuthorityExternal Link Disclaimer have evaluated aspartame and also consider it safe at current permitted use levels.
Sweetness Intensity of Sweeteners Compared to Table Sugar ;
The image below shows the sweetness intensity of sweeteners compared to table sugar or sucrose

CDSCO Regulatory guidelines in sweetening agents;
Consideration of the question to ban the use of Saccharin in diabetic preparations. The Director, CIPL, Gha/.ibid informed that in the Fourth edition o f I.P. the cautionary remarks has been included under the monograph Saccharin that “ not to be allowed in paediatric preparations”. Item No. 3
SUMMARY :
The effect of artificial sweeteners on human metabolism and their role of diabetes is controversial amongst the research community and its educators. Of the five artificial sweeteners discussed ; aspartame , saccharin, acesulfame, neotame , sucralose, the FDA approach and supports their uses when consumed under the recommended guidelines. (34). Artificial sweeteners may not be a healthy alternative, has noted by a research study that show a gradient risk after many years of consumption and per the quantity of artificial sweeteners are consumed each day (34,35] consumption of artificial sweetener above the recommended FDA guideline may have catastrophic effects and may play a larger role any development of obesity , leading to diabetes(36) both health care professionals and individuals with diabetes can benefit from learning more about artificial sweetener to help make informed decisions about their uses based on available evidence,(34). Has various limitations exit in clinical study design furtheploration is required with well design large scale studies in the general population to perhaps determine artificial role in diabetes[36].
CONCLUSION :
Group a patients who consumed artificial sweetening agents had higher in insulin resistance has compared to group B patients. However , further studies are required to conclude a direct correlation of artificial sweetener with decreased insulin sensitivity. Artificial sweetener has been reported to be associated with and increased risk of weight gain, obesity, and type2 diabetes. Further researching the adequate health and nutrition reference standards needed for their quantity and health complications.
REFERENCES
- AH.Barnett Type 2 Diabetes. 2nd ed. Oxford University Press; 2012. p. 162.
- E Loghmani – ‘Guidelines for adolescent nutrition services’, 2005; 167-81
- D. Daneman, Type 1 diabetes, Lancet 367, (2006), 847–58
- M. A. Atkinson, G.S. Eisenbarth, A.W.Michels, Lancet,383, (2014), 69–82.
- R. Taylor, ‘Type 2 diabetes- Etiology andReversibility’, Diabetes care, 36, (2013), 1047-55
- A.Hussain..Lin, Poveda V, Plant derived sweetening agents: Saccharides and polyolconstituents of some sweetening plants. J. Ethnopharmacology. 28, 1990, 103-11
- jprsolutions.info/newfiles/journal-file-56e3aa1b78a4b5.63523461. (accessed on Dec 12, 2018)
- www.healthline.com/health/food-nutrition/stevia-side-effects, overview(accessed on Dec 12, 2018)
- www.medicalnewstoday.com/articles/287251.php. (accessed on Dec28, 2018
- J.C. Maloney, Sugar and Sweeteners. Colorado State University ,6, 2016.
- S.L. Roberts, (2011). Sugar substitutes. 2
- www.greenfacts.org/en/aspartame/aspartame-greenfacts-level2.pdf
- https://caloriecontrol.org/ (accessed Nov. 6,2017).
- S. Chattopadhyay, U. Raychaudhuri, R. Chakraborty, Artificial sweeteners – a review. J. Food Sci. Technol. 51 (4), (2014), 611-621.
- WHO Food Additive Series 28: Acesulfame Potassium (1990). (www.inchem.org/documents/jecfa/jecmono/v28je13.htm accessed on April 8, 2018)
- S.E. Swithers T.L. Davidson, A role for sweet taste: Calorie predictive relations in energy regulation by rats, Behav Neurosci. 122 (2008), 161–73.
- T. Hampton, Sugar substitutes linked to weight gain. JAMA 299, (2008), 2137–8.
- A. Ferland, P. Brassard, and P. Poirier, Is aspartame really safer in reducing the risk of hypoglycemia during exercise in patients with type 2 diabetes? Diabetes Care, 30(7), (2007), 59.
- D.L. Arnold, Toxicology of saccharin. Fund and Applied Toxicol. 4(5), (1984), 674- 685.
- C.R. Whitehouse, J. Boullata, and L.A. McCauley, The potential toxicity of artificial sweeteners. Aaohn Journal, 56(6), (2008), 251-261..
- J. Bowen, Splenda is not splendid! 2003. (accessed 2 December 2018).
- Y.F. Sasaki, S. Kawaguchi, A. Kamaya, M. Ohshita, K. Kabasawa, K. Iwama, K. Taniguchi, S. Tsuda S. The comet assay with 8 Shwide-Slavin C, Swift C, Ross T. Nonnutritive sweeteners: Where are we today? Diabetes Spectrum.2012; 25: 104-10.
- D Jones; Dosage form and Design pharmaceutical press, London, Chicago, 2012;17-18
- "High-Intensity Sweeteners". U.S. Food and Drug Administration. May 19, 2014.
Retrieved September 17, 2014.
- http://en.wikipedia.org/wiki/Encyclopedia
- S.S. Schiffman et al., Brain Res. Bull. 36 (5) 505-513 (1995).
- M. Behrens, K. Blank, and W. Meyerhof, Cell Chem. Biol. 24 (10) 1199-1204 (2017).
- Avicel CE-15 www.pharma.dupont.com/pharmaceutical-brands/avicelr-forsuspensions.html, accessed April 14, 2020.
- S. Kimura et al., Int. J. Pharm. 484 (1-2) 156-162 (2015).
- K. Dziemidowicz et al., AAPS PharmSciTech. 19, 2646–2657 (2018).
- M. C. Ambros et al., Pharm. Dev. Technol. 3 (4) 509-515 (1998).
- M. Lanz et al., Drug Dev. Ind. Pharm. 40 (12) 1623-1631 (2014).
- N.Gupta, Chidambaram, and M. A. Khan, Drug Dev. Ind. Pharm. 41 (2) 239-243 (2015).
- F.Imamura O’Connor L, Ye Z, et al. Consumption ofsugar sweetened beverages, artificially sweetenedbeverages, and fruit juice and incidence of type 2 diabetes:Systematic review, metaanalysis, and estimation ofpopulation attributable fraction BMJ. 2015; 351:1-12.
- ssG. Fagherazzi Gusto G, Affret A, et al. Chronicconsumption of artificial sweetener in packets or tabletsand type 2 diabetes risk: Evidence from the E3N-Europeanprospective investigation into cancer and nutrition study.Ann Nutr Metab. 2017; 70:51-58.
- KR.Tandel . Sugar substitutes: Health controversy overperceived benefits. J Pharmacol Pharma other. 2011; 2:236- 43.


 B.V.Ramana*
B.V.Ramana*







 10.5281/zenodo.10798404
10.5281/zenodo.10798404