Abstract
A monoclonal antibody called dostarlimab (TSR-042) targets the checkpoint protein programmed death-1 (PD-1), which prevents T-cell activation. Dostarlimab improves the immune system’s capacity to identify and combat cancer cells by inhibiting PD-1. The preclinical and clinical development of dostarlimab as an Immunotherapeutic drug is examined in this study, with an emphasis on its application in the treatment of advanced cancers, including endometrial cancer, solid tumors lacking mismatch repair, and other cancers. We go review the results of important clinical trials, including its effectiveness, safety, and possible benefits over competing PD-1 inhibitors.In order to further maximize treatment outcomes, the study also highlights current research examining combination treatments and biomarker-driven patient selection. Although dostarlimab’s full clinical impact will depend on long-term data and wider implementation in a variety of cancer types, its encouraging profile highlights its potential as a game-changing medication in oncology.
Keywords
Dostarlimab, PD1 immunoglobulin, Endometrial cancer, Rectal cancer.
Introduction
Cancer immunotherapy has revolutionized the treatment landscape for many malignancies by harnessing the body’s immune system to target and eliminate cancer cells. One of the most significant advances in this field has been the development of immune checkpoint inhibitors, which block regulatory proteins that prevent immune cells from attacking tumor cells. Among these, antibodies targeting the programmed cell death-1 (PD-1) receptor have emerged as a cornerstone of modern cancer therapy, demonstrating clinical efficacy across a range of cancers, including melanoma, non-small cell lung cancer (NSCLC), and more recently, endometrial cancer. Dostarlimab (TSR-042), a novel humanized monoclonal antibody that targets PD-1, has gained attention for its promising clinical outcomes and manageable safety profile. Mismatch repair-deficient (dMMR) and microsatellite instability-high (MSI-H) tumors are a subset of cancers that respond especially well to immunotherapy. Dostarlimab was first created to treat these tumors. In solid tumors where alternative treatment choices have been limited, such as colorectal and endometrial cancer, early-phase clinical trials have shown that it can provide long-lasting responses (Chavez et al., 2020; Le et al., 2020). Dostarlimab has demonstrated considerable promise in monotherapy settings, in contrast to several PD-1 inhibitors, indicating that it might provide therapeutic32 benefits for patients with particular molecular . The purpose of this study is to give a summary of dostarlimab's preclinical and clinical development while assessing its safety, effectiveness, and possible role in cancer treatment. Its mode of action, current clinical trials, and the changing function of PD-1 inhibitors in precision oncology will also be covered.
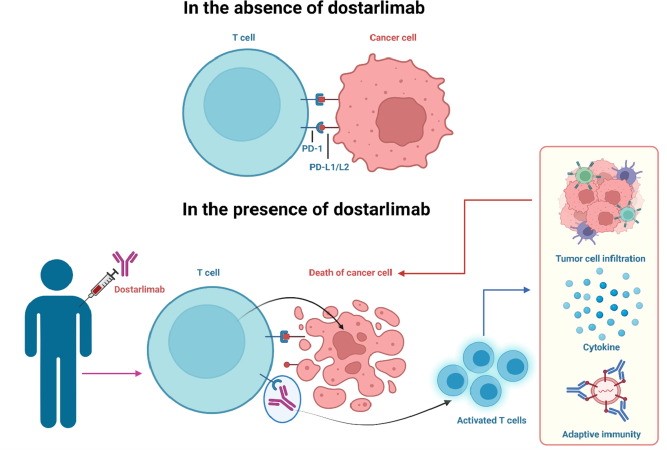
Fig 1 :-Promise of dostarlimab in cancer therapy: Advancements and cross-talk considerations
|
Monoclonal antibody
|
|
|
Type
|
Whole antibody
|
|
Source
|
Humanised
|
|
Target
|
PDCD 1
|
|
Clinical data
|
|
|
Trade name
|
Jemperli
|
|
Other names
|
TSR-042,WBP-258
Dostarlimab-gxly
|
|
Route of administration
|
Intravenous
|
|
Drug class
|
Antineoplastic
|
|
ATC code
|
L01FF07(WHO)
|
|
Clinical and Physical Data
|
|
|
Formula
|
C6420H9832N16901O2014S44
|
|
Molar masss
|
144325.73 g/mol
|
The many inactive substances include water for injection, sodium chloride, sodium citrate, dihydrate, citric acid, monohydrate, and polysorbate 80. Rectal cancer is a type of tumor that develops in the lower portion of the digestive system. The medication exhibits high bioavailability since it circumvents metabolism. Approved for use in patients with recurring cancers in August 2021. For six months, individuals with stage II or III rectal adenocarcinoma that lacked mismatch repair received a single dosage of dostarlimab every three weeks. Standard chemotherapy, radiotherapy, and surgery were to be administered after this treatment.
Dostarlimab And Its Development:-
GlaxoSmithKline (GSK) created the unique humanized IgG4 monoclonal antibody dostarlimab (JemperliTM), which functions as a PD-1 antagonist. It has a molecular mass of 144 KDa and is produced in CHO cells using the recombinant DNA method. It has a strong affinity for the PD-1 receptor on T cells (KD = 300 pM). The PD-1 receptor’s interaction with the ligands is blocked as a result of this binding, which in turn activates T-cells, increases immunity, and stops cancer cells from evading the T-cells’ immune response. Although it is marketed under the Jemperli brand, dostarlimab is also known by the names TSR-042, WBP-285, and dostarlimab-glxy. IgG4 is Jemperli’s isotype.A mouse hybridoma was used to create the humanized mAb, which functions as a PD-1 receptor antagonist in a variety of cancer therapeutic approaches. The Jemperli single dose vial contained 500 mg of Jemperli in 10 mL of solution along with 50 mg of citric acid monohydrate (0.48 mg), sodium chloride (1.81 mg), trisodium citrate dehydrate (6.68 mg), L-arginine hydrochloride (21.07 mg), Dostarlimab-glxy, polysorbate 80 (0.2 mg), and injection water (USP.29–31). Jemperli (Dostarlimab) is a single-dose vial of a disinfected, yellow solution that is clear to slightly opalescent and devoid of suspension. With the molecular weight of 144.2 kDa (non-glycosylated) and the chemical formula C6420H9832N1680O2014S44 (nonglycosylated), it is a member of the antineoplastic medication class.Adult patients with MMRd endometrial carcinoma and recurring or advanced solid tumors can now get dostarlimab thanks to approval in the US and EU. Until the disease develops or there is medication toxicity, dostarlimab should be administered intravenously (IV) at a dose of 500 mg every three weeks (Q3W) for four cycles, and then 1000 mg every six weeks (Q6W). 150 patients with endometrial cancer had their dostarlimab pharmacokinetic characteristics assessed. Dostarlimab is an ICI medication that was produced from mouse monoclonal antibodies utilizing somatic hypermutation-based technology (SHM-XEL). With 12 intra-chain and 4 inter-chain disulfide bonds, it is a glycosylated homodimer comprising two identical light and heavy chains. Light chains in this medication are responsible for steric inhibition of PD-L1 binding, whereas heavy chains contribute to the binding affinity between PD-1 and dostarlimab. Each of the two heavy chains has a serine to proline substitution (S228P) to improve the stability of disulfide bonds between them. The chain that is both hefty and lightDostarlimab was humanized by using a complement-determining segment on the germline variable section framework of their closest CONTACT human species orthologs.34 Figure 2 provides a brief overview of Dostarlimab's preparation and key characteristics. Dostarlimab is a PD-1 inhibitor that stops ligands from attaching to receptor proteins on T-cell surfaces. In a humanized mouse model system, dostarlimab demonstrated anticancer effectiveness with decreased tumor growth and increased immune cell infiltration, however in mice, it showed no affinity for PD-1.
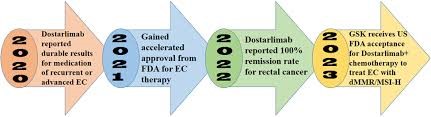
Fig 2:-Annual progress in Dostarlimab status.

Fig 3 :- Preparation and attributes of Dostarlimab
Dostarlimab's PK profile indicates that its exposure is contingent on clinically indicated dosages and did not exhibit any differences according to hepatic impairment, age, sex, ethnicity, or tumor type. Given that dostarlimab primarily targets the defense system throughout its activity rather of directly engaging with malignant cells, this result may be explained by the drug's mode of action.34,39,40 With a clearance rate of 0.007 l/h and an average terminal elimination half-life of 25.4 days, dostarlimab is metabolized by a catabolic mechanism.40 Additionally, dostarlimab has been tested for a variety of cancer types, including ovarian carcinoma, fallopian tube tumors, pancreatic carcinoma, squamous cell lung cancer (SCLC), and non-squamous cell lung cancer (NSCLC).
Pharmacological Profiles:-
A humanized monoclonal antibody called dostarlimab prevents the ligand PD-L1 from activating the programmed cell death protein-1 (PD-1) receptor. Immune responses specific to malignancy are suppressed by the checkpoint receptor PD-1.
Dostarlimab Mode Of Action:-
Dostarlimab is an approved humanized programmed death (PD-1) receptor.The source of these entire antibodies is humanized, which refers to antibodies derived from non-human animals whose protein sequences have been altered to make them more comparable to antibody varieties that humans naturally create. PDCD1, or program cell death protein 1, is the drug’s target. Jemperli is an antibody that blocks the programmed death receptor-1 (PD-1). The PD-1 receptor on T cells is bound by the PD-1 ligands PD-L1 and PD-L2, which prevents T cell proliferation and cytokine production. Certain cancers exhibit upregulation of PD-1 ligands, and signaling via this route may help to prevent active T cell immune surveillance of malignancies. By binding to the PD-1 receptor and preventing its interaction with PD-L1 and PD-L2, dostarlimab-gxly, a humanized monoclonal antibody of the IgG4 isotype, releases PD-pathway-mediated suppression of the immune response, including the anticancer immune response. When the PD-1 ligands PD-L1 and PD-L2 attach to the T cell PD-1 receptor, the receptor is activated. T cell growth and cytokine generation are inhibited. Since PD-1 ligands are elevated in certain cancers, signaling via this pathway may help to inhibit T-cell immunological responses. 7. Keeping an eye on cancers— One humanized IgG4 monoclonal antibody is dostarlimab. An isotype that binds to the PD1 receptor and prevents it from connecting with the PD-L1 and PD-L2 receptors releases immune response repression mediated by the PD-1 pathway, including the immune system's antitumor response. By preventing PD-1 activation, tumor development is inhibited in syngeneic animal tumor models. Using the body’s defenses against cancer, immunotherapy is one of the newer forms of cancer treatment. Dostarlimab, a member of the ICI medication class, stimulates the immune system by preventing T-cell surface PD-1 receptor protein from interacting with PD-L1. The body’s defense system is a complex network of organs, cells, and proteins that work together to protect the body against various disease-causing agents.44–46 When it comes to triggering an immunological response, lymphocytes are essential. B-cells and T-cells are two types of lymphocytes. Bone marrow produces and matures B-cells, sometimes referred to as the immune system’s memory, whereas bone marrow produces T-cells, which subsequently migrate toward the thymus gland to mature
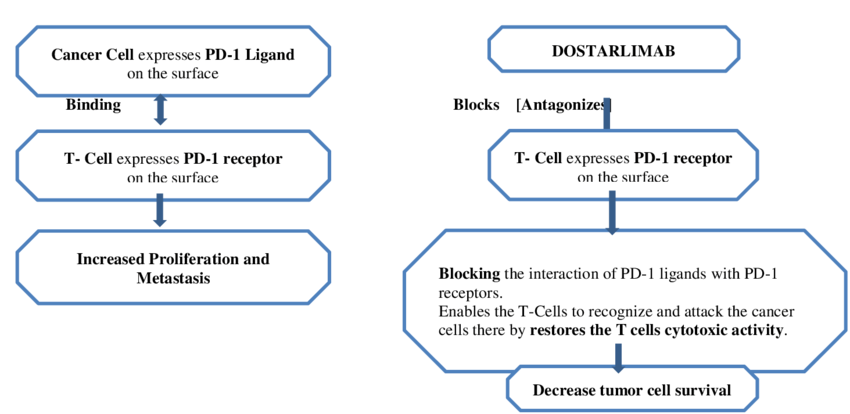
Fig4: Mode of Action of Dostarlimab
By improving effector T-cell function, dostarlimab, a strong anti-PD-1 receptor antagonist that contains humanized monoclonal antibodies of the IgG4 isotype, helps the body regain immunity against tumor cells. The literature review states that dostarlimab functions by attaching itself to PD-1 and preventing it from interacting with PD-L1. As shown in Figure 3, an inhibitory signal is prevented and T-cells successfully target and eliminate cancer cells by disrupting the PD-L1/PD-1 connection. The body's immunological reaction is advantageous in this situation. Normally, when bacteria or any other germ enter the body, the immune system detects it and the B-cells are stimulated to manufacture antibodies by the supporting T-cells. The presence of antigens on the invaders' surface makes this identification possible.48 The antibody's constant end adheres to killer T-cells, while its variable end aids in the destruction of harmful cells by T-cells.49 On the other hand, malignant cells use a ligand called PD-L1 on their surface to trick the immune system. Normal cells aren't tumor cells. The T-cell surface has the PD-1 receptor, which aids in the immune system's healthy operation. By decreasing effector T-cell activation through a decrease in proliferation, cytokine production, and cytotoxic activity, the interaction between PD-1 and PD-L1 sends inhibitory signals to T-cells.
Dose And Adminstration:-
Dostarlimab-gxly injection is a liquid solution that a medical institution can administer intravenously (via a vein) over the course of 30 minutes. Typically, it is administered once every three weeks for four cycles, and then once every six weeks for as long as the doctor prescribes. Jemperli is provided as an intravenous injection. The dosage that is advised Is for four doses, patients got an intravenous infusion of 500 mg of Jemperil every three weeks. After that, they received 1000 mg every six weeks until the disease progressed or the toxicity became intolerable. According to a blinded impartial central evaluation, the results displayed the overall response rate and response duration. The findings indicate that, of the 71 evaluable responses, the total response rate was 42.3%, with a complete response rate (CR) of 12.7% and a partial response rate (PR) of 29.6%.
In a medical institution or infusion center, a doctor or nurse will provide dostarlimab-gxly injection as a solution, which is a liquid, intravenously, meaning into the vein over a 30-minute period. Dostarlimab-gxly received accelerated FDA approval for the treatment of adult patients. Until the fourth dose, dostarlimab 500 mg should be given every three weeks. Dosage schedule: 1,000 mg every six weeks after the fourth dosage, beginning three weeks later (Doe 5 forward). It is recommended to administer dostarlimab intravenously for 30 minutes using either 5?xtrose solution or normal saline.
Chemistry Of Dostarlimab :-
A monoclonal antibody called dostarlimab, often referred to as Jemperli, is used to treat endometrial cancer.
a. Other names: TSR-042, WBP-285, dostarlimab-gxly
b. Drug class: Antineoplastic
c. Formulae: C6420H9832N1690O2014S44
d. Molar mass: 144325.73 g·mol?1
Structural Formula:-
A humanized IgG4 monoclonal antibody is called dostarlimab. With 12 intra-chain disulfide links and 4 inter-chain disulfide bonds, it is a glycosylated homodimer made up of two identical heavy and two identical light chains. Dostarlimab's physicochemical characteristics include being a clear to slightly opalescent, colorless to yellow solution that is virtually particle-free.
Phamarcokinetic :-
500 mg of dostarlimab is administered intravenously every three weeks. During the first cycle, dostarlimab-gxly’s mean maximum concentration (Cmax) was 171 mcg/mL, and its AUC (0-tau) was 35,730 mcg.h/mL. At 1000 mg every six weeks, the average Cmax and AUC (0-tau) are 309 mcg/mL and 95,820 mcg.h/mL, respectively. The terminal elimination half-life of dostarlimab is 25.4 days on average. Its exact metabolism is unknown, although catabolic processes are expected to break it down into smaller peptides and amino acids.
Pharmacodynamic:-
Dostarlimab coupled to human and cynomolgus monkey PD-1 with high affinity, according to surface plasmon resonance, flow cytometry using cell lines overexpressing recombinant PD-1, or binding to the natural protein on peripheral blood mononuclear cells (PBMC). Additionally, PD-L1 and PD-L2 were unable to bind with the receptor due to the antibody. Dostarlimab functioned as a potent functional antagonist in a human CD4+ mixed lymphocyte response assay, leading to increased IL-2 production. Dostarlimab’s activity in this test was enhanced by the addition of anti-TIM3 or anti-LAG3 antibodies. When human PBMCs were incubated with dostarlimab alone, there was no discernible activation of cytokine release.
Adverse Effects Of Dostarlimab:-
In addition to the intended effects, a medicine may have unintended side effects. Not all of these adverse effects are expected to happen, but if they do, you might need to see a doctor. Reduced back or side pain, muscle cramps and stiffness, pale skin, slow heartbeat, sore tongue, difficulty breathing, unusual bleeding or bruising, unusual weakness or fatigue, weight gain, constipation, depression, difficult, burning, or painful urination, dry skin and hair, feeling cold, frequent urge to urinate, hair loss, hoarseness or husky voice, and loss of appetite are some of the side effects that have been observed during treatment.Anxiety, irritability, lethargy, muscle twitches, nausea, nervousness, seizures, chest pain or tightness, chills, coma, confusion, coughing up mucus, decreased urine output, diarrhea, dizziness, fever, overall feeling of being ill, sweating, facial, foot, lower leg, ankle, or hand swelling, thickening of bronchial secretions, and difficulty breathing. “Tenderness in the back or legs, black or bloody stools, gum disease, edema, blue or pale skin, blurred vision, burning, tingling, or pain in the hands, arms, feet, or legs, a burning feeling in the chest or abdomen, a change in vision, chest pain that may radiate to the left arm, neck, or shoulder, black urine, darkening of the skin, fatigue, dry mouth, eye pain, fainting, fast heartbeat, edema throughout the body, and difficulty moving the
"arms or legs, indigestion, joint pain, light-colored stools, light headedness, loss of consciousness, decreased energy, muscle cramps, anguish, tenderness, or frailty, bloody noses, tingling or numbness in the fingers, face, or feet, pains in the lower abdomen, side, or abdominal muscles, a severe headache, skin rash, erythema, soreness, or pruritus, lesions, welting, or blisters, stabbing pain, neck stiffness or back, stomach discomfort or upset, sudden numbness and weakness in the arms and legs, possibly radiating to the back, rapid, shallow breathing, pinpoint red spots on the skin, redness of the eye, pins and needles, and swollen, painful lymph nodes."
Distribution, Elimination And Metabolism Of Dostarlimab:-
Dostarlimabis’s mean (% CV) volume of distribution in steady state is 5.3 L, or 12%. Dostarlimab has a terminal elimination half-life of 25.4 days and a mean (% CV) clearance of 0.007 L/h (31%), which is anticipated to be broken down into small peptides and amino acids by catabolic mechanisms in the case of metabolism.
Contraindications:-
|
Adverse reaction
|
severity
|
|
Hepatitis with no tumor involvement of the liver
|
AST or ALT increases to more than 3 and upto 8 times ULN or total bilirubin increase to more than 1.5 and up to 3 times the ULN
|
|
Hepatitis with tumor involvement of the liver
|
Baseline AST or ALT is more than 1 and up to 3 times ULN and increases to more than 5 and up to 10 times ULN
|
|
Nephritis with renal dysfunction
|
Increase blood creatinine
|
This medication should be avoided in the following circumstances. If you experience any of the following symptoms, you should see a doctor: An overactive thyroid gland, low thyroid hormone levels, type 1 diabetes, interstitial pneumonitis, hepatitis, an inflammatory condition of the liver, an inflammatory condition of the kidney, an inflammatory condition of the pituitary gland, high blood sugar, pregnancy, a patient who is nursing or nursing, an overactive thyroid gland, and low thyroid hormone levels.
AST- aspartate aminotransferase
ALT- alanine aminotransferase
ULN- upper limit of normal
Effecacy: The median age of the patients was 66 years old (range 40-83), and the majority were white (92.5%) and male (59.7%). Ninety-four percent of patients had stage IV illness, and the majority (77.6%) had an ECOG performance level of one. The majority of patients (92.0%) with nonsquamous histology had adenocarcinoma, while 25.4% of patients had squamous histology at diagnosis. Ten patients had more than three previous lines of treatment, 16 patients had two prior therapies, and the majority of patients had only one prior anticancer therapy.
|
Characteristics
|
All patient
|
|
Median age, years(range)
|
66(40-80)
|
|
<65>
|
40
|
|
< or>
|
37
|
|
Male
|
40
|
|
Female
|
27
|
|
White/black
|
60/1
|
Toxicity Of Dostarlimab :-
“Pneumonitis (symptoms include coughing or shortness of breath), colitis (symptoms include diarrhea; cytomegalovirus infection/reactivation has occurred in patients with corticosteroid-refractory immune-mediated colitis), hepatitis, endocrinopathies, and nephritis were the most common immune-mediated adverse reactions, which also occurred in less than 5% of the patients.
Clinical Trials Testing The Combination Of Drug Dostarlimab With Other Therapies:-
|
Target population
|
Combination
|
Clinical trial
|
|
Endometrial cancer
|
Dostarlimab and niraparib
|
NCT03016338
|
|
Head and neck cancer
|
Dostarlimab and niraparib
|
NCT04313504
|
|
Localised unresectable adult primary liver cancer
|
Dostarlmab and TSR-022
|
NCT03680508
|
|
Melanoma stage III or IV
|
Dostarlmab and TSR-022
|
NCT04139902
|
|
Endometrial or ovarian carinosarcoma
|
Dostarlimab and niraparib
|
NCT03651206
|
|
Recurrent ovarian cancer
|
Dostarlimab and niraparib
|
NCT03806049
|
|
Stage III or IV nonmucinous
|
Standard of care ± Dostarlimab and niraoarib
|
NCT03602859
|
|
Advanced (unresectable)or metastatic solid tumor
|
Dostarlmab and TSR-022 (anti-TIM-3)
|
NCT02817633
|
|
Advanced (unresectable)or metastatic solid tumor
|
Dostarlimab and anti-LAG-3
|
NCT03250832
|
|
Mainly NSCLC or any other metastatic cancer
|
Platinum-based doublet chemotherapy, bevacizumab and niraparib, and the combination of dostarlmab and TSR-022
|
NCT03307785
|
|
Recurrent ovarian cancer
|
Dostarlimab, niraparib and bevazizumab
|
NCT03574779
|
|
Advance and metastatic NSCLC
|
Niraparib + pembrolizumab/dostarlimab
|
NCT03308942
|
|
Ovarian advanced cancer
|
Dostarlimab and niraparib
|
NCT03955471
|
|
Triple negative breast cancer
|
Dostarlimab and niraparib and radiation therapy
|
NCT04837209
|
|
Advanced nonsmall cell lung cancer
|
Dostarlimab and Cobolimab
|
NCT04655976
|
|
Non-squamous nonsmall cell lung cancer with metastases
|
Dostarlimab and chemotherapy (pemetrexed,cisplantin, and carboplantin)
|
NCT04581824
|
|
Relapsed/ Refractoy multiple myeloma
|
Dostarlimab and belantamab mafodotin
|
NCT04126200
|
Clinical Trials:-
Dostarlimab in monotherapy: The largest open-label, multi-center, I-phase clinical research investigating dostarlimab monotherapy for advanced solid tumors is called GARNET. Part 1 of the study, which started in March 2016, examined the safety, pharmacokinetics, and pharmacodynamics of increasing intravenous doses of dostarlimab at weight-based, ascending doses of 1 mg/kg, 3 mg/kg, and 10 mg/kg until the maximum tolerated dose of 20 mg/kg was reached. Part 2 is composed of two subparts. Part 2A evaluates the safety and tolerability of dostarlimab at fixed dosages. Its clinical activity in five cohorts (A1, A2, E, F, and G) of participants with particular types of advanced solid tumors—dMMR/MSI-H EC (A1), MMRp/MSI-H EC (A2), non-endometrial dMMR/MSI-H and POLE mutant cancers (F), and platinum-resistant ovarian cancer BRCA-wild type (G)—is examined in Part 2B. The 500 mg dose is administered every three weeks (Q3W) or 1,000 mg every six weeks (Q6W). Patients with advanced or recurrent EC in groups A1 and A2 who had not previously received immune checkpoint inhibitor (ICI) therapy and who had advanced on or after platinum-based chemotherapy were included in the study. Additionally, detectable illness at baseline verified by blinded independent central review (BICR) was a crucial inclusion criterion. Every histological subtype was suitable, with the exception of sarcoma and carcinosarcoma. Patients were given 500 mg Q3W in the second section for the first four cycles, followed by 1,000 mg Q6W. Immuno-related Response Evaluation Criteria in Solid Tumors (irRECIST) was used to measure anti-tumor activity. The duration of response (DOR) and objective response rate (ORR) were set as the main goals. First released in 2018 and 2019, the preliminary findings were then published again in 2020. The most recent findings, which comprised the largest patient group under study, were finally presented in early 2022.
Uses In Special Populations
Before administering dostarlimab, the patient’s physician or pharmacist should ascertain whether the patient has any other allergies or is allergic to the drug. This product contains inactive chemicals that may cause allergic reactions or other problems. Prior to prescribing this drug, the physician or pharmacist should confirm the patient’s medical history, especially about organ transplants, stem cell transplants using donor cells, pregnancies, or plans to get pregnant. Because dostarlimab may harm the fetus, patients should avoid getting pregnant while taking it. It is best to request a pregnancy test prior to beginning medication. A trustworthy method of birth control should be used both during the course of this medicine and for four months after treatment ends.You should call the doctor immediately if you become pregnant. Because of the possible risks to the unborn child, breastfeeding is not recommended while taking this medicine or for four months after stopping therapy. A doctor should be consulted before starting nursing.
- Pregnancy: Because of the way it functions, JEMPERLI may be hazardous to the fetus if taken by a pregnant woman. Information regarding the use of JEMPERLI in pregnant women is currently unavailable. Blocking the PD-1/PD-L1 pathway increases the risk of immune-mediated embryonic rejection, which can cause fetal death, as shown in animal studies (see Data). Because human IgG4 immunoglobulins (IgG4) are known to cross the placental barrier, dostarlimab-gxly may also do so. Inform moms of the danger to a fetus. The estimated background risks of miscarriage and major birth defects in clinically recognized pregnancies are 15–20 percent and 2-4 percent, respectively.
- Animal data: “No research has been done on how JEMPERLI affects fetal development and reproduction in animals. The mother’s immune tolerance to the fetus is maintained by PD-1/PD-L1 signaling, which is necessary for the pregnancy to continue. JEMPERLI treatment during pregnancy may increase the risk of abortion or stillbirth since suppression of PD-L1 signaling has been shown to decrease tolerance to the fetus and increase fetal loss in murine models of pregnancy. PD-1 and PD-L1 17 knockout mice had immune-mediated issues, in contrast to research in the literature that found that the suppression of PD-1/PD-L1 signaling resulted in defects in these animals’ offspring. Dostarlimab-gxly’s mode of action suggests that fetal exposure may change the immune system’s normal response or increase the risk of immunological-mediated illnesses.
- Lactation: Dostarlimab-presence gxlys in human milk, its impact on breastfed children, and its influence on milk production are all unknown. Because of the potential for severe adverse effects in a breastfed infant, women are recommended not to breastfeed throughout treatment and for four months after the final dosage of JEMPERLI.
- Elderly patient: 50.7% of the 515 patients who received dostarlimab monotherapy were younger than 65, 37.9% were between 65 and 75, and 11.5% were older than 75, per the study. Overall, the study found no differences between younger patients (those under 65) and older patients (those over 65).
CONCLUSION:-
By binding to the PD-1 receptor and preventing it from interacting with PD-L1 and PD-L2, dostarlimab, a humanized monoclonal antibody of the IgG4 isotype, suppresses the PD-1/PD-L1 immune response, including the anticancer immune response, via the PD-1 pathway. The public should have access to dostarlimab and a medical team that can monitor patients, like in the trial NCT04165772, and take appropriate treatment if the tumor returns. We are confident that we are headed in the correct direction to find a dramatic match for the other tumors because dostarlimab has a strong effect on patients with rectal cancer. We think that an approach based on cancer type and subtype will be the way that cancer is treated in the future
REFERENCES
- Chavez, R. J., et al. (2020). Dostarlimab in advanced mismatch repair–deficient or microsatellite instability–high solid tumors: A phase 1 study. Journal of Clinical Oncology, 38(15), 1635-1643.
- Le, D. T., et al. (2020). Pembrolizumab for microsatellite instability–high advanced colorectal cancer. New England Journal of Medicine, 372(26), 2509-2520.
- https://searchusan.amaassn.org/usan/documentDownloadUri=/unstructured/binary/usan/dostarlimab.pdf (2017).
- Jeong T-J, Lee H-T, Gu N, et al. The high-resolution structure reveals remarkable similarity in PD-1 binding of Cemiplimab and Dostarlimab, the FDA-approved antibodies .For cancer immunotherapy. Biomedicines 2022; 10: 3154.
- Kumar S, Ghosh S, Sharma G, et al. Preclinical characterisation of dostarlimab, a therapeutic anti-PD-1 antibody with Potent activity to enhance immune function in in vitro celluLar assays and in vivo animal models. mAbs 2021; 13:1954136.
- Sullivan MR, Ugolini GS, Sarkar S, et al. Quantifying the Efficacy of checkpoint inhibitors on CD8+cytotoxic T Cells for immunotherapeutic applications via single-cell Interaction. Cell Death Dis 2020; 11: 979.
- Kaplon H, Muralidharan M, Schneider Z, et al. Antibodies to Watch in 2020. mAbs 2020; 12: 1703531.
- Kaplon H, Chenoweth A, Crescioli S, et al. Antibodies to Watch in 2022. In: MAbs. Taylor & Francis, 2022, pp.2014296.
- Issafras H, Fan S, Tseng C-L, et al. Structural basis of HLX10 PD-1 receptor recognition, a promising anti-PD-1 Antibody clinical candidate for cancer immunotherapy. Plos One 2021; 16: e0257972.
- Oaknin A, Gilbert L, Tinker AV, et al. LBA36 Safety and Antitumor activity of dostarlimab in patients (pts) with Advanced or recurrent DNA mismatch repair deficient (dMMR) or proficient (MMRp) endometrial cancer (EC): results from GARNET. Ann Oncol 2020; 31: S1166.
- Alkholifi FK and Alsaffar RM. Dostarlimab an inhibitor of PD-1/PD-L1: a new paradigm for the treatment of cancer. Medicina 2022; 58: 1572.
- Oaknin A, Gilbert L, Tinker AV, Brown J, Mathews C, Press J, et al.Safety and antitumor activity of dostarlimab in patients with advanced Or recurrent DNA mismatch repair deficient/microsatellite instability-High (dMMR/MSI-H) or proficient/stable (MMRp/MSS) endometrial Cancer: interim results from GARNET—a phase I, single-arm study.Journal for ImmunoTherapy of Cancer. 2022;10(1):e003777. Available. From: https://doi.org/10.1136/jitc-2021-003777.
- Subramanian J, Moreno V, Bosch-Barrera J, Pikiel J, Kristeleit R, Guo W, et al. 1399P Safety and efficacy of dostarlimab in patients (pts) with Recurrent/advanced non-small cell lung cancer (NSCLC). Annals of Oncology. 2020;31(31):S886–S887. Available from: https://doi.org/10. 1016/j.annonc.2020.08.1713.
- FDA Approved Drug Products: Jemperli (dostarlimab-gxly) for intravenous injection.
- Eno, J. Immunotherapy Through the Years. J. Adv. Pract. Oncol, 2017; 8: 747.
- Wikipedia Foundation. Dostarlimab. Wikipedia; 2022. Available:https://en.m.wikipedia.org/wiki/D Dostarlimab
- Murtaza A, Laken H, Correia JS, McNeeley P, Altobell LJ, Zhang JG,Et al. Discovery of TSR-022, a novel, potent anti-TIM-3 therapeutic Antibody. Eur J Cancer. 2016;69:32901–32902. Available from: https://doi.org/10.1016/S0959-8049(16)32903-3.
- Lizardo DY, Kuang C, Hao S, Yu J, Huang Y, Zhang L. Immunotherapy Efficacy on mismatch repair-deficient colorectal cancer: From bench To bedside. Biochimica et Biophysica Acta (BBA) – Reviews on Cancer.2020;1874(2):188447–18844. Available from: https://doi.org/10.1016/j.bbcan.2020.188447.
- CHMP. Committee for Medicinal Products for Human Use (CHMP) Assessment Report, 2021. Available online: https://www.ema.europa.eu/en/news/meeting-highlights- Committee-medicinal-products-human-use-chmp-13-16-december-2021
- FDA Approved Drug Products: Jemperli (dostarlimab-gxly) for intravenous injection Jemperli (dostarlimab) dosing, indications, interactions, adverse effects, and more, 2021. Available: https://www.webmd.com/drugs/2/ drug-181367/dostarlimab-gxly
- Lizardo DY, Kuang C, Hao S, Yu J, Huang Y, Zhang L. Immunotherapy Efficacy on mismatch repair-deficient colorectal cancer: From bench To bedside. Biochimica et Biophysica Acta (BBA) – Reviews on Cancer. 2020;1874(2):188447–18844. Available from: https://doi.org/10.1016/j.bbcan.2020.188447.
- Yi M, Jiao D, Xu H, Liu Q, Zhao W, Han X, et al. Biomarkers For predicting efficacy of PD-1/PD-L1 inhibitors. Molecular Cancer. 2018;17(1):129–142. Available from: https://doi.org/10.1186/s12943-018-0864-3.
- Kumar S, Ghosh S, Sharma G, Wang Z, Kehry MR, Marino MH, et al.Preclinical characterization of dostarlimab, a therapeutic anti-PD-1 Antibody with potent activity to enhance immune function in in vitroCellular assays and in vivo animal models. mAbs. 2021;13(1):1954136. Available from: https://doi.org/10.1080/19420862.2021.1954136.
- Available:https://www.webmd.com/drugs/2/Drug-181367/dostarlimab-gxly-Intravenous/details/list-contraindications Available:https://www.drugs.com/pregnancy/dostarlimab.html.
- Intravenous/details/list-contraindicationsAvailable:https://www.drugs.com/pregnancy/dostarlimab.html
- Oaknin A, Ellard SL, Leath Iii, et al. Preliminary safety, efficacy, and PK/PD characterization from GARNET, a phase I clinical trial of the Anti-PD-1 monoclonal antibody, TSR-042, in patients with recurrent Or advanced MSI-H endometrial cancer. Ann Oncol. 2018;29(1):viii334. Doi.org/10.1093/annonc/mdy285.144
- Oaknin A, Duska LR, Sullivan RJ, et al. Preliminary safety, efficacy, and Pharmacokinetic/pharmacodynamic characterization from GARNET, A phase I/II clinical trial of the anti–PD-1 monoclonal antibody, TSR-042, in patients with recurrent or advanced MSI-h and MSS Endometrial cancer. Gynecol Oncol. 2019;154(1):17. Doi.org/10.1016/j.Ygyno.2019.04.044
- Oaknin A, Tinker AV, Gilbert L, et al. Clinical Activity and Safety of The Anti-Programmed Death 1 Monoclonal Antibody Dostarlimab For Patients With Recurrent or Advanced Mismatch Repair-Deficient Endometrial Cancer: A Nonrandomized Phase 1 Clinical Trial. JAMA Oncol. 2020;6(11):1766–1772. Doi:10.1001/jamaoncol.2020.4515
- European Medicines Agency, EMA. An overview of Jemperli (Dostarlimab) and why it is Authorized in the EU. Jemperli (dostarlimab), 2021; 3: 134553.
- Lu S, Bowsher RR, Clancy A, Rosen A, Zhang M, Yang Y, et al. An integrated analysis Of dostarlimab immunogenicity. The AAPS Journal, 2021; 23(5): 96. Available:https://doi.org/10.1208/s12248- 02100624
- Available:https://gskpro.com/content/dam/global/hcpportal/en_US/Prescribing_Inform Ation/Jemperli/pdf/JEMPERLI-PI


 Sanap Tushar *
Sanap Tushar *
 Shinde Jayesh
Shinde Jayesh
 Dhomase Rohan
Dhomase Rohan




 10.5281/zenodo.14185327
10.5281/zenodo.14185327