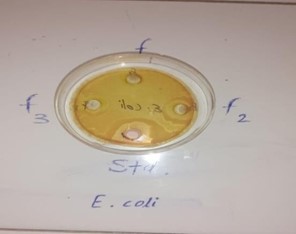
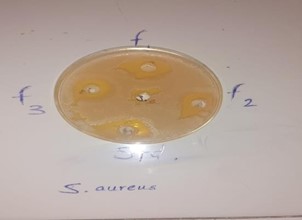
Hand washing with soap is important because it is proven to clean hands from germs and bacteria and chemicals which can cause personal harm or disease. This is an essential habit for the general population as well as for those who handle food or work in the medical industry. In response to this concern, we formulated the antibacterial Polyherbal Paper soap with combination of Azadirachta indica, Ocimum tenuiflorum, Curcuma longa, Aloe barbadensis and evaluate their parameter like physicochemical, pH, foam height, form retention, total fatty matter, anti-bacterial. Based on these tests, F2 soap Formulation code was selected as the best formulation among the others. The designed formulation F2 consisting 2.0gm gel of Aloe barbadensis, 3.0gm Azadirachta indica Extract, 0.5gm Curcuma longa Extract, 1.0gm Ocimum tenuiflorum (Tulsi oil), 2.0gm Sodium lauryl sulphate (SLS), orange oil 1.0gm, & Glycerin Soap Base (q.s.), found to be promising polyherbal soap with antibacterial properties. Finally, concluded that the formulated herbal soap used as a most promising, eco-friendly, low-cost formulation alternative to commercial chemical containing soaps.
Polyherbal paper soaps, Antibacterial activity, Soap
Human skin is the body's outermost covering and the first line of defense against numerous diseases. As the skin communicates with the environment, it is constantly subjected to various environmental stimuli, resulting in skin damage. Similarly, damaged skin will normally build scar tissue. Because the hand is a component of the body that is exposed to viruses even while working on a daily basis, soap was developed as a formulation that is commonly used in our daily lives to combat various diseases. A soap is the potassium salt (or sodium salt) of a long chain carboxylic acid (fatty acid) that cleanses water. It is a salt of a strong base (NaOH) and a weak acid (carboxylic acid), hence a solution of soaps in water is basic in nature.[1]
SOAP
Generally speaking, soaps are formed by reacting alkaline chemicals with fatty acids in fats or oils to form salts of free fatty acids. In order to create the many kinds of soap that we have, additional ingredients are added to this soap base, or salt of free fatty acid. Typically, they are utilised for bathing and cleaning. Thus, individuals use synthetic soaps extensively in their daily lives. Because of the soap's scent, appearance, colour, and advertisements, it gains popularity. These artificial soaps serve as dish soap. Thus, using those soaps damages the skin and increases the risk of illnesses such as rashes, skin allergies, fungus, psoriasis, acne, and a decrease in the colour and appearance of the skin. Human skin is the body's outermost covering and the first line of defense against numerous diseases. As the skin communicates with the environment, it is constantly subjected to various environmental stimuli, resulting in skin damage.[2]
TYPES OF SOAP [3]
- Natural/Herbal Soap
- Liquid soap
- Moisturizing Soap
- Anti-bacterial Soap
- Chemical Free Soap
- Foam Soap
- Bar Soap
- Body Soap
MATERIALS AND METHODS
Table 1: Materials
METHODOLOGY EVALUATION TESTS FOR THE NEEM AND CURCUMIN
- Foreign Matter Test [5]
Take the weighed quantity of crude medication (500 gm root stem/dark, 250 gm leaves blossoms). 50 grammes of chopped plant material and seed fruit. Spread it out thinly, then use a 10x magnifying lens, an appropriate sieve, or visual inspection to sort out any foreign material. To eliminate dust (mineral admixture), run the remaining sample through a filter with a number of 250. Calculate the percentage of weight in the sorted foreign materials by weighing it.If the foreign substance looks like plant material, use a combined sample and perform microscopy or physical/chemical testing. Calculate the percentage of foreign matter by adding together all the percentages that don't pass the test.
DETERMINATION OF LOSS ON DRYING (LOD) [5]
Gravimetric Method:
Weigh approximately 1.5 grams of the powdered medication into a flat and thin porcelain dish.Dry in the oven at 100°C or 105°C until the difference between two successive weighings is less than 0.5 mg. Cool in desiccators and weigh. Weight loss is frequently reported as moisture.
Calculation:-
Weight of sample = gm
Weight of porcelain dish +sample before drying = gm Weight of porcelain dish +sample after 1st drying = gm Weight of porcelain dish +sample after 2nd drying = gm
Difference between LOD = sample after 1st drying - sample after 2nd drying
DETERMINATION OF ASH VALUES OF A CRUDE DRUG[5] DETERMINATION OF TOTAL ASH VALUE:
Weigh and light a thin, flat porcelain dish. Put roughly 2 grammes of the drug's powder into the crucible or dish. Place a pipe- clay triangle on top of a retort stand ring to support the dish. Using a hob, place the dish about 7 cm over the flame and heat it until the vapours almost completely disappear. Then, lower the dish and increase the heat until all of the carbon is burned off. Use a desiccator to cool. Weigh the ash and determine the proportion of total ash by comparing it to the crude drug sample that has been air dried.
Calculation:-
Weight of empty dish (x) = gm. Weight of dry powder taken (y) = gm.
Weight of empty dish + dry powder = gm. Weight of dish + ash (z) = gm.
Weight of ash = z – x gm.
DETERMINATION OF ACID-INSOLUBLE ASH VALUE
Follow the directions that are provided in the process for establishing the total ash value of a crude medication. Furthermore Wash the ash from the dish used for total ash into a 100 ml beaker and boil for five minutes using 25 ml of diluted hydrochloric acid. Cover a Bunsen burner with just gauze and let it boil for five minutes. Use 'ashless' filter paper and give the residue two hot water washes. A crucible should be lit, cooled, and weighed. Place the residue and filter paper into the crucible; heat the mixture slowly at first until the vapours stop evolving, and then more vigorously until the carbon has been eliminated. Use a desiccator to cool. Calculate the acid-insoluble ash of the crude medication by weighing the residue and using the air-dried sample as a point of reference.
Calculation:-
Sample weight ws = gm Crucible weight wc = gm Crucible + ash weight wf = gm
Acid insoluble ash = wf - wc×100 / ws
DETERMINATION OF WATER SOLUBLE ASH VALUE
This is calculated similarly to acid insoluble ash, but instead of using diluted hydrochloric acid, 25 ml of water are used.
4. DETERMINATION OF EXTRACTIVE VALUES/EXTRACTABLE MATTER[5] DETERMINATION OF ALCOHOL-SOLUBLE EXTRACTIVES
Weigh around 4 g of the coarsely ground medication in a weighing bottle and transfer it to a dry 250 ml conical flask. The solvent (90 percent alcohol) should be added to a 100 ml graduated flask until it reaches the delivery point. Rinse the weighing bottle and transfer the washings to the conical flask along with the remaining solvent. Shake often for a full day after corking the flask. (Chewing). Strain into a 50 ml cylinder. Once enough fiitrate has gathered, transfer 25 ml of the filtrate to a thin porcelain plate that has been weighed and used to determine the ash levels.Dry completely on a water bath, then finish drying for six hours at 105 degrees Celsius in an oven. Allow to cool for half an hour.
DETERMINATION OF WATER-SOLUBLE EXTRACTIVES
Weigh around 4 g of the coarsely ground medication in a weighing bottle and transfer it to a dry 250 mL conical flask.The solvent (10 percent chloroform and 90 percent water) should be added to a 100 ml graduated flask until it reaches the delivery point. Rinse the weighing bottle and transfer the washings to the conical flask along with the remaining solvent. Shake often for a full day after corking the flask. (Chewing). Strain into a 50 millilitre cylinder. Once enough fiitrate has gathered, transfer 25 millilitres of the filtrate to a thin porcelain plate that has been weighed and used to determine the ash levels.Dry completely on a water bath, then finish drying for six hours at 105 degrees Celsius in an oven. Allow to cool for half an hour.
5. EXTRACTION OF CURCUMIN BY SOXHLET APPARATUS[6]
The Soxhlet chamber was filled with 35 grams of curcumin powder in a thimble. The distillation procedure started after 300 ml of the chosen solvent (methanol) was prepared for the Soxhlet extractor and put in a round-bottom flask. The solvent was evaporated by placing the extractor and solvent on a water bath once the extraction process was finished.
6. EXTRACTION OF NEEM BY SOXHLET APPARATUS[6]
35 gm of neem powder was placed into the thimble and placed in the soxhlet chamber. 300 ml of selected solvent (Methanol) were placed in a round bottom flask and assembled for soxhlet extractor then the distillation process was begun. After completed the extraction process, the solvent was evaporated in water bath.

Fig 1: - Extraction of Neem
Fig.2.: - Extraction of Curcumin
PRELIMINARY PHYTOCHEMICAL SCREENING OF ACTIVE INGREDINTS[5] TESTS FOR CARBOHYDRATES
- General test
Molisch's test (General test): Add a few drops of an alcohol-based alpha-naphthol solution to 2-3 ml of aqueous extract, agitate, and then add concentrated H2SO4 from the test tube's sides. When two liquids come together, a violet ring forms.
b. Tests for reducing sugars:
1. Fehling's test:
Combine 1 ml each of Fehling's A and B solutions, then boil for a minute. Pour in the same amount of test solution. Take a 5-to 10-minute hot water bath . Brick red and then yellow ppt. are seen.
- Benedict's test:
In a test tube, combine an equal volume of Benedict's reagent and test solution. Take a 5 minute hot water bath. The color of the solution changes to green, yellow, or red based on the quantity of reducing sugar in the test solution.
c. Test for non-reducing polysaccharides (starch) :
1. Iodine test:
Mix 3 ml test solution and few drops of dilute Iodine solution. Blue colours appears, it disappears on boiling and reappears on cooling.
2.Tannic acid test for starch:
With 20% tannic acid, test solution give ppt
TESTS FOR PROTEINS
- Biuret test (General test):
To 3 ml T.S. add 4% NaOH and few drops of 1% CuSO4 solution. Violet or pink colours appears.
- Millon's test (for proteins):
Mix 3 ml T.S. with 5 ml Million's reagent. White ppt. Warm. ppt. turns brick red or the ppt. dissolves giving red coloured solution.
TESTS FOR GLYCOSIDES
1. TESTS FOR CARDIAC GLYCOSIDES:
a. Baljit’s test:
A section shows yellow to orange colours with sodium picrate.
b. Liebermann's test (Test for bufadenoloids): After adding 2 ml of the plant extract to 2 ml of acetic anhydride, the mixture cooled in ice. Carefully sulfuric acid was added along the test tube's side. When the colour changes from violet to blue-green, it means that the cardiac glycoside's glycone portion—the steroid nucleus—is present.
TESTS FOR ANTHRAQUINONE GLYCOSIDES:
- Borntrager's test for anthraquinone glycosides:
To 3 ml extract, add dil. H?SO?. Boil and filter. Add the same amount of chloroform or benzene to the cold filtrate. Shake well. Separate the organic solvent. Add ammonia. Ammoniacal layer turns pink or red.
3. TESTS FOR SAPONIN GLYCOSIDES:
a. Foam test:
Through water, mix the drug extract or dry powder. Stable, long-lasting foam was noted.
4. TESTS FOR CYANOGENETIC GLYCOSIDE:
a. Add 3% aqueous mercurous nitrate solution to dry drug powder or extract. Metallic mercury forms.
TESTS FOR TANNINS AND PHENOLIC COMPOUNDS
Incorporate a few drops of each of the following reagents in 2-3 ml of alcoholic or aqueous extract:
a. 5?Cl3 solution: deep blue-black colours
b. Lead acetate solution: white ppt.
TESTS FOR ALKALOIDS
Evaporate the aqueous, alcoholic and chloroform extracts separately. To residue, add dilute HCI. Shake well and filter. With filtrate, perform following tests:
- Dragendorff's test:
Add a few drops of Dragendorff's reagent to 2-3 ml of filtrate. A brown-orange ppt. forms.
- Mayer's test:
2-3 ml of filtrate and a few drops of Mayer's reagent gives ppt.
TESTS FOR AMINO ACIDS
- Ninhydrin test (General test):
Heat 3 ml T.S. and 3 drops 5% Ninhydrin solution in boiling water bath for 10 min. Purple or bluish colours appears.
- Test for tyrosine:
Heat 3 ml T.S. and 3 drops Millions reagent. Solution shows dark red colour.
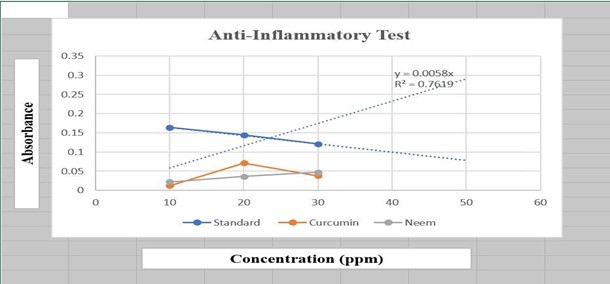
ANTI-INFLAMMATORY ACTIVITY[7]
The process of applying an external stressor or chemical to proteins causes them to lose their secondary and tertiary structures, a phenomenon known as protein denaturation. such as heat, an organic solvent, a strong acid or base, or a concentrated inorganic salt. When denatured, the majority of biological proteins cease to function biologically.
Procedure:
- The reaction mixture consists of 2.8 ml normal saline solution.
- Add 0.2 ml fresh albumin solution.
- Add 2 ml sample plant extract as given ppm.
- Extracts were incubated for 20 min at 37? and then heated at 51? for 20 min.
- Measure the turbidity of sample extracts after cooling.
- Experiment performs in triplicate.
Calculate the percentage of inhibition equivalent by the following formula.
Percentage inhibition= (Abs control - Abs sample / Abs control) 100
Procedure:
- Preparation of stock solution 2 gm of extract mix ethanol.
- Prepare 100 ppm solution dissolving 1ml of stock solution in 10ml of ethanol.
- Then prepare dilution of 10 ppm, 20 ppm, 30 ppm
- Reaction mixture of were prepared using 2.8ml of saline ,0.2ml of egg albumin, then 2ml of extract were mixed gently from each different concentration.
- The samples then filtered for UV spectroscopy.
UV SPECTROSCOPY
- First, let the machine of UV spectroscopy starts.
- Then prepare the cuvette with ethanol to clear the baseline and note the absorbance.
- Then one of the ethanol cuvettes is replaced with sample of different dilutions and note the absorbance of all three samples.
- Lastly calculate the % of absorbance.
- Protein denaturation test (pain killer)
Preparation of reference drug (positive control)
NSAID (ibuprofen) were used as reference drug Ibuprofen was crushed into fine powder. About 0.2 gm of Ibuprofen drug powder was measured using a digital analytical balance and was added to 20.0 ml of distilled water. The solution was mixed well.
- Serial dilutions
Serial dilution was carried out for three sample extracts and reference medications (ibuprofen) from 1000 ppm to 0.01 ppm. Each sample had a total volume of 5.0 millilitres. 2.8 ml of phosphate-buffered saline (pH 6.4) and 0.2 ml of fresh hen's egg albumin were used to create reaction solutions. Subsequently, reaction mixtures were gently combined with 2 millilitres of extract from each concentration. Ibuprofen was one of the reference medications, and they were used using a comparable process.
Modified method:
Reagents:
Fresh hen’s egg albumin, Phosphate-buffered saline, samples.
Instruments:
Colorimeter.
Formula:
Percentage inhibition = (Abs. control – Abs. sample / Abs. control) 100.
Absorbance taken on 650 nm.
PREPARATION OF ANTIBACTERIAL SINGLE USE SOAP [8,9]
PROCEDURE
COLD PROCESS METHOD:
Step 1
Solidified basic glycerin soap was weighed and broken down into smaller pieces.
Step 2 -
It was transferred into a vessel and then heated in a water bath to melt the soap base and stirred continuously with
a glass rod.
Step 3 -
The powder compositions were added to the base after the soap base was liquefied.
Step 4 -
The mixture was stirred continuously to avoid lumps.
Step 5 -
There was continuous agitation with a glass rod for 15 minutes until the molten mixture became homogeneous.
Step 6 -
The homogenous mixture was removed from the water bath, and orange oil was added.
Step 7 -
It was stirred slowly.
Step 8 -
The paper soap strips were prepared by dipping technique using modified disintegration apparatus and air dried
overnight at 37 ± 2°C.
Step 9 –
For this purpose butter paper were dipped one after another into the soap solution of various concentrations and air
dried overnight.
Three herbal soap formulations (F1, F2 & F3) were prepared by cold press method given in table no.2.2


 Ankit P. Gondane*
Ankit P. Gondane*
 Samir R. Pakhidde
Samir R. Pakhidde
 Sanket R. Ganvir
Sanket R. Ganvir
 Tushar C. Bisen
Tushar C. Bisen
 Devesh R. Chourasia
Devesh R. Chourasia


















 10.5281/zenodo.11062953
10.5281/zenodo.11062953