Sunscreen is essential for protecting skin from UV rays that can cause sunburn, premature aging, and an increased risk of skin cancer. Because of their ability to absorb UV light and convert it into a less harmful energy, benzophenones are a common constituent in sunscreens. But concerns have been voiced regarding benzophenones' potential effects on the environment and human health. It has been linked to endocrine disruption, which can interact with hormones like oestrogen and androgens and perhaps cause issues with development and reproduction. Lower head circumferences and lower birth weights have been associated with higher urine concentrations of benzophenone derivatives; studies on animals have also suggested potential effects on male reproductive health. The fact that some benzophenone compounds may be carcinogenic adds to the concerns about extended exposure. Because benzophenone derivatives are poisonous and have a tendency to bioaccumulate, their persistence in ecosystems poses a threat to the environment. Biomonitoring studies show that over 95% of Americans, including pregnant women and premature babies, have detectable levels of benzophenones in their urine, suggesting widespread exposure. The fact that benzophenone can be transferred to offspring through the placenta or breast milk further complicates the drug's possible effects on new-borns. The worries about prolonged exposure are heightened by the possibility that some benzophenone chemicals are carcinogenic. Benzophenone derivatives are hazardous to the environment because they are toxic and have a propensity to bioaccumulate in ecosystems. Almost 95% of Americans, including expectant mothers and premature infants, have measurable levels of benzophenones in their urine, according to biomonitoring studies, indicating widespread exposure. The potential effects of benzophenone on neonates are further complicated by the fact that the substance can be passed to children through the placenta or breast milk.of benzophenones in their urine, according to biomonitoring studies, indicating widespread exposure. The potential effects of benzophenone on neonates are further complicated by the fact that the substance can be passed to children through the placenta or breast milk. complicated by the fact that the substance can be passed to children through the placenta or breast milk.
Benzophenones, Benzophenone derivatives, Liquid Chromatography-Tandem mass spectrometry, Biofluids
Using sunscreen is an essential public health practice to guard against UV radiation, which lowers the risk of sunburn, premature aging, and skin cancer. Sunscreen usage varies greatly despite its benefits because of a number of factors, including socioeconomic position, cultural customs, awareness, and accessibility. Commonly found in sunscreens, benzophenones absorb UV photons and transform them into less dangerous energy. But worries about their safety continue, especially in light of their endocrine-disrupting effects. These substances have the ability to mimic or interact with hormones, especially androgens and oestrogen, upsetting the balance of hormones and perhaps causing problems with development and reproduction. For example, lower birth weight and smaller head circumference in babies have been associated with higher urine levels of these derivatives, and animal studies have suggested possible effects on male reproductive health and body organ weights. Concerns over their long-term exposure dangers have been raised by the classification of several benzophenone compounds as potential carcinogens. Furthermore, these substances may result in skin sensitivity and allergic responses, which in sensitive people may result in dermatitis. These substances may have long-term health effects since they can enter the bloodstream and be discovered in biofluids such as breast milk, urine, and blood. Benzophenone derivatives are persistent in the environment and pose a threat to ecosystems and wildlife because of their potential toxicity and bioaccumulation, which extends beyond human health concerns. Studies on biomonitoring have shown that there is widespread exposure and that different populations' urine contains measurable amounts of the substance. Over 95% of urine samples from U.S. individuals, including pregnant women and premature infants, contained benzophenones. This finding suggests significant exposure and possible transmission to children through the placenta or breast milk. Benzophenone's modest anti-androgenic and estrogenic properties raise questions regarding potential endocrine disruption. Mothers who have elevated urinary levels of benzophenone and have been associated with lower birth weights and head circumferences; animal studies have also suggested possible effects on body, liver, and kidney weights.
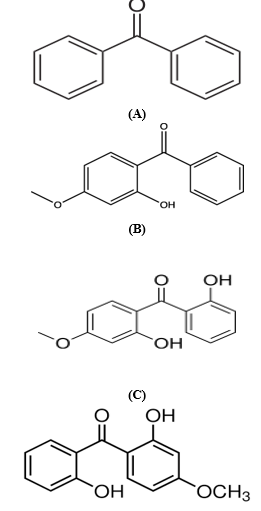
Figure1: Structure of Benzophenone (a), Benzophenone-3(b), Benzophenone-8 (c), 2,2?-Dihydroxy-4-methoxybenzophenone (d)
EXTRACTION OF SAMPLES FROM BIOFLUIDS:
In biomedical research and clinical diagnostics, sample extraction of biofluids, such as blood, urine, or saliva, is a crucial procedure that aims to isolate particular analytes, such as hormones, medications, metabolites, or proteins, from complicated biological matrices. Because biofluids are complex mixtures of biomolecules, precise extraction methods are necessary to guarantee consistent and correct analytical outcomes. These methods include protein precipitation (PPT), liquid-liquid extraction (LLE), solid-phase extraction (SPE), and QuEChERS (Quick, Easy, Cheap, Effective, Rugged, and Safe). Microextraction techniques such as liquid-phase microextraction (LPME) and solid-phase microextraction (SPME) are examples of further methods. A new extraction method called online turbulent flow chromatography (TFC) allows complicated samples—like breast milk—to be directly injected without first being purified. High flow rates create a turbulent flow inside the column that enables the stationary phase to selectively retain tiny molecules while excluding and washing away bigger molecules like lipids and proteins. In case of human placenta, after being lyophilized and spiked with surrogates, a sample of human placenta was put onto a polypropylene cartridge, mixed with C18 sorbent, and extracted with 20 milli litres of ethyl acetate. After being evaporated, the extract was reconstituted in a solution of methanol, water, and ammonia, vortexed, centrifuged, and made ready for chromatographic analysis. Following the thawing of frozen plasma samples, 150 µL was introduced to 96-well Phospholipid Removal plates, and the mixture was quenched using acetonitrile that included internal standards. Following centrifugation and vortexing, mobile phase A was combined with the filtrate, and 5 µL of the prepared sample was introduced into the mass spectrometer. Human serum was mixed with acetone, then centrifuged and vortexed to remove serum proteins. A 5% NaCl solution was used to dilute the supernatant, and 0.1 M HCl was used to bring the pH down to 2.0.
Liquid chromatography-tandem mass spectrometry, or LC-MS/MS, is an advanced analytical method that combines the precision mass analysis of tandem mass spectrometry (MS/MS) with the separation powers of liquid chromatography (LC). Different compounds can be isolated by their retention duration in this procedure, which uses LC to first separate complicated mixtures of chemicals based on their interactions with a stationary phase and a liquid mobile phase. The chemicals are separated, ionized, and then subjected to analysis by the mass spectrometer. The ions are broken up into smaller ion fragments in a collision cell after the mass-to-charge ratio (m/z) of the ions is measured by the first mass analyser (MS1). The second mass analyser (MS2) then examines these pieces, providing in-depth structural information about the chemicals. Due to its exceptional sensitivity and specificity, LC-MS/MS is widely used in a wide range of industries, including clinical diagnostics, pharmaceuticals, environmental monitoring, and food safety. Its vital importance in modern analytical chemistry is highlighted by its adaptability, capability for comprehensive structural elucidation, and ability to identify and quantify tiny quantities of chemicals in complicated biological matrices.
Determination of Benzophenone Derivative Levels in Breast Milk:
In an investigation to find the amount of UV filters in breast milk triple quadrupole mass spectrometer, the TSQ Vantage, with an electrospray ionization (ESI) source, was connected to a turbulent flow chromatograph, which was equipped with a PAL auto sampler. Turbo flow TFC columns, Cyclone (50 mm × 0.5 mm, 60 µm particle size, 60 Å pore size), Cyclone MAX (50 mm × 0.5 mm, 60 µm particle size, 60 Å pore size), Cyclone P (50 mm × 0.5 mm, 60 µm particle size, 60 Å pore size), and C18 XL (50 mm × 0.5 mm, 60 µm particle size, 60 Å pore size) were tested to ensure good extraction efficiency. A C18 Purosphere® STAR RP18 end capped (125 × 2.0 mm, 5 µm) was consistently utilized as an analytical LC-column. The components of the mobile phase were methanol and water, both at 0.1% in formic acid. Positive ionization and selective reaction monitoring (SRM) were used for the analyses. The following general MS/MS parameters were found: 400 °C for the capillary, 350 °C for the vaporizer, 40 m Torr for the sheath gas pressure, 20 arbitrary units for the auxiliary gas (N2), 0.5 arbitrary units for the ion sweep gas (N2), 3.8 kV for the spray voltage, and 30 m sec for the dwell period. The daily intake (EDI) of UV filters taken by new-borns was determined using the equation, Dose = Cf × Im, where Cf is the concentration of pollutants in ng g?1 of milk and Im is the intake of breast milk in g milk d?1 kg body weight?1. The daily rate of milk consumption per new-born during the initial days of breastfeeding was calculated using a value of 500 g of milk d?1. With the limited information that the mothers could provide, this approximation was used. To evaluate the method's quality and determine which TFC column to use for the analysis, a set of milk samples were spiked at 25, 50, and 100 ng g?1 of milk (n=7 for each level). With the exception of Cyclone P, which was able to recover every target compound, all of the tested TFC columns were able to recover the majority of the compounds. Benzophenone recovery rates (%R) for cyclone columns were good, with values above 60% for Cyclone MAX and Cyclone P, respectively. Cyclone MAX had the highest values, approaching 70%. Finally, because Cyclone P offered the best recovery rates overall, achieved acceptable precision between replicates, and offered the greatest spectrum of chemicals for analysis, it was chosen as the TFC column for the analysis of UV filters residues in breast milk. A correlation factor ranging from 0.993 to 0.999 was used to investigate the linearity between 1 and 500 ng g?1[1]. The range of the instrumental limits of quantification (ILOQ) was 5–65 pg [1], whereas the range of the instrumental limits of detection (ILOD) was 1–20 pg. For N = 7, intraday and inter-day precision was consistently less than 15%. The range of the method limits of quantification (LOQ) was from 0.3 to 5.1 ng g?1 milk [1], whereas the method limits of detection (LOD) were between 0.1 and 1.5 ng g?1 milk [1]. The breast milk sample was analysed using the newly developed TFC-HPLC-MS/MS technique. A mean quantity of 144.8 ng g?1 milk was found in 23% of the samples containing hydroxy methoxy benzophenone [1], often known as benzophenone-3. The quantities of benzophenone-3 metabolites 4-hydoxybenzophenone (3%) and 4-dihydroxy benzophenone (10%) were shown to be lower than 43.3 ng g?1 [1] milk.
Determination of Benzophenone Derivative Levels in Human Placenta:
An UPLC C18 column (50 mm × 2.1 mm, 1.7 µm particle size) was used for chromatographic separation in a study to identify benzophenone (BP) filters in human placenta. A gradient mobile phase containing 0.1% ammonia in methanol (solvent B) and 0.1% ammonia in an aqueous solution (solvent A) was used for the separation. The gradient conditions were as follows: a total run time of 10.0 minutes; 0.0–3.5 minutes at 60% B, 3.5–4.0 minutes from 60% to 100% B, 4.0–6.5 minutes at 100% B, and 0.1 minutes back to 60% B. The column temperature was kept at 40°C, the injection volume was set at 10 µL, and the flow rate was fixed at 0.25 mL/min. The tandem mass spectrometer was running in multiple reactions monitoring (MRM) mode while electrospray ionization (ESI) was carried out in both negative and positive ion modes during the same run. The mass spectrometric settings were continuously infused with standard solutions (1 mg/L) to maximize the results for every analyte. In addition to the ion source temperature of 150°C, the instrument's other settings were the capillary voltage of 0.60 kV, the de-solvation temperature of 500°C, and the cone gas flow rate of 150 L/h. Argon was utilized as the impact gas and nitrogen as the cone and de-solvation gas. For every analyte, the collision energies (CE) and cone voltages (CV) were adjusted, and dwell periods were fixed at 25 ms. Using an MSPD extraction method and a UHPLC–MS/MS analysis, the free quantities of four parabens and six benzophenones in human placental tissue samples were successfully identified and quantified. Analyte isolation from samples was precisely tuned, and the process was verified. The suggested technique was employed to identify these compounds in samples taken at the time of birth from ten randomly chosen mothers [1].
Determination of Benzophenone Derivative Levels in Human Plasma:
Phospholipid removal pre-treatment was used in conjunction with UHPLC-MS/MS in a study to evaluate benzophenones in human plasma. UHPLC system and triple-quadrupole mass spectrometer were utilized in the analysis. A 2.1 × 50 mm, 1.8 µm C18 column was injected with a 5 µL sample. An isocratic mobile phase of 10 mM ammonium formate, 0.1% formic acid, and methanol (24:76, v/v) at a flow rate of 0.7 mL/min was used to separate the analytes. The autosampler was kept at 5°C and the column at 35°C. A robust response was achieved by optimizing a number of mass spectrometry parameters, such as De-clustering Potential (DP), Collision Energy (CE), Collision Cell Exit Potential (CXP), gas flows, source temperature, and ion spray voltage, after the solution was introduced into an electrospray ionization (ESI) source. The hydroxy methoxy benzophenone calibration curve showed suitable linearity within the concentration range of 0.4–300.0 ng/mL [3], indicating the accuracy of the method in terms of quantification.
Determination of Benzophenone Derivative Levels in Human Serum:
A novel technique was created to measure the benzophenone UV filters present in human serum samples using liquid chromatography-tandem mass spectrometry in conjunction with dispersive liquid-liquid microextraction. Utilizing an UPLCs C18 (50 mm 2.1 mm i.d., 1.7 ?m particle size), chromatographic separation of substances was carried out. A gradient mobile phase comprising 0.1% (v/v) ammoniacal aqueous solution (solvent A) and 0.1% (v/v) ammonia in methanol (solvent B) was used to separate the standards and samples. The gradient conditions were as follows: 60?or 0.0–3.5 minutes; 60%–100?or 3.5–4.0 minutes; 100?or 4.0–6.5 minutes; and 60?gain for 0.1 minutes [4]. The flow rate was 1 mL per minute. There was a 10 mL injection volume. The temperature of the column was kept constant at 40?. A total of 10.0 minutes were run. Positive ion mode was used for ESI. Q1 and Q3 quadrupoles were set at unit mass resolution, and the tandem mass spectrometer was run in the selected reaction monitoring (SRM) mode. One way to adjust the mass spectrometric conditions for each drug was to constantly infuse standard solutions (1 mg/ L). 150? was the constant temperature of the ion source. The capillary voltage was 0.60 kV, the source temperature was 150?, the de-solvation temperature was 500?, the cone gas flow was 150 L h-1, the de-solvation gas flow was 500 L h-1, the collision gas flow was 0.15 mL min-1, and the nebulizer gas flow was 7.0 bar. Cone and de-solvation gases were nitrogen (99.995%), while collision gas was argon (99.999%). The concentrations of free and total benzophenone-UV filters were measured in 20 human serum samples from unidentified men and women residing in Linares using the suggested methodology. In 70% of the examined samples (n=14/20), benzophenone-3 was found, and in 40% (n=8/20), it was measured [4].
Determination of Benzophenone Derivative Levels in Sprague-Dawley Rat Serum:
Using HPLC tandem mass spectrometry, the serum levels of benzophenone and its three metabolites—2,4-dihydroxybenzophenone [DHB], 2,3,4-trihydroxybenzophenone [THB], and 2,2-dihydroxy-4-methoxybenzophenone [DHMB]—were determined in Sprague-Dawley rats. 40 litres of serum were treated to protein precipitation using 100 litres of acetonitrile (ACN) and an internal standard of 1 g/ml reserpine. Using a UFLC, and C18-LC column (2 ? × 50 mm) and guard column, chromatographic separation was accomplished. Before adding a linear gradient that increased to 63?N over 6 minutes and kept for 3 minutes, the initial gradient conditions were set at 5?N with 0.1% formic acid and held for 1 minute [5]. For every analysis, the temperature of the column was 40°C, and the flow rate was set at 0.3 ml/min. A 3200 QTRAP mass spectrometer running in the electrospray mode was used to find the analyte. Every day, six concentrations of each analyte were produced as serum calibration curves, which were then utilized to compute the levels in the study samples. Benzophenone had a limit of detection (LOD) of 0.005 g/ml [5]. When rat dams and their pups were administered benzophenone maternally and lactatorily, very few negative effects were seen at dosages of 10,000 ppm or less. It is unclear whether benzophenone caused a delay in postnatal growth in the higher dose groups, which would have had a negative impact on the development of reproductive organs.
Determination of Benzophenone Derivative Levels in Human Plasma and Mouse Serum:
A technique was created to measure the amount of benzophenone in human plasma and mouse serum. The UHPLC apparatus was used in the studies. Fast-scanning fluorescence detector (FSFD), UV-Vis diode array detector (DAD), auto-sampler, degasser membrane, oven column compartment, and binary pump were among its features. The Open-Lab CDS Chem-station software package, was used to control the instrument and to acquire and analyse the data. A 2.7 ?m C18 analytical column (3.0 × 50 mm), was used to create two different separation techniques. A mixture of water and acetonitrile (50:50) with a flow rate of 0.60 mL min?1 and a column temperature of 30 °C comprised the mobile phase of the chromatographic system for method A, while a mixture of water and acetonitrile (35:65) with a flow rate of 0.70 mL min?1 and a column temperature of 35 °C comprised the mobile phase for method B. The injection volume was 50 ?L in each instance. By using chemometric methods, it was possible to determine benzophenone-3 in just two minutes, with remarkable recovery and precision outcomes [6].
Determination of Benzophenone Derivative Levels in Rat Plasma:
Rat plasma samples were subjected to analysis using a Triple Quad 5,500 mass spectrometer coupled to a High-Performance Liquid Chromatography (HPLC). The m/z 227?211, 213?135, 229?151, and 243?94 for HMB and m/z 232?215 for internal standards hydroxy methoxy benzophenone-d5 were the transitions that were observed. Analyte separation was performed using a C18 column (50 × 2 mm2, 5 ?), with a flow rate of 400 ?L/min and the gradient described below: 1 mM ammonium acetate/0.02% formic acid in (A) and acetonitrile in (B); 0–0.2 min, 20% B; 0.2–2.9 min 95% B, 2.9–4.0 min 95% B; 4.0–4.5 min 20% B; 4.5–9.5 min 20% B. Negative ion mode was used to run the electrospray ionization interface. The ion spray voltage, 4,500 or 3,000 V; source temperature, 550 or 450°C; sheath and auxiliary gas pressures, 50 or 45 arbitrary units; and collision energy, 30 eV were the parameters under which the mass spectrometer operated. By analysing plasma calibration standards over the concentration range of 15–150 ng/mL for free hydroxy methoxy benzophenone and dihydroxy benzophenone, 200–2,000 ng/mL for total hydroxy methoxy benzophenone and trihydroxy benzophenone, and 40–400 ng/mL for total 2,5- dihydroxy methoxy benzophenone and dihydroxy benzophenone, the linearity, intra- and inter-day precision, and accuracy of the method were assessed [7]. For both free and total analytes, the coefficient of determination (r2) for the plasma standard curves was ?0.98 in all accepted runs [7].
Determination of Benzophenone Derivative Levels in Umbilical Cord Blood:
The objective of this study was to develop a sensitive analytical method for the simultaneous analysis of paraben preservatives and hydroxy methoxy benzophenone-type UV filters in umbilical cord blood. The compounds were separated chromatographically in a C18 (50 mm × 2.0 mm, 5 ?m) column using a Symbiosis TM Pico instrument, and the compounds were detected in a 4000 Q TRAPTM hybrid quadrupole-linear ion trap mass spectrometer. The injection volume was set up to 20 ?L. Electrospray ionization in positive (ESI+) and negative (ESI-) modes were used for increased sensitivity and selectivity, tandem mass spectrometry detection (MS/MS) was carried out in selected reaction monitoring (SRM) mode. For the quantification (the most intense, or first transition) and confirmation (the second most intense, or second transition) of each molecule, the two most intense transitions were chosen. Serum extracts were combined with analytical standards, reagent blank samples, and quality control solutions in each analysis batch. In order to create a representative combination of umbilical cord serum which was required to validate the suggested method, a number of the received samples were combined. For every chemical, a broad linearity interval of 1–700 ng/mL was found, with r2 > 0.9969[8]. The MLODs for the procedure were in the range of 0.01–0.42 ng/mL blood, indicating great sensitivity [8].
Determination of Benzophenone Derivative Levels in Human Urine:
A study was conducted utilizing the secondary sex ratio and the urine concentrations of hydroxy methoxy benzophenone-type UV filters in pairs. Benzophenone-3 and its metabolic by-products (BP-1, BP-2, BP-8, and 4-OH-BP) were used to identify five benzophenone-type UV filters through the analysis of urine samples obtained at the time of enrolment. These substances' urinary quantities (ng/mL) were measured by triple quadrupole mass spectrometry coupled with isotopic dilution high performance liquid chromatography. The target analytes' recoveries varied from 95 to 107%. The quantification of benzophenone type UV filters was done using established standard operating procedures, which included continuous quality assurance and control processes. Benzophenone-type UV filters have limits of detection (LODs) ranging from 0.01 to 0.02 ng/mL [9]. To avoid bias brought about by this approach, all concentrations measured by the instrument were included for analysis; amounts below the LOD were not substituted for. Using the Creatinine Plus Assay and a Roche/Hitachi Model 912 clinical analyzer, urinary creatinine concentrations (mg/dL in 0.15 mL of urine) were measured [9]. This study offers the first documentation of the relationship between the SSR and specific metabolic derivatives of BP-3, such as BP-2 and 4-OH-BP. Our findings are consistent with some but not all previous studies on the relationship between paternal exposure to other estrogenic substances and the SSR, indicating that paternal preconception exposure to BP-2 may be linked to the reversal of the SSR resulting in an excess of female births
Table 2: Different types of samples with their solvent, analytical method used an LOD obtained
CONCLUSION:


 SANOOJA P.K*
SANOOJA P.K*
 PRASANTH S.S
PRASANTH S.S
 AJAY A
AJAY A
 MOHAMED FAROOQ. P
MOHAMED FAROOQ. P
 SIBINA M.K
SIBINA M.K
 JISHA .U
JISHA .U
 K.T. AKSHARA
K.T. AKSHARA
 RIYA RAJAN
RIYA RAJAN


 10.5281/zenodo.13337326
10.5281/zenodo.13337326