Helminthic infections are mainly caused by parasites, remain a major public health burden in developed countries, and affect millions around the world every year. In the present investigation, the anthelmintic potential of aqueous and ethanolic pod extract of Leucaena leucocephala was assessed at different concentrations. Antiparasitic medicine, Albendazole was used as a standard drug. The aqueous and ethanolic extraction of pods was performed using hot decoction and Soxhlation, respectively. A different group of adult earthworms (Pheretima posthuman) was used to evaluate the anthelmintic potential of the investigated extract and the time taken to the paralysis and death of worms was recorded as the end point of study. Both extracts at a concentration of 60 mg/ml caused paralysis of worms in a shorter time compared with standard drugs. Thereafter, the death of worms was observed. The result demonstrated that both aqueous and ethanolic extract of Leucaena leucocephala pod would be used as an anthelmintic drug. The phytochemical screening and microscopic study were also performed to understand the plant's cellular structures. The present investigation elucidates the active compounds of the extract responsible for deworming this conclusion.
Leucaena leucocephala, Anthelmintic activity, Pheretima posthuman, Albendazole, Earthworms.
Since ancient times, physicians around the world have been treating most of the diseases with the help of plants and their components. Plant-based compounds are usually considered safe and free from toxicity. However, some reports reveal that some nutritive plants such as Annona squamosa, Glycine max, and Leucaena leucocephala are considered toxic plants in many literature (Becker?Ritt et al., 2004) (Chandrakar et al., 2024; Yeligar et al., 2023) Leucaena leucocephala is a leguminous tropical tree commonly known as wild tamarind or white lead tree belonging to the Fabaceae family. It is a medium-sized, fast-growing mimosoid tree that is usually grown in tropical and subtropical climates (Alvim et al., 2023). It has been introduced worldwide due to its multiple uses such as soil fertilizer, firewood, livestock fodder, medicine, etc(Ojo et al., 2023). The leaves and dry seeds of the plant are rich in protein content. Mushy and silvery fibers are obtained from the wood of the plant, having excellent quality to make paper and board. This plant is widely used in livestock feed, especially for ruminants like sheep cattle, and goats. This tree can fix nitrogen from the atmosphere and help to enhance soil fertility(Marie-Magdeleine et al., 2020). Leucaena leucocephala is efficient in reducing soil erosion on slopes and near riverbanks due to its rapid growth and extensive root system. This tree contributes to carbon sequestration by absorbing atmospheric carbon dioxide and storing it in its biomass(Sithole et al., 2021). The taxonomical position of the Leucaena leucocephala is Kingdom: Plantae, Order: Fabales, Family: Fabaceae, Subfamily: Mimosoideae, Genus: Leucaena, Species: Leucaena. Leucocephala(Pendyala, 2010). Different parts of Leucaena leucocephala have been used in traditional medicine in various communities around the world for the treatment of various ailments. The decoction of root and bark is used to induce abortion. The roasted seeds are used as an emollient. The freshly crushed leaves of the tree are used to treat wounds in humans as well as animals. It is used as animal feed to increase milk production in cattle(Bhatia et al., 2014).
Different parts of Leucaena leucocephala are consumed in various countries like Thailand, Java, Indonesia, and America. In Thailand, America, and Indonesia young pods, leaves, and flowers are consumed in the form of soups, and seeds are popped like popcorn. In Java, seeds are eaten as sprouts (Dablin et al., 2021). The roasted seeds are used to prepare coffee-like beverages. Fresh young pods are consumed as a salad. The mucilage extracted from the fresh leaves is acidic and complex in structure. The mucilage obtained from the leaves resembles gum tragacanth and gum Arabic which did not contain mannose. The gum and mucilage of the plant are used as disintegrating agents in tablet formulation, and hydrogels in modified-release dosage forms(Pendyala, 2010).
Apart from their health benefits, seeds process some toxic effects due to the presence of the nonprotein amino acid namely mimosine, which produces a large number of toxic effects such as cataracts, alopecia, goiter, decreased fertility, growth retardation, and mortality in non-ruminant animals (Balderas-León et al., 2021). In Indonesia, where seeds are eaten from time to time, a large number of the population including children and adults experience alopecia associated with hair loss in the scalp and eyebrows. The localized edema of the scalp was also reported if the food is cooked in iron pots, it detoxifies the mimosine by forming a complex with it (Poonam Sethi and Pushpa R. Kulkarni, 1995). Although acclaimed traditionally as an antimicrobial and anthelmintic activity there is no scientific evidence regarding the effect therefore, the present study was designed to investigate the anthelmintic activity of aqueous and ethanolic extract of Leucaena leucocephala pods to generate the scientific report.
MATERIAL AND METHODS
Collection and authentication of plant
The pods of Leucaena leucocephala were collected from the minor forest area of Durg Chhattisgarh, India in March 2023. The leaves samples were identified as available gally on internet sources as well as local botanists.
Extraction methodology
The fresh pods of Leucaena leucocephala were washed with distilled water and cut into small pieces. About 400 gm pods of Leucaena leucocephala were extracted with 600 ml ethanol in a soxhlet apparatus at 80?C. A three-cycle of extraction runs within 48 hours. Before extraction defatting was done using petroleum ether by shocking. The ethanolic extract was dried by solvent evaporation in a water bath. In another set, 400 grams of pods of Leucaena leucocephala were extracted by using a hot decoction process on heating metal for 48 hours using water as menstruum and further concentrated by evaporating solvent. After complications of extraction, the extract was filtered to obtain and kept in a desiccator for further study(Jones & Kinghorn, 2012; XU et al., 2017).
Microscopic and Phytochemical screening
A cross-section of the sample was cut and prepared slide by conventional method. A conventional single stain was performed on the sample and after washing visualized in a light microscope at 100-time magnification(Harun et al., 2022). A procedure for screening phytochemicals is reported here in short. Test for alkaloids: A Dragendroff regent was used in the detection of alkaloids in the sample. A few drops of the test solution were treated with potassium bismuth iodide which produced an orange or brown precipitate, confirming the presence of an alkaloid. Test for carbohydrates: Heat the test solution with 2-3 drops of Barfoed’s reagent in a boiling water bath for 5-6 minutes, then cool under the running water. The presence of monosaccharide was confirmed by the production of a brick-red precipitate. Test for cardiac glycosides: 1ml of test solution is treated with a few drops of sodium picrate or picric acid, development of an orange color shows the presence of cardiac glycoside. Test for flavonoids: In a test tube, 1 ml of extract solution was added, followed by a few drops of diluted sodium hydroxide solution. With the addition of a few drops of diluted hydrochloric acid, the color changed from deep yellow to colorless, indicating the presence of flavonoids. Test for protein: About 1 ml of extract was poured into a test tube. Then the test solution was treated with an equal volume of 5% sodium hydroxide solution and copper sulfate. The development of the blue color confirms the presence of the protein in the sample. Test for steroids and terpenoids: Added a small amount of sulfur powder to the test solution; it sinks to the bottom, indicating the presence of steroids and terpenoids. Test for saponin: Added 5 ml of distilled water to the test sample in a test tube, shaken well, and a stable froth developed for 15 minutes, showing the presence of saponin. Test for amino acid: The presence of the amino acid was checked by using a ninhydrin reagent. Take 1 ml of ninhydrin with a similar quantity of the sample and subsequently heat it. The formation of purple color confirms the presence of the amino acid. Test for tannin: A ferric chloride solution is used to test the presence of the tannin. Take an extract into a test tube and add ferric chloride dropwise. The development of green or blue color shows the presence of tannin.(Dutta et al., 2023; Mohanasundaram & Saral, 2023)
Worm collection
In the present investigation, anthelmintic activity was assessed using the adult earthworm Pheretima posthuman. The earthworms are caught by the government undertaking Gowthan Samiti (A specialized form of development of worm compost) situated in Durg, Chhattisgarh India. near a swampy area in Nehru Nagar Bhilai, Chhattisgarh. The worms were washed with a standard saline solution to remove all filth. For the experiment, a 5 to 6 cm long earthworm was used.
Drugs and chemicals used
Albendazole (Alkem, India) was used as the reference standard. All other chemicals were purchased from Himedia Mumbai India.
Evaluation of anthelmintic activity
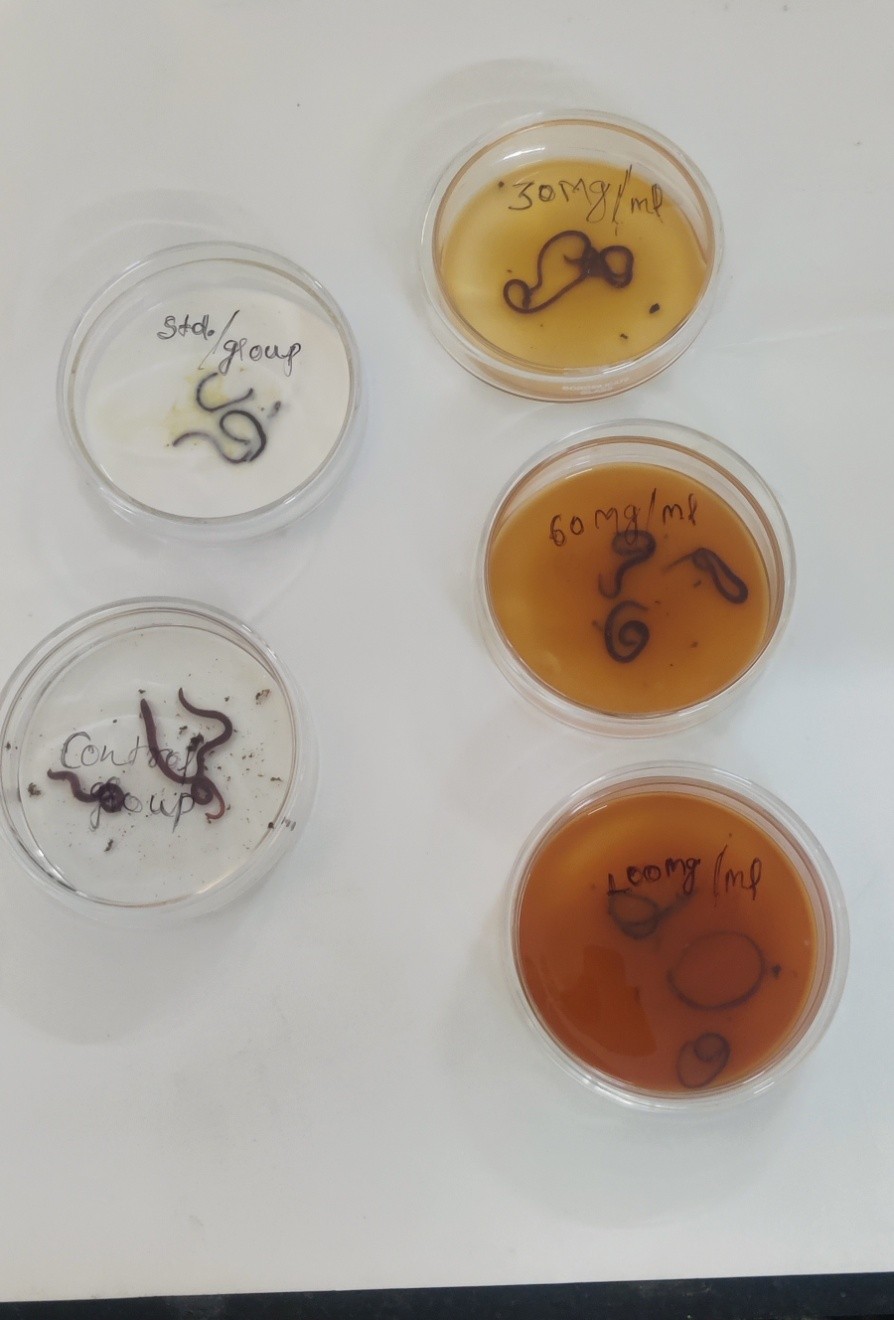
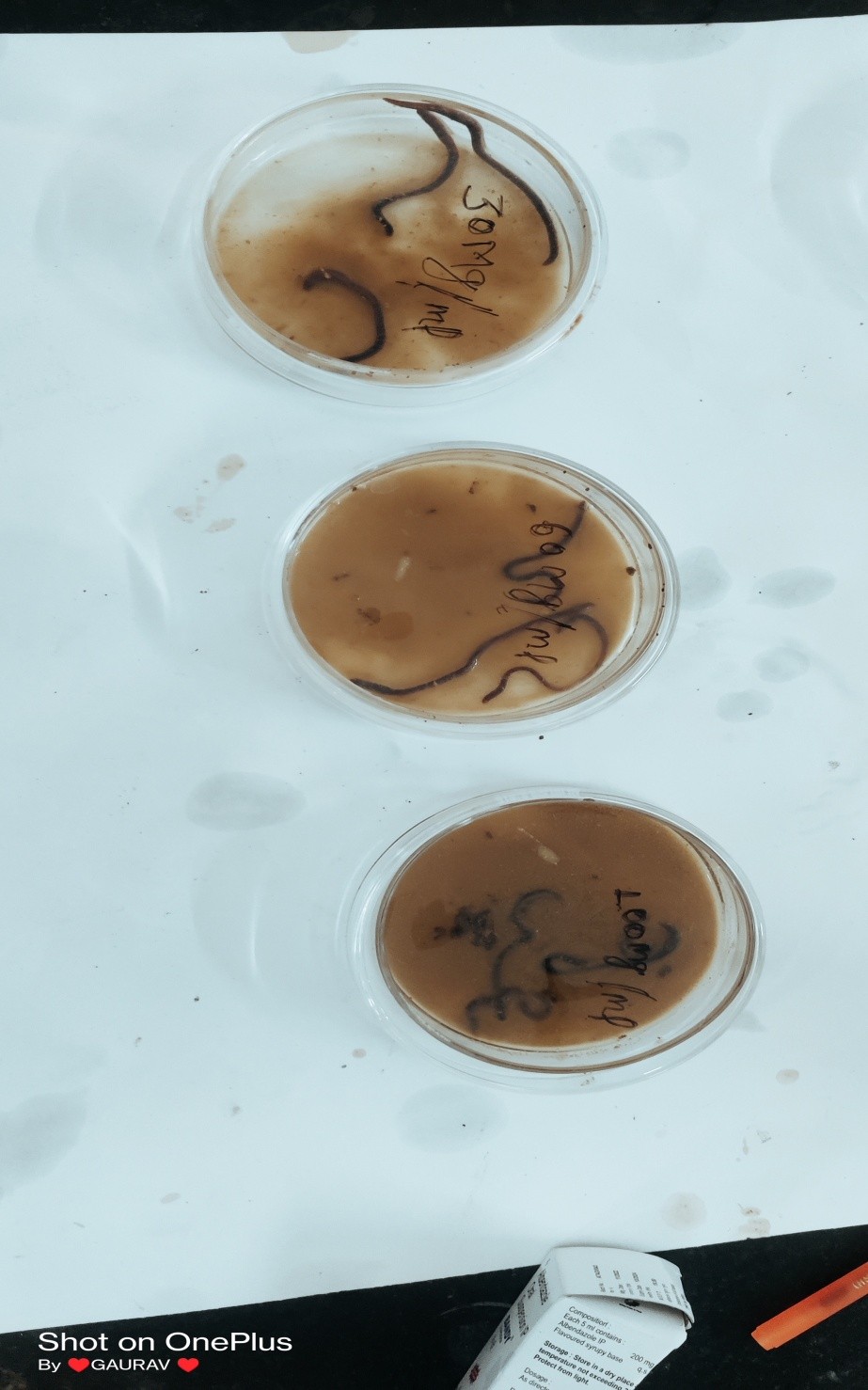
A group, each consisting of three earthworms of approximately the same size was released into 2 ml of sample at room temperature. Each group was first treated with distilled water and then treated with one of the following solutions. The standard drug Albendazole in suspension (30 mg/ml) and the test solutions i.e. ethanol and aqueous extract (30 mg/ml, 60 mg/ml, and 100 mg/ml) were evaluated for Anthelmintic activity(Sarwa et al., 2017). Observations were made for the time taken for paralysis/immobility and death/ morbidity of individual worms. The mean time for the paralysis (P) of worms in minutes was recorded when the worms lost their movement, except when the earthworms were shaken vigorously considered as death time. Death time (D) in minutes was noted after ascertaining the earthworms lost their movement followed by their body color fading away(Dkhil et al., 2020; Jamkhande & Barde, 2014). All anthelmintic experiments are repeated thrice. All the results were expressed as mean ± SEM of 3 worms in each group.
RESULTS AND DISCUSSION
Microscopic characters
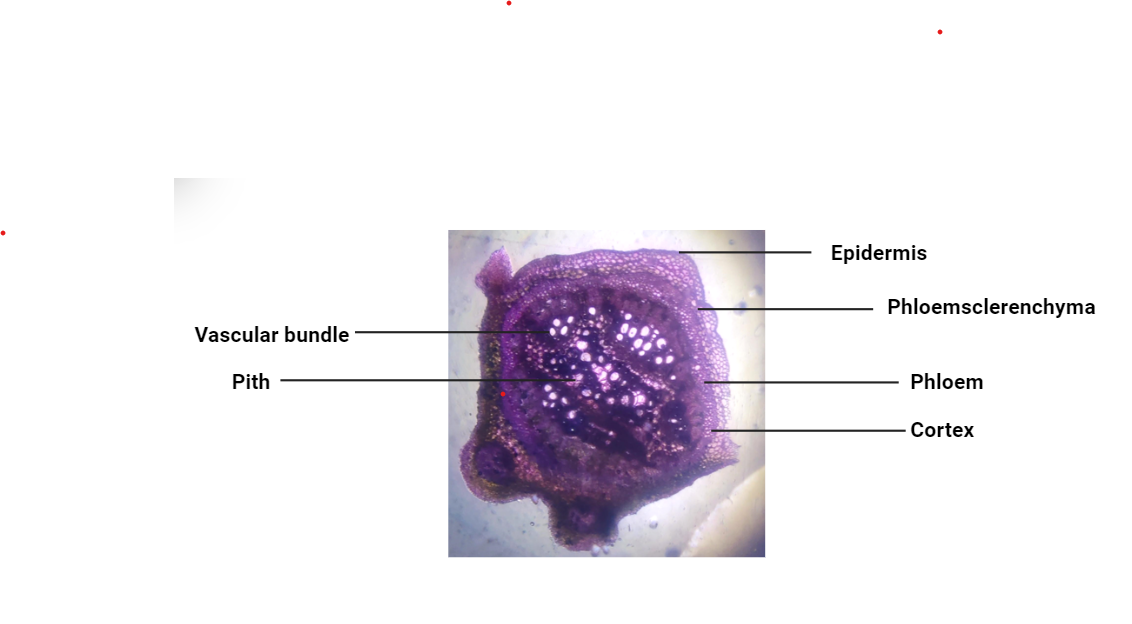
The transverse section of the Leucaena leucocephala stem shows the epidermis, sclerenchyma, cortex, phloem, vascular bundle, pith, etc. The epidermis is referred to as the outermost layer of stem cells. The cortex is situated between the epidermis and vascular bundles. The cross-section of Leucaena leucocephala stem is shown in Figure 1.

Figure 2 Phytochemical screening of aqueous pods extract of the Leucaena leucocephala

Figure 3 Phytochemical screening of ethanolic pods extract of the Leucaena leucocephala
Preliminary phytochemical screening of various extracts revealed the presence of different
primary and secondary metabolites. Phyllanthus
emblica leaves were found to contain steroid Preliminary phytochemical screening of various extracts revealed the presence of different primary and secondary metabolites. Phyllanthus emblica leaves were found to contain steroid.
Phytochemical screening of ethanolic and aqueous pod extract of Leucaena leucocephala
Preliminary phytochemical screening of the ethanolic and aqueous pod extract of Leucaena leucocephala revealed the presence of phytochemicals tabulated in Table 1. The findings indicate the extract contains alkaloids, cardiac glycosides, saponin glycosides, protein, tannins, steroids, and triterpenoids.


 Khomendra Sarwa* 2
Khomendra Sarwa* 2
 Manisha Chandrakar 1
Manisha Chandrakar 1


 10.5281/zenodo.14211686
10.5281/zenodo.14211686