Abstract
Chalcones are the biogenetic precursors of flavonoids and Isoflavonoids, which are abundant in plants. Chalcones are active lead molecules in medicinal chemistry for the discovery of new drugs. Here, we review properties, biosynthesis and structural diversity of natural chalcones. Chalcones are a group of naturally occurring compounds that have biological effects that include anti-inflammatory, anti-cancer, and antibacterial properties. Chalcones are active lead molecules in medicinal chemistry for the discovery of new drugs. Chalcone is a privileged species with medicinal significance as it consists of reactive keto ethylenic moiety–CO–CH=CH– belonging to flavonoids. The presence of a reactive ?, ?-unsaturated carbonyl function in chalcone and its derivatives is the reason for its pharmacological activities. Chalcones exhibit a wide spectrum of pharmacological effects such as antioxidant, antibacterial, anthelmintic, antiulcer, antiviral, insecticidal, antiprotozoal, anticancer, anti-inflammatory, antidiabetic. Chalcones can be synthesized by Claisen–Schmidt’s condensation, Heck’s reaction, Suzuki’s reaction. Multifaceted and complex underlying mechanisms of chalcone actions demonstrated their ability to modulate a number of cancer cell lines, to inhibit a number of pathological microorganisms and parasites, and to control a number of signalling molecules and cascades related to disease modification.
Keywords
Chalcone, Anticancer, Antimicrobial, Antiviral, Antibacterial, Antioxidant, Anti-inflammatory, Anti HIV.
Introduction
Chalcone is the organic compound and ?, ? unsaturated ketone. A variety of important biological compounds are known collectively as chalcones or chalconoids. They are widely known bioactive substances, fluorescent materials, and chemical intermediates. [1] Chalcones are 1, 3-diphenyl-2-propene-1-one, in which two aromatic rings are linked by a three carbon ?, ?-unsaturated carbonyl system as

These are abundant in edible plants and are considered to be precursors of flavonoids and isoflavonoids. Chalcones possess conjugated double bonds and a completely delocalized ?-electron system on both benzene rings. Chalcone (1,3-diaryl-2-propen-1-ones) are flavonoids found in fruits and vegetables, that attracted attention because of their pharmacological activities such as anti-inflammatory, antiviral, antibacterial, antifungal, antioxidant, antineoplastic. Most of aromatic rings of natural chalcones are found as hydroxylated, Chalcones, dihydrochalcones and aurones are composed of pigments whose colour changes from yellow to orange in some Coreopsis and Asteraceae taxa species. These compounds are found not only in flowers but also in lots of different tissues of the plants. Free radical scavenging properties of phenol groups of chalcones increased the interest in consumption of plants that included chalcones. [2] Chalcones are included dimer, oligomer, Diels-Alder adducts and different conjugates. At the same time because of being precursors of all of other flavonoid groups, chalcone are very important biosynthetic compounds. Essential property that separates chalcones and dihydrochalcones from the other flavonoids is that an open chain with three carbon molecules binds to A and B ring instead of C ring of flavonoids. Chalcones turn to flavanones with a stereospecific reaction catalyzed by chalcone isomerase enzyme in plants. Close biogenetic and structural relation between chalcones and flavanones is the reason for these compounds usually found together in natural products. This is the cause of the identification of chalcone, and dihydroflavonol generally. Chalcones are called as minor flavonoids for chalcones doesn’t seem appropriate because of increasing of new species of flavonoids. [3]
SYNTHESIS OF CHALCONE DERVATIVES
Scheme-1: Synthesis of Chalcone derivatives of 2-Acetyl Naphthalene
Chalcones 1a–g were constructed by treating 2-acetyl naphthalene with benzaldehyde /or substituted benzaldehyde in methanol and potassium hydroxide, and the constructed derivatives exhibited antibacterial and antifungal activities. [4]
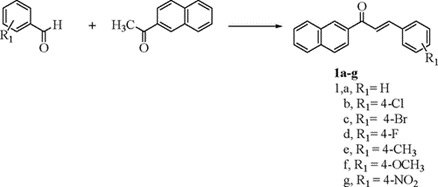
Scheme-2:
The trisubstituted triazines 6a–f were constructed by treating aniline with cyanuric acid at 0–5 °C to yield monosubstituted triazine, which was reacted with substituted amine at RT to produce disubstituted triazine 4. Treating the latter 4 with 4-aminoacetophenone afforded the corresponding trisubstituted triazine 5, which was reacted with different aldehydes to provide chalcone derivatives 6a–f. [5]

Scheme-3: Synthesis of Acetamido Chalcone Derivatives
Treatment of 4-acetamidoacetophenone (9) with substituted aldehydes in potassium hydroxide as the base in ethanol and sonication for 10–15 min using a water bath of ultrasonic cleaner afforded chalcone derivatives 10a–f. [6]

Scheme-4: Synthesis of Methoxyamino Chalcones
The chalcone derivatives 13–29 were synthesized via treatment of acetophenone derivative 11 and benzaldehyde derivative 12 in equal amounts using ethyl alcohol in 40% NaOH solution at 10 °C and stirred for 1 h and then at RT for 4 h; the reaction proceeded via Claisen–Schmidt reactions. [7]

Scheme-5: Synthesis of Sappanchalcone
Chalcone derivatives 32–44 were constructed via a Claisen–Schmidt reaction by treatment of benzaldehyde derivatives and acetophenone derivatives in methanol and potassium hydroxide and subject to ultrasonic irradiation for 8 h, utilizing a water bath at 80 °C, and exhibited XO inhibitory activity. [8]
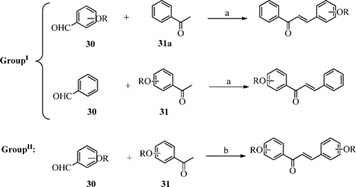
Scheme-6: Synthesis of Chalcone Derivatives from 1, 3-Diacetylbenzene and 1,4-Diacetylbenzene
Construction and biological potential of chalcone derivatives 45–47 were reported. These compounds were synthesized through acid-catalyzed one-step condensation of 1, 3- and/or 1,4-diacetylbenzene and 1,3,5-triacetylbenzene with 4-hydroxy-3-methoxybenzaldehyde in the presence of acids as acetic acid, concentrated hydrochloric acid, phosphoric acid, and concentrated sulfuric acid. The best result was achieved in case using concentrated sulfuric acid in ethanol. [9]
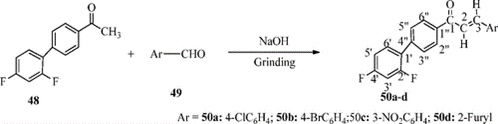
Scheme-7: Synthesis of Chalcone Derivatives from 1-(2?,4?-Difluorobiphenyl-4-yl) ethanone
The chalcone derivatives 50a–d were prepared in good yield via solvent-free Claisen–Schmidt condensation reaction of equal amounts of 1-(2?,4?-difluorobiphenyl-4-yl)ethanone with many aldehydes in 40% NaOH, leading to the formation of a sodium adduct which was neutralized by diluted HCl in cold water to afford the corresponding chalcone derivatives 50a–d. [10]

Scheme-8: Synthesis of Chalcone from Acetophenone Derivatives
Treatment of hydroxyacetophenone 51 with benzaldehyde derivative 52 in 50% KOH provided the corresponding chalcones 53–56, and the highest yield of chalcones ranged from 93 to 97%. (41) On the other hand, chalcone 53 was prepared in a low 32% yield in the presence of KOH as the catalyst, (42) whereas chalcone B was synthesized with BF3-Et2O catalyst in a high yield 90%. (43) Reaction of veratraldehyde with 4-hydroxyacetophenone yielded chalcone 54 in a high 97% yield. (41) However, treatment of 2, 4-dihydroxyacetophenone provided the corresponding chalcone 55 in 96% yield and 56 in 93% yield. [11]

Scheme 9: Synthesis of Chalcones
Chalcone derivatives 59–99 were constructed by treating aldehyde 57 with acetophenone 58 in ethanol containing NaOH 40% or few drops of hydrochloric acid. [12]

Scheme 10:
Treatment of benzaldehyde and acetophenone derivatives in acid catalyst or alkaline conditions at 50–100°C in presence of liquid solvent led to 100 (Claisen–Schmidt Condensation). [13]

Scheme 11: Synthesis of Chalcone from Phenyl Halide
Chalcone 100 was prepared by reaction of phenyl halide and styrene in carbon monoxide and pelladium catalyst. [14]

Scheme 12: Treatment of phenylacetylene with benzaldehyde in (BmimOTs) and HBr at 100 °C for 12 h provided the corresponding chalcone 99. The reaction proceeds via a coupling reaction. [15]

Scheme 13: Synthesis of Chalcone 100 via a Coupling Reaction
Chalcone derivative 101 was constructed from the reaction of propargyl alcohol and phenyl halide using microwave irradiation utilizing PdCl2 (pph3)2 in THF. [16]

Scheme 14: Synthesis of Chalcone Derivatives from Benzoyl Chlorides
Ynones were synthesized by reaction of benzoyl chlorides and phenylacetylenes using Sonogashira conditions described in a literature procedure. Ynones undergo deuterations using the H-Cube system using D2O instead of water. [17]

Scheme 15: Chalcone 100 was synthesized by reaction of benzoyl chloride with styrylboronic acid 103 in was 100 anhydrous toluene and in Chalcone presence of Pd(PPh3)4 and CsCO3. Also from the reaction of cinnamoyl chloride 104 and phenylboronic acid in anhydrous toluene and in the presence of Pd (PPh3)4 and CsCO3, the reaction proceeds via the Suzuki–Miyaura coupling reaction. [18]
Scheme 16: Synthesis of Chalcone via One-Pot Synthesis
Treatment of phenylmethanol 105 and acetophenone using CrO3 as the oxidizing agent provided chalcone derivative 100. [19]
Scheme 17: One-Pot Synthesis of Chalcone 100
Treatment of benzaldehyde and phenylacetylene under microwave irradiation using heterogeneous acid in a catalytic amount in 1, 2-dichloroethane as solvent provided the corresponding chalcone 100. [20]
Scheme 18: Some Chalcones from Natural Product
Chalcones occur in natural products derived from plant species, such as Macaranga denticulata, which contains compound 106. Also Uvaria siamensis roots contain compound 107; compound 108 is derived from Stevia lucida, and compound 109 is found in Pongamia pinnata (L.). Hydroxychalcone-based sugar functionalities 110–112 were separated from Coreposis lanceolata flowers. [21]

BIOLOGICAL ACTIVITES
Anticancer Activity
Some chalcones from synthetic as well as natural origin were acknowledged as active against tumor cells along with antioxidant principles, by inhibiting superoxide production and lipid peroxidation. An anticancer chalcone, Millepachine (5) is isolated from Millettia pachycarpa. Licochalcone A (6) isolated from Glycyrrhiza inflate is another anticancer chalcone which exhibited toxicity towards L1210 leukemia and B16 melanoma cells. A new class of chalcone (7) proposed as an anti-mitotic agent by increasing the survival of mice inoculated with L1210 leukemia with doses range of 2.65-5.0 mg/kg. Butein (8) is another natural chalcone which can suppress the several human cancers including, breast cancer, colon carcinoma, osteosarcoma, and hepatic stellate cells in vitro. [22]
Antimicrobial Activity
Antimicrobial activity of chalcones are believed owing to ?, ?-unsaturated carbonyl function. Isobavachalcone (9) and bavachalcone (10) are two important chalcones isolated from Psoralea corylifolia reported as antibacterial agents from the natural source. A synthetic scaffold, 3-(Carboxyalkyl) rhodanine (11) is an antimicrobial chalcone which displays potent inhibition at low concentration (1?g/mL) against human pathogens and document as antibacterial as well as antifungal agent. Another, ring fused chalcone (12) is proposed as an antimicrobial scaffold for the treatment of oral infections. A hybrid chalcone comprises pharmacophore fluconazole (13) showed potent inhibitionwith 0.12 ?g/mL IC50 concentration against Candida albicans and patented as antifungal agent. [23]
Anti-HIV
Few prominent chalcones were reported from natural and synthetic origin as active against Human Immune Virus (HIV). The natural chalcone, xanthohumol (4) is isolated from Hops Humulus exhibits anti-HIV properties. Nakagawa and Lee (2006) isolated a unique ?-hydroxy chalcone (14) from the genus Desmos show good anti-HIV activity. Another chalcone (15) was isolated from the leaves of Maclura tinctoria (Moraceae) showed inhibitory activity against pathogens Candida albicans and Cryptococcus neoformans related to AIDS. An adamantly chalcone (16) was approved as HIV patent which showed trivial activity against HIV disease [24].
Antidiabetic Activity
Chalcones were reported as potent inhibitors of ?-glucosidase, dipeptidyl peptidase-4 (DPP4), peroxisome proliferator-activated receptors (PPAR) and protein tyrosine phosphatase 1B (PTP1B), aldose reductase and are significant agents for the treating diabetes mellitus. Isoliquiritigenin (17), echinatin (18),licochalcone A (6), lichochalcone C (19) and lichochalcone E (20) were isolated from Glycyrrhiza inflata and its synthetic derivatives are reported as PTP1B which play vital role in the treating of type II diabetes and obesity, as a negative regulator of the insulin and leptin signaling pathway. A novel chalcone, abyssinone-VI- 4-O-methyl ether (21) isolated from the root bark of Erythrina mildbraedii showed potent antidiabetic activity by inhibition of PTP1B were reported new sulfonamide chalcones (22-29) as a strong inhibitor of the ?-glucosidase enzyme. [25]
Anti-Inflammatory activity
Naringenin-chalcone (30) is a well-known natural compound which exhibits anti-inflammatory activity by inhibiting the production of cytokines, a pro-inflammatory agent. Isoliquiritigenin (31) isolated from Nepalese propolis and butein (32) isolated from Rhus verniciflua are another significant natural chalcones which exhibit potent anti-inflammatory activity by inhibiting LPS-induced iNOS and COX-2 expression. A reduced chalcone Naringenin (33) which identified as anti-inflammatory agent by inhibiting the production of NO induced by LPS and INF in murine microphage-like cell lines. Another synthetic hetero chalcone (34) had reported as a potent cytokine inhibitor used for the management of anti-inflammatory ailments. [26]
Anti-leishmanial Activity
Licochalcone A (6) is a renowned natural antiparasitic agent used to treat various abdominal spasmodic symptoms by Japanese. Kanzonol C (35), isolated from the licorice roots (Glycyrrhiza eurycarpa, Leguminosae) showed strong antileishmanial activity. Crotaramosmin (36) is another important chalcone isolated from Crotolaria rosmosissima, which showed potent antileishmanial activity. A dihydrochalcone (37) synthesized showed trivial antileishmanial activity. A new class of dihydropyrimidine derivates, and the compound (38) displayed antileishmanial activity against promastigotes of Leishmania major and L. donovani with the inhibitory concentration of 0.47 ?g/mL and 1.5 ?g/mL, respectively. [27]
Antioxidant Activity
Numerous free radicals are produced in the human body during the metabolic process may capable to damage the biomolecules such as DNA, proteins and lipids through oxidation which results several oxidative damage related diseases suchas cancers, non-inflammatory tumors, digestive ulcers, rheumatoid arthritis and aging. A penta-oxygenated chalcone (39) isolated from Glycyrrhiza uralensis (Leguminosae) exhibits potent DPPH radical activity and used as traditional medicine in northeastern China. Cedredipronone (40), is another chalcone isolated from the extracts of fruits and seeds of Cedrelopsis grevei (Ptaeroxylaceae) showed strong superoxide scavenging properties. The prenylated chalcone glycoside (41), isolated from the bark of Maclura tinctoria (Moraceae) showed the radical scavenging activity in different antioxidant principles. An allylated chalcones (42-44) which shows good antioxidant activity than non-allylated chalcones by inhibiting the free radicals. [28]
Anti-tuberculosis Activity
Nardoaristolone A (45) is a novel terpenoid chalcone with unusual skeleton, isolated from Nardostachys Chinensis exhibited promising antituberculosis activity. Fluorine substituted synthetic chalcone (46) has reported as antitubercular agents against Mycobacterium tuberculosis strain. [29]
Antiviral Activity
Naringenin-chalcone (30) is widely distributed in citrus fruits and described to possess antiviral properties. Another natural chalcone, Myrigalone G (47) is isolated from Leptospermum recurvum (Myrtaceae) exhibits antiviral activity against the herpes simplex virus. Iryantherin K (48) and L (49) are the two antiviral chalones isolated from Iryantheria megistophulla showed strong inhibition against potato virus and moderate inhibition against acetylcholinesterase. The Compounds 48 and 49, are C-benzylated dihydrochalcone-lignan conjugate diastereo isomers, and this occurrence is very rare in nature. [30]
Antiulcer Activity
Kanzonol C (35) is incidence from the natural source as well as from the synthetic origin. A synthetic derived chalcone (35), exhibits potent antiulcer activity. Sophoradin (50) is a natural occurring prenylated chalcone, and its derivatives were displayed antiulcer activity. Synthetic analogs (51-53) of sophoradin were showed highest antiulcer activity as similar potency with sophoradin. [31]
CONCLUSION:
The present review emphasis primarily on the novel chalcone derivatives with promising applications reported from the natural source as well as synthetic origin. Furthermore, this comprehensive review describes the biosynthesis of chalcones, structural significance of chalcones to be as fluorescent materials, various synthetic approaches for the preparation chalcones, therapeutic applications. It is an interesting note to mention that the chalcone derivatives have a privileged template with ?, ?-unsaturated carbonyl system and easily allow for the structural modifications. For this reason, the researchers make much attention on skeletal modification of chalcones in the design of new and novel materials with diverse applications. Hence, chalcone derivatives are an innovative scaffold and plays a significant role in the drug discovery. REFERENCES
- Tomás?Barberán, Francisco A., and Michael N. Clifford. "Flavanones, chalcones and dihydrochalcones–nature, occurrence and dietary burden." Journal of the Science of Food and Agriculture 80.7 (2000): 1073-1080.
- Opletalova, V., et al. "Conformational analysis of 2-hydroxy-2?, 5?-diazachalcones." Journal of pharmaceutical and biomedical analysis 23.1 (2000): 55-59.
- Veitch, N. C., and R. J. Grayer. "Chalcones, dihydrochalcones, and aurones." (2006): 1003-1100.
- Arora, V. A. R. U. N., Arora, P., & Lamba, H. (2012). Synthesis and biological activities of some 3, 5-disubstituted pyrazoline derivatives of 2-acetyl naphthalene. International J. Pharm. and Pharmaceut. Sci, 4(4), 2012.
- SG, P., et al. "Synthesis, Characterization, Antimicrobial studies and Evaluation of Stability constants of Cu (II), Ni (II) and Co (II) complexes with the ligands derived from chalcone derivatives." Appl. Organomet. Chem 36.2 (2022): e6465.
- Suwito, Hery, et al. "Design and synthesis of chalcone derivatives as inhibitors of the ferredoxin—Ferredoxin-NADP+ reductase interaction of plasmodium falciparum: Pursuing new antimalarial agents." Molecules 19.12 (2014): 21473-21488.
- Bui, T. H.; Nguyen, N. T.; Dang, P. H.; Nguyen, H. X.; Nguyen, M. T. T. Design and synthesis of chalcone derivatives as potential non-purine xanthine oxidase inhibitors. SpringerPlus. 2016, 5 (1), 1789.
- Jung, J.-C.; Lee, Y.; Min, D.; Jung, M.; Oh, S. Practical synthesis of chalcone derivatives and their biological activities. Molecules. 2017, 22 (11), 1872.
- Fathimunnisa, M.; Manikandan, H.; Sivakumar, D. An efficient, solvent free synthesis of some chalcone derivatives and their biological evaluation. Asian J. Chem. 2018, 30 (4), 807– 810.
- Anwar, C.; Prasetyo, Y. D.; Matsjeh, S.; Haryadi, W.; Sholikhah, E. N.; Nendrowati, N. Synthesis of chalcone derivatives and their in vitro anticancer test against breast (T47D) and colon (WiDr) cancer cell line. Indonesian Journal of Chemistry. 2018, 18 (1), 102– 107.
- Wang, J.; Huang, L.; Cheng, C.; Li, G.; Xie, J.; Shen, M.; Chen, Q.; Li, W.; He, W.; Qiu, P.Design, synthesis and biological evaluation of chalcone analogues with novel dual antioxidant mechanisms as potential anti-ischemic stroke agents. Acta Pharmaceutica Sinica B 2019, 9 (2), 335– 350.
- Mahapatra, D. K.; Bharti, S. K.; Asati, V. Chalcone scaffolds as anti-infective agents: Structural and molecular target perspectives. European journal of medicinal chemistry 2015, 101, 496– 524.
- Wu, X.-F.; Neumann, H.; Spannenberg, A.; Schulz, T.; Jiao, H.; Beller, M. Development of a general palladium-catalyzed carbonylative Heck reaction of aryl halides. J. Am. Chem. Soc. 2010, 132 (41), 14596–14602.
- Xu, L. W.; Li, L.; Xia, C. G.; Zhao, P. Q. Efficient coupling reactions of arylalkynes and aldehydes leading to the synthesis of enones. Helvetica chimica acta 2004, 87 (12), 3080–3084.
- Takahashi, S., et al. "A convenient synthesis of ethynylarenes and diethynylarenes." Chemischer Informationsdienst 11.47 (1980).
- Hsieh, C.-T.; Otvos, S. B.; Wu, Y.-C.; Mandity, I. M.; Chang, F.-R.; Fulop, F. Highly Selective Continuous-Flow Synthesis of Potentially Bioactive Deuterated Chalcone Derivatives. ChemPlusChem. 2015, 80 (5), 859– 864.
- Otvos, S. B.; Hsieh, C.-T.; Wu, Y.-C.; Li, J.-H.; Chang, F.-R.; Fulop, F. Continuous-flow synthesis of deuterium-labeled antidiabetic chalcones: Studies towards the selective deuteration of the alkynone core. Molecules. 2016, 21 (3), 318.
- Mahapatra, D. K.; Bharti, S. K.; Asati, V. Chalcone scaffolds as anti-infective agents: Structural and molecular target perspectives. European journal of medicinal chemistry 2015, 101, 496– 524.
- Rueping, M.; Bootwicha, T.; Baars, H.; Sugiono, E. Continuous-flow hydration–condensation reaction: Synthesis of ?, ?-unsaturated ketones from alkynes and aldehydes by using a heterogeneous solid acid catalyst. Beilstein journal of organic chemistry. 2011, 7 (1), 1680– 1687.
- Salae, A.-W.; Chairerk, O.; Sukkoet, P.; Chairat, T.; Prawat, U.; Tuntiwachwuttikul, P.; Chalermglin, P.; Ruchirawat, S. Antiplasmodial dimeric chalcone derivatives from the roots of Uvaria siamensis. Phytochemistry. 2017, 135, 135– 143.
- Wu, Wenshuang, et al. "Millepachine, a novel chalcone, induces G 2/M arrest by inhibiting CDK1 activity and causing apoptosis via ROS-mitochondrial apoptotic pathway in human hepatocarcinoma cells in vitro and in vivo." Carcinogenesis 34.7 (2013): 1636-1643.
- Rammohan, Aluru, et al. "Chalcone synthesis, properties and medicinal applications: a review." Environmental Chemistry Letters 18 (2020): 433-458.
- Wang Q, Ding ZH, Liu JK, Zheng YT (2004) Xanthohumol, a novel anti-HIV-1 agent purified from Hops Humulus lupulus. Antiviral Res 64: 189?194.
- Yoon, Goo, et al. "Inhibitory effect of chalcones and their derivatives from Glycyrrhiza inflata on protein tyrosine phosphatase 1B." Bioorganic & medicinal chemistry letters 19.17 (2009): 5155-5157.
- Hirai, Shizuka, et al. "Inhibitory effect of naringenin chalcone on inflammatory changes in the interaction between adipocytes and macrophages." Life sciences 81.16 (2007): 1272-1279.
- Rashid, Umer, et al. "Structure based medicinal chemistry-driven strategy to design substituted dihydropyrimidines as potential antileishmanial agents." European journal of medicinal chemistry 115 (2016): 230-244.
- Cioffi, Giuseppina, et al. "Antioxidant Chalcone Glycosides and Flavanones from Maclura (Chlorophora) t inctoria." Journal of Natural Products 66.8 (2003): 1061-1064.
- Guantai, Eric M., et al. "Enone–and chalcone–chloroquinoline hybrid analogues: in silico guided design, synthesis, antiplasmodial activity, in vitro metabolism, and mechanistic studies." Journal of medicinal chemistry 54.10 (2011): 3637-3649.
- KYOGOKU, KAZUAKI, et al. "Anti-ulcer effect of isoprenyl flavonoids. II. Synthesis and anti-ulcer activity of new chalcones related to sophoradin." Chemical and Pharmaceutical Bulletin 27.12 (1979): 2943-2953.
- Sasajima, M., et al. "Studies on the anti-ulcer effects of isoprenyl flavonoids (1). The anti-ulcer effects of isoprenyl chalcone extracted from Sophora subprostrata (author's transl)." Nihon yakurigaku zasshi. Folia pharmacologica Japonica 74.8 (1978): 897-905.
- Rammohan, Aluru, et al. "Chalcone synthesis, properties and medicinal applications: a review." Environmental Chemistry Letters 18 (2020): 433-458.


 A.Sri Priya*
A.Sri Priya*
 K. Govinda Rao
K. Govinda Rao










 10.5281/zenodo.13941625
10.5281/zenodo.13941625