Abstract
The majority of potential medications in the pharmaceutical industry have limited water solubility and permeability. SEDDS are relatively new lipid-based technological breakthroughs that show great potential for enhancing the oral bioavailability of poorly water-Soluble medications. This technology has the potential to improve oral bioavailability, allow for dosage reduction, provide a more consistent temporal profile of drug absorption, selectively target drugs in the stomach, and Protect drugs from a hostile environment. this review gives an understanding about Mechanism of sedds, Rationale, Suitable drug candidate, Component of sedds and Characterization of sedds, Solidification method of sedds, Challenges and limitation of sedds.
Keywords
Self-Emulsifying Drug delivery System (SEDDS), Solubility, Permeability, Bioavailability, Droplet size, Solid SEDDS (S- SEDDS).
Introduction
The pharmaceuticals are frequently supplied orally; however, around 40% of new drug competitors have poor-water solvency, and the oral distribution of such medications is challenging because to their low bioavailability, high intra- and inter-subject variability, and an absence of dose proportionality. The needed concentration of any drug to elicit its pharmacological reaction is primarily dependant on its solubility, making it extremely difficult for formulation scientists to maintain the pharmacological range of lipophilic medicines. (1) Emulsions are used as drug carriers in pharmaceutical preparations, despite the fact that they have poor absorption profiles that may increase oral bioavailability of the treatment. One of the most common ways for improving the stability of orally delivered APIs is to use lipid-based drug delivery vehicles. According to the literature, the terminology for lipid-based approaches is heavily contested. (2) A self-emulsifying drug delivery system (SEDDS) is a lipid-based formulation including an isotropic mixture of surfactants, oil phase, co-solvents, and medication that forms a milky emulsion with submicrometric droplet size after modest agitation in water or gastrointestinal fluid. The small globules formed enhance the interfacial area, allowing for a faster release of medications. This can increase the intestinal permeability of a number of drugs by boosting lymphatic transport and skipping the metabolism of the first step, thus improving drug bioavailability. (3)
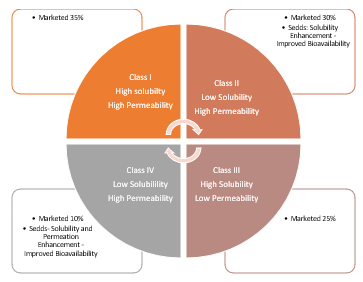
Fig. Biopharmaceutical Classification System
The size of the initial droplet is the most important factor in determining micro and nanoemulsions (SMEDDS and SNEDDS). SEDDS is a broad term that often refers to emulsions with droplet sizes ranging from a few nanometers to several micrometers. "Selfmicro-emulsifying drug delivery systems" (SMEDDS) refers to formulations that produce clear microemulsions with oil droplets ranging from 100 to 250 nm. The phrase "self-nano-emulsifying drug delivery systems" (SNEDDS) refers to globules smaller than 100 nm.(4) SEDDS are isotropic and thermodynamically stable systems consisting of oil, surfactant, cosolvent/co-surfactant, and drug components, which can form an oil/water microemulsion when mixed with water at low speed.” Oil and surfactant mixtures can be diluted in excess GIT fluid to generate these systems. This distinct construction mechanism makes these systems ideal for oral administration.(5) SEDDS improves dissolving rate by causing the emulsion to develop spontaneously in the gastrointestinal tract with slight agitation caused by stomach motility. The medication is supplied in a solubilised condition, and the droplet's small size provides a large interfacial surface area for drug absorption. The SEDDS can influence medication absorption in a variety of ways, including as increasing drug solubility, permeability, and lymphatic uptake. When a SEDDS is taken orally, it enters the GIT's (gastrointestinal tract) lumen and interacts with the GI fluid to form micro or nanoemulsion. Oil droplets flow swiftly through the stomach, increasing drug distribution throughout the GIT, and lymphatic absorption allows the medicine to avoid first-pass metabolism, improving drug oral bioavailability. (6)
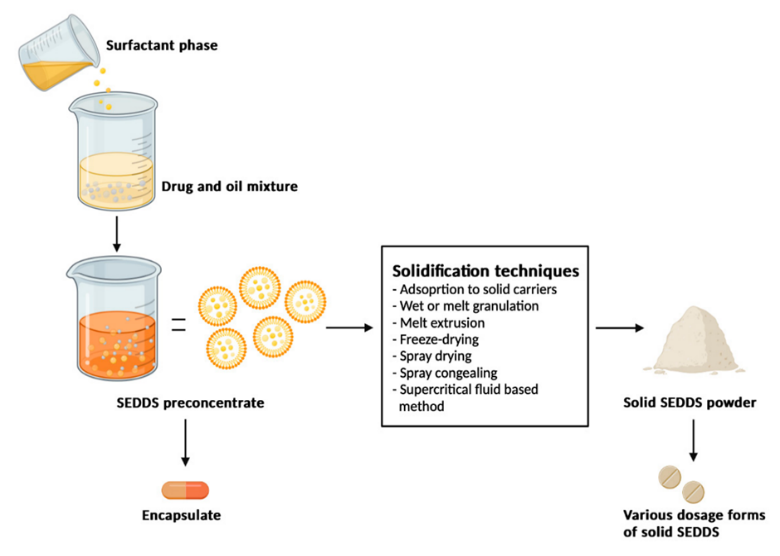
Fig. Self-Emulsifying Drug Delivery System
Recently, formulation scientists in the pharmaceutical business have faced intriguing hurdles when it comes to formulating poorly soluble molecules. The pharmaceutical industry has found up to 40% of new chemical entities to be lipophilic or poorly soluble, which results in substantial intra- and inter-subject variability, lack of dosage proportionality, and low oral bioavailability. Many methods, including reducing particle size, using wetting agents, co-precipitation, and creating solid dispersions, have been tried in the oral formulation of these chemicals in an effort to alter the dissolution profile and increase the absorption rate. Among the various delivery methods, such as incorporating pharmaceuticals into oils, emulsions, liposomes, and surfactant dispersion, one of the most widely used strategies is the use of self-emulsifying drug delivery systems (SEDDS).(7)
Rational Of Sedds (8) (9)
When BCS class II or IV drugs are administered orally to the gastrointestinal system, their dissolution rate is often restricted. There is presently no one, easy answer to the problem. Various formulation techniques can be utilized for this. Indeed, in some circumstances, these tactics have proved effective. However, these approaches have drawbacks.
- Salt generation from neutral substances is not possible, and the synthesis of weak acid and weak base salts may not always be practicable. Furthermore, the salts generated may revert to their original acid or basic forms, causing aggregation in the gastrointestinal system.
- Particle size reduction may be undesirable in instances where very tiny particles are difficult to handle and have poor wettability.
- The problem with micronization is chemical/thermal stability; many drugs deteriorate and lose bioactivity when micronized using standard methods.
- The number of carriers employed in solid dispersion is frequently substantial, thus if the active component dose is high, the tablets or capsules created will be huge in volume and difficult to swallow. Furthermore, the carriers employed are often costly, and the freeze-drying or spray-drying methods necessitate specific facilities and processes, resulting in high manufacturing costs.
- Complexation using cyclodextrin methods is not suitable for medicinal compounds that are insoluble in both aqueous and organic solvents.
The discovery that the oral bioavailability of low water soluble medications can be increased when delivered with a fat-rich meal has sparked renewed interest in the formulation of poorly water soluble pharmaceuticals in lipids. (10)
Mechanism:
A single hypothesis cannot account for every facet of the creation of microemulsions. The surfactant and co-surfactant were thought to have formed a complex layer at the oil-water interface, which was the cause of the spontaneous creation of micro emulsion droplets. When the entropy change favoring dispersion is larger than the energy needed to enhance the dispersion's surface area and the free energy (A?G) is negative, emulsification takes place, according to the thermodynamic theory of microemulsion production.(11)
The free energy of a standard emulsion formulation is directly proportional to the energy required to construct a new surface between the oil and water phases. Equation describes the thermodynamic relationship of net free energy change.
?G =?Ni?ri2?
where: ?G is the free energy associated with the operation, ri is the radius of droplets, Ni is the number of droplets, and ? is the interfacial energy. The two phases of the emulsion tend to separate with time, reducing the interfacial area and hence lowering the free energy of the system(s). Conventional emulsifying agents stabilize emulsions formed by aqueous dilution by establishing a monolayer surrounding the emulsion droplets, lowering interfacial energy and creating a barrier to coalescence. In contrast, emulsification occurs spontaneously with SEDDS because the free energy required to create the emulsion is modest, whether positive or negative. Emulsification requires the interfacial structure to provide low or no resistance to surface shearing.(12)
Formulation Consideration for Development of Sedds
1. The drug's ability to dissolve in various oils, surfactants, and co-solvents.
2. Choosing the oil, surfactant, and co-solvent according to the drug's solubility and the phase diagram's creation
3. Making the SEDDS formulation by dissolving the medication in a mixture of co-solvent, surfactant, and oil.
4. The digestion rate.
5. The digested formulation's solubilization ability.
6. The temperature that self-emulsification takes place at. (13)
Suitable Drug Candidate Identification for Sedds
Lipinski's rule of five has been widely used as a qualitative predictive model for oral absorption patterns. In the discovery situation, the 'rule of five' predicts that poor absorption or poor permeation is more likely when there are more than five H-bond donors, more than ten H-bond acceptors, the molecular weight exceeds 500, and the computed log P is greater than 5. The question is whether solubility and log P alone are adequate to identify prospective medication candidates for such formulations. Although categorization techniques like the BCS and Lipinski's rule of five are useful, especially for preliminary screening, they have drawbacks. It is thought that the rule of five only applies to molecules that are not substrates for active transporters, and with growing evidence indicating that most medications are substrates for either efflux or uptake transporters, this constraint may be significant. Aqueous solubility and/or log P alone are unlikely to be sufficient to determine the feasibility of a lipid-based formulation technique because they do not accurately predict potential in vivo (i.e. physiological) impacts.(14)
Components Of Sedds: -
- Oils
- Surfactant
- Co-solvent
- Oil:- Oil is an important component of a self-emulsifying drug delivery system; the oil phase should be lipophilic and low viscosity. A huge number of excipients are utilized in the process of developing a self-emulsifying drug delivery system. Normally, oils with long and medium triglyceride chains and different numbers of double or triple bonds are utilized to formulate self-emulsifying drug delivery systems. Edible oils provide a "natural" base for lipid-containing compounds, but they are no longer used in the preparation of self-emulsifying drug delivery systems due to their low solubility and low self-emulsification efficiency. As a result, modified or hydrolyzed vegetable oils are preferred for self-emulsifying drug delivery system formulation. Oils can solubilize the required dose of the lipophilic drug and facilitate self-emulsification, as well as increase the fraction of the lipophilic drug transported via the intestinal lymphatic system, thereby increasing absorption from the GI tract, depending on the triglyceride's molecular nature.(15)
- Surfactant: - The second required component in SEDDS is surfactants, which are amphiphilic molecules having a hydrophilic head and a hydrophobic tail. These surfactants are included in SEDDS formulations because of their capacity to lower surface tension and build a monolayer between the oil and aqueous phases. This approach makes SEDDS more stable and allows them to stay in the GIT for longer periods of time in soluble state, which aids medication absorption. When selecting a surfactant for SEDDS, two important factors to consider are the drug's maximal solubility in the surfactant and the hydrophilic-lipophilic balance (HLB). HLB values and surfactant content have a significant influence on emulsion droplet size. The droplet size of the emulsions decreased and then increased with increasing surfactant concentration because at first, the amount of adsorbed surfactant around a droplet's oil-water interface increases, resulting in a decrease in the system's interfacial tension and the formation of fine droplets. However, when the surfactant concentration increases, extra water penetrates the bulk oil, generating substantial interfacial disturbance and ejection of droplets into the bulk aqueous phase.(16)
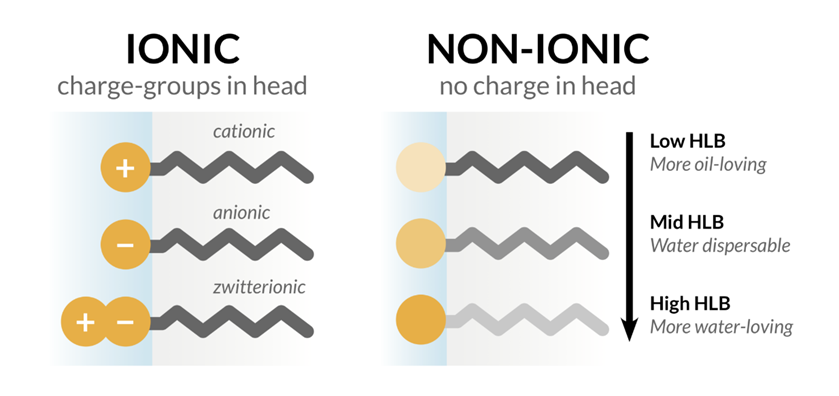
Fig. Types of Surfactant
The most commonly advised surfactants to be used in SEDDS are nonionic ones with relatively high HLB values, such as lecithin and fatty acid ethoxyl and sorbitan esters, which have HLB values between 4 and 15. Examples of these include Brij-30, Labrafac CM 10, Tween 20, Tween 80, Cremophor RH40, Pluronic L-64, and Emulphor El-620.(17) In addition to fine globule formation, surfactant inhibition of the efflux transporter is thought to contribute to increased bioavailability by reducing the possibility of the molecule being effluxed back into the intestinal lumen. Some nonionic surfactants as Cremophore EL, Cremophore RH40, Span, and Tween have been shown to block P-gp (P-glycoprotein), one of the generally recognized efflux transporters. (18)(19)
- Co- Solvent :- The formation of an optimal SEDDS requires rather high concentrations of surfactants (usually more than 30% w/w), hence surfactant concentrations can be decreased by using a cosurfactant. The role of the cosurfactant, in conjunction with the surfactant, is to reduce interfacial tension to a very low, even transitory negative value. At this value, the interface would expand to create finely dispersed droplets, which would then absorb additional surfactant and surfactant/co-surfactant until their bulk condition was reduced enough to restore positive interfacial tension. The microemulsion is formed by a process known as'spontaneous emulsification'. Many non-ionic surfactants do not require the addition of a cosurfactant in self-emulsifying systems.(20) The choice of surfactant and co-surfactant is critical not only to the creation of SEDDS, but also to the drug's solubility in the SEDDS. Organic solvents suitable for oral administration (ethanol, propylene glycol (PG), polyethylene glycol (PEG), etc.) may aid in the dissolution of large amounts of either the hydrophilic surfactant or the drug in the lipid base and can act as a cosurfactant in self-emulsifying drug delivery systems, though alcohol-free self-emulsifying microemulsions have also been described in literature. Indeed, such systems may exhibit some advantages over previous formulations when incorporated in capsule dosage forms, because alcohol and other volatile co-solvents in conventional self-emulsifying formulations are known to migrate into the shells of soft gelatin or hard sealed gelatin capsules, resulting precipitation of the lipophilic drug.(21) Co-solvents are added in the form of aqueous solvents such as Triacetin (an acetylated derivative of glycerol), glyceryl triacetate, or other appropriate solvents. Triacetin is appropriate since it is miscible in the oil and lipid phases and may be utilized to solubilize a hydrophobic medication.(33) In SEDDS, co-surfactants with HLB values of 10-14 are commonly utilized. Hydrophilic co-surfactants are preferred alcohols of intermediate chain length such as hexanol, pentanol, and octanol, which are known to lower the oil-water interface and allow the spontaneous creation of microemulsions.(34)
Table 1: Examples of oils, surfactants, co surfactant and co solvents
|
Oils
|
Surfactant
|
Co-Surfactant/Co-Solvent
|
|
Cotton Seed Oil
|
Polysorbate 20 (Tween 20)
|
Span 20
|
|
Soybean Pil
|
Polysorbate 80(Tween 80)
|
Span 80
|
|
Corn Oil
|
D- alphaTocopheryl polyethylene glycol 1000 succinate (TPGS)
|
Capryol 90
|
|
Sunflower Oil
|
Polyoxy-40-castor oil(Cremophor RH 40)
|
Lauroglycol
|
|
Castor oil
|
Polyoxy-40-hydrogenated castor oil (Cremophor RH40)
|
Transcutol
|
|
Sesame oil
|
Labrasol
|
Capmul
|
|
Peanut Oil
|
|
Ethanol
|
|
Labrafac
|
|
Polypropylene glycol
|
|
Labrafil
|
|
Polyethylene glycol
|
Ternary Phase Diagram
The phase behavior of various formulation components is described using ternary phase diagrams. Facilitating water penetration into the surfactant layer around the oil droplets' surface is linked to facilitating self-emulsification. (22) The spontaneous emulsification method (phase titration method) is used to create micro emulsions, which can then be represented using phase diagrams. The creation of a phase diagram is an effective method for studying the complicated series of interactions that might occur when different components are combined. Because a quaternary phase diagram (four component system) is time-consuming and difficult to comprehend, a pseudo ternary phase diagram is frequently used to identify different zones, including the microemulsion zone, in which each corner of the diagram indicates 100% of the specific component. When four or more components are explored, pseudo-ternary phase diagrams are employed, with each corner representing a binary mixture of two components, such as surfactant/co-surfactant, water/drug, or oil/drug. The number of various phases present in a given combination can be visually determined. Figure shows a very schematic (pseudo) ternary phase diagram exhibiting these qualities. (23)
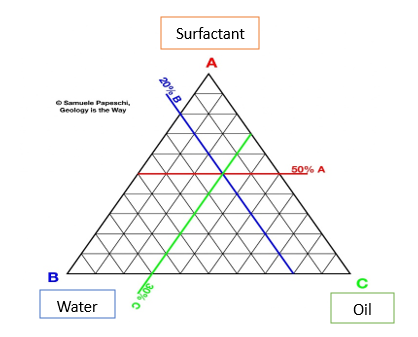
Fig. Ternary Phase Diagram
Characterization: -
1) Turbidity measurement
This determines if the di
spersion finds equilibrium quickly and consistently. Turbidity meters, most notably the Hach and Orbeco-Helle turbidity meters, are used to take these measurements. This device is linked to a dissolution apparatus, and optical clarity of formulation is measured every 15 seconds to determine the clarity of the nano or micro emulsion generated and the emulsification time. Turbidity can also be assessed in terms of spectroscopic evaluation of optical clarity (i.e. the absorbance of properly diluted aqueous dispersion at 400 nm).(14)
2) Visual Evaluation:
Visual observation aids in the evaluation of self-emulsification. The presence of a clear, isotropic, transparent solution after water dilution of SEDDS implies microemulsion formation, whereas an opaque, milky white appearance indicates macroemulsion evolution. A lack of precipitation and/or phase separation indicates the formulation is stable. (24)
3) Droplet size :-
Droplet size has a key role in self-emulsification performance, affecting drug release rates and emulsion stability. Photon correlation spectroscopy and microscopic methods are mostly utilized to determine the size of the emulsion droplets. Reducing droplet size to less than 50 ?m creates steady, isotropic, and transparent o/w dispersion.
4) Zeta potential measurements:
This identifies the charge of the droplets. The presence of free fatty acids in typical SEDDSs causes the charge on an oil droplet to be negative.(7)
5) Thermodynamic stability studies:
The physical stability of a lipid-based formulation is also important for its performance, which might be compromised by drug precipitation in the excipient matrix. Furthermore, inadequate formulation physical stability can cause excipient phase separation, which affects both formulation performance and visual appearance. In addition, incompatibilities between the formulation and the gelatin capsule shell can result in brittleness or deformation, delayed disintegration, or inadequate drug release. Heating/cooling cycle: The study examines six cycles ranging from refrigerator temperature (40C) to 450C, with storage at each temperature lasting at least 48 hours. Centrifugation tests are performed on formulations that remain stable at these temperatures.(25)
6) Determining self-emulsification :-
The efficiency of emulsifying Tween 85/medium chain triglyceride systems was measured using a revolving paddle in a crude nephelometer (24). This allowed an estimate of the time required for emulsification. After emulsification was completed, samples were collected for particle size using photon correlation spectroscopy, and self-emulsified systems were compared to homogenized systems. Light microscopy was used to observe the self-emulsification process. It was obvious that emulsification occurred through the erosion of a fine cloud of microscopic particles off the surface of giant droplets, rather than a steady reduction in droplet size.(26)
7) Dispersibility test:-
The rate of dispersion is assessed visually. Light microscopy is used to study the process of self-emulsification. The USP XXII dissolving equipment can be used to evaluate the effectiveness of oral nanoemulsions or microemulsions. The sample formulation (1 mL) is combined with 500 mL of water at 37±1°C. For continuous agitation, a stainless steel dissolving paddle was used at a stirring speed of 100 rpm, and the time for emulsion formation was measured. The precipitation and phase separation of the resulting mixture are monitored at various time intervals (2, 4, 6, 8, 12, 24 hours). The grading method used to evaluate the in vitro performance is shown below.(27)
Grade A: Rapidly developing nanoemulsion that forms in less than a minute and produces a bluish-colored clear solution.
Grade B: Rapidly produce a bluish-white nanoemulsion. Grade C: Create a delicate milky nanoemulsion in 2 minutes.
Grade D: Formation of a dull, grayish-colored emulsion with an oily appearance that emulsifies gradually and takes longer than 2 minutes.
Grade E: Weak emulsification, resulting in big oil globules near the surface.
8) The phase separation technique:-
The phase separation technique is a simple way for determining SEDDS stability. The diluted samples with distilled water were centrifuged at a certain rpm for a set period of time to evaluate phase separation. The estimation of cloud point in SEDDS containing non-ionic surfactants is an important instrument. At the cloud point, rising temperature causes irreversible phase separation. The cloudiness of the preparation has a detrimental impact on the absorption of the integrated medicine because SEDDS components are dehydrated. Hence, the cloud point of self-emulsifying systems must be above 37 degrees Celsius, unless phase separation cannot be prevented in the gastrointestinal tract.(22)
9) viscosity:-
Internal friction inside a fluid, known as viscosity, can assist both flow resistance and spontaneous emulsification. For these reasons, determining viscosity profiles for SEDDSs intended for topical/transdermal drug delivery is considered critical, as these results can help identify the influence of divergent oil phases as well as different surfactant concentrations on viscosity and self-emulsification potential. The attraction forces inside fluids that determine viscosity are sensitive to temperature fluctuations. In addition, certain systems can represent reversible or irreversible structural changes caused by fluid movement. Flow behavior is characterized as Newtonian or non-Newtonian.(28)
Solidification Methods:
Solid SEDDS were generated primarily by adsorption onto solid carriers, spray drying, melt extrusion, dry emulsion, solid dispersion, and so on. These solid SEDDS can be formed into pellets, pills, or capsules.
1) Adsorption on Solid Carriers
A) Physical Adsorption
B) Spray Drying
2) Melt Extrusion
3) Lyophilization
1) Adsorption on solid carriers
A) Physical Adsorption
These solid carriers have the ability to absorb liquid/semisolid formulations as a self-emulsifying system. It's a straightforward technique in which SES is mixed into a free-flowing powder substance with adsorption properties. The mixture is equally adsorbed after being mixed in a blender. This solid combination is either poured into a capsule or mixed with extra excipient to be compressed into tablets. The aforesaid combination was consolidated into powder form utilizing three types of adsorbents: microporous calcium silicate (FloriteTMRE), magnesium aluminum silicate (NeusilinTMUS2), and silicon dioxide (SylysiaTM 320).(29)
B) Spray drying
This process involves spraying a formulation containing oil, surfactant, medication, and solid carrier into a drying chamber using a nozzle. The volatile vehicles evaporate, leaving behind little solid particles that may be crushed into tablets or capsules. The procedure is depicted in Figure 3. This technique involves eliminating water from an emulsion to create dry emulsions. Nimodipine self-micro emulsifying formulation was created using spray drying with dextran as a solid carrier.(30)
2) Melt Extrusion/Extrusion Spheronization
Extrusion Spheronization process is based on the ability of materials to be readily extruded and spheronized. These approaches do not require liquid excipients, but continuous temperature and pressure must be maintained to obtain high drug loading. Melt extrusion guarantees content consistency and is a popular method for producing pellets and granules. Extrusion converts raw materials with plastic qualities into homogenous pellets of variable sizes, which are determined by the size of the extruder aperture. A self-nanoemulsifying ubiquinone formulation was created utilizing the extrusion Spheronization process. [49] This approach also improves the bioavailability of propranolol.(31)
3) Lyophilization
Lyophilization, often known as freeze drying, is a revolutionary process for solidifying SEDDS. Lyophilization makes SEDDS more stable, soluble, and patient-friendly. This process freezes the liquid SEDDS and converts it to a dry powder by sublimation at low temperatures and pressure. Dry powder may simply be reconstituted into a fine emulsion. Lyophilization is a three-stage process that begins with quick freezing, followed by a main drying phase when most of the water content is sublimated out. The third stage, secondary drying, removes any remaining water molecules, creating a stable and dried powder.(32)
Challenges And Limitations of Sedds
(SEDDS) have emerged as an effective technique for increasing the solubility and bioavailability of weakly water-soluble medicines, particularly those classified as BCS Classes II and IV. However, their widespread adoption is hampered by a number of issues and limits that must be solved. Stability concerns are among the most serious challenges, particularly in liquid SEDDS. These formulations are prone to phase separation, drug precipitation, and oxidative degradation during storage, especially in environments with changing temperatures and humidity. Furthermore, encapsulating liquid SEDDS in gelatin capsules frequently results in leaking, which compromises their stability and shelf life. Such disadvantages highlight the need for better stabilization solutions, including the creation of solid SEDDS, which offer greater physical and chemical stability but come with their own set of challenges.(35) Another key constraint is the high surfactant concentration necessary for effective self-emulsification. While surfactants are necessary for decreasing interfacial tension and creating tiny emulsions, excessive use—often ranging from 30% to 60% of the formulation—can cause gastrointestinal discomfort and cytotoxicity. This is especially important for chronic therapy, as repeated exposure to high surfactant levels may compromise patient compliance and safety. Furthermore, pharmaceutically acceptable, non-toxic surfactants with an adequate hydrophilic-lipophilic balance (HLB) must be addressed in formulation design.(38) Manufacturing scalability is a further difficulty, since the move from lab-scale to industrial-scale production necessitates precise control over excipient ratios, emulsification efficiency, and batch consistency. Spray drying, hot-melt extrusion, and adsorption onto solid carriers are all viable techniques for solid SEDDS, but they add complexity, expense, and possible compatibility difficulties.(37) Finally, whereas SEDDS have demonstrated tremendous promise for lipophilic medicines, their applicability to hydrophilic and macromolecular pharmaceuticals is restricted. Recent advances, such as ionic liquids and hybrid delivery methods, provide potential answers but require additional investigation. Despite these obstacles, breakthroughs in computer modeling, new excipient engineering, and hybrid drug delivery methods continue to push the limits of SEDDS formulations.(36) Addressing these constraints through a multidisciplinary approach will be critical to maximizing their clinical potential and extending their place in current pharmaceutical sciences.
CONCLUSION
Self-emulsifying drug delivery systems (SEDDS) have transformed the area of drug delivery by tackling the essential issues of solubility and bioavailability in poorly water-soluble medicines. This study has thoroughly explored the many facets of SEDDS, including their fundamental principles, formulation methodologies, characterisation techniques, and diverse therapeutic uses. SEDDS are classified into conventional SEDDS. The change from liquid to solid SEDDS increased the delivery system's adaptability. Solid SEDDS, created using processes such as spray drying, adsorption onto carriers, and hot-melt extrusion, overcome the stability, mobility, and production issues inherent in liquid SEDDS. This move has allowed for better scalability, increased shelf-life, and improved patient compliance while still keeping the benefits of self-emulsifying devices. SEDDS has several applications other than increasing bioavailability. These systems have proved their flexibility to a wide range of pharmaceutical issues, from controlled and targeted drug release to the stabilization and delivery of biologics and peptides. Emerging technologies, such as hybrid drug delivery systems, 3D-printed SEDDS, and supersaturable SEDDS (Su-SEDDS), are pushing the frontiers of this technology, resulting in higher therapeutic impact and patient-centric solutions. While SEDDS show great promise, they also confront several hurdles. Excipient toxicity, drug precipitation, and scalability are all ongoing issues that highlight the importance of continual innovation and cooperation. Computational modeling and in silico techniques are already playing an important role in solving these issues by speeding up formulation development and assuring precision in excipient selection. Finally, by addressing unmet requirements and exploiting developing technology, SEDDS are positioned to continue to play an important role in the development of next-generation drug delivery systems, therefore determining the future of modern healthcare.
REFERENCES
- Baytok N, Saka OM. SELF EMULSIFYING DRUG DELIVERY SYSTEMS- An Ovierview. Ank Univ Eczacilik Fak Derg [Internet]. 2023 Feb 17 [cited 2024 Dec 12]; Available from: https://dergipark.org.tr/en/doi/10.33483/jfpau.1244378
- Salawi A. Self-emulsifying drug delivery systems: a novel approach to deliver drugs. Drug Deliv. 2022 Dec 31;29(1):1811–23.
- Mishra V, Nayak P, Yadav N, Singh M, Tambuwala MM, Aljabali AAA. Orally administered self-emulsifying drug delivery system in disease management: advancement and patents. Expert Opin Drug Deliv. 2021 Mar 4;18(3):315–32.
- Oh DH, Kang JH, Kim DW, Lee BJ, Kim JO, Yong CS, et al. Comparison of solid self-microemulsifying drug delivery system (solid SMEDDS) prepared with hydrophilic and hydrophobic solid carrier. Int J Pharm. 2011 Nov 28;420(2):412–8.
- Abdulkarim M, Sharma PK, Gumbleton M. Self-emulsifying drug delivery system: Mucus permeation and innovative quantification technologies. Adv Drug Deliv Rev. 2019 Mar 1;142:62–74.
- Maji I, Mahajan S, Sriram A, Medtiya P, Vasave R, Khatri DK, et al. Solid self emulsifying drug delivery system: Superior mode for oral delivery of hydrophobic cargos. J Controlled Release. 2021 Sep;337:646–60.
- Kumar A, Sharma S, Kamble R. SELF EMULSIFYING DRUG DELIVERY SYSTEM (SEDDS): FUTURE ASPECTS. 2.
- Vilas PC. A REVIEW ON SELF MICROEMULSIFYING DRUG DELIVERY SYSTEM. 2013;
- document.pdf [Internet]. [cited 2024 Dec 24]. Available from: https://citeseerx.ist.psu.edu/document?repid=rep1&type=pdf&doi=9dd9f0c644aca487e181696192601b974c18abda
- Tang J ling, Sun J, He ZG. Self-Emulsifying Drug Delivery Systems: Strategy for Improving Oral Delivery of Poorly Soluble Drugs. Curr Drug Ther. 2007 Jan 1;2(1):85–93.
- Sebastain G, Rajasree PH, George J, Gowda DV. Self-micron emulsifying drug delivery systems (SMEEDS) as a potential drug delivery system-novel applications and future perspectives: a review. Int J Pharm. 2016;6:105-.
- Self-Emulsifying Drug Delivery Systems (SEDDS): Formulation Development, Characterization, and Applications - Critical ReviewsTM in Therapeutic Drug Carrier Systems, Volume 26, 2009, Issue 5 - Begell House Digital Library [Internet]. [cited 2024 Dec 19].
- Revathi S, Raju MD. Self-emulsifying drug delivery system: A review. World J Pharm Pharm Sci. 2012 Dec 8;2:89-107.
- Kohli K, Chopra S, Dhar D, Arora S, Khar RK. Self-emulsifying drug delivery systems: an approach to enhance oral bioavailability. Drug Discov Today. 2010 Nov;15(21–22):958–65.
- P. Shabeera* VM. Pneumonia And Types & An Over View On Aspiration Pneumonia. 2024 Jan 26 [cited 2024 Dec 21]; Available from: https://zenodo.org/doi/10.5281/zenodo.10570770
- Chatterjee B, Hamed Almurisi S, Ahmed Mahdi Dukhan A, Mandal UK, Sengupta P. Controversies with self-emulsifying drug delivery system from pharmacokinetic point of view. Drug Deliv. 2016 Nov 21;23(9):3639–52.
- Fernandez-Tarrio M, Yañez F, Immesoete K, Alvarez-Lorenzo C, Concheiro A. Pluronic and Tetronic Copolymers with Polyglycolyzed Oils as Self-Emulsifying Drug Delivery Systems. AAPS PharmSciTech. 2008 Mar 14;9(2):471–9.
- Zhang H, Yao M, Morrison RA, Chong S. Commonly used surfactant, Tween 80, improves absorption of P-glycoprotein substrate, digoxin, in rats. Arch Pharm Res. 2003 Sep;26(9):768–72.
- Katneni K, Charman SA, Porter CJH. Impact of Cremophor-EL and Polysorbate-80 on Digoxin Permeability across Rat Jejunum: Delineation of Thermodynamic and Transporter Related Events Using the Reciprocal Permeability Approach. J Pharm Sci. 2007 Feb;96(2):280–93.
- Thakare P, Mogal V, Borase P, Dusane J, Kshirsagar S. A review on self-emulsified drug delivery system. Pharm Biol Eval. 3.
- Patel AR, Vavia PR. Preparation and in vivo evaluation of SMEDDS (self-microemulsifying drug delivery system) containing fenofibrate. AAPS J. 2007 Sep;9(3):E344–52.
- Ujhelyi Z, Vecsernyés M, Fehér P, Kósa D, Arany P, Nemes D, et al. Physico-chemical characterization of self-emulsifying drug delivery systems. Drug Discov Today Technol. 2018 Jul;27:81–6.
- Reddy S, Katyayani T, Navatha A, Ramya G. Review on self micro emulsifying drug delivery systems. 2011;
- Bhargava P, Bhargava S, Daharwal SJ. Self Emulsifying Drug Delivery System: An Approach to Improve the Solubility of Poorly Water-Soluble Drug. 1.
- Solanki N, Prajapati S. Self-emulsifying drug delivery system (SEDDS): A review. The International Journal of Pharmaceutical Research and Bio-Science. 2012;1(1).
- Rahman MdA, Hussain A, Hussain MdS, Mirza MohdA, Iqbal Z. Role of excipients in successful development of self-emulsifying/microemulsifying drug delivery system (SEDDS/SMEDDS). Drug Dev Ind Pharm. 2013 Jan;39(1):1–19.
- Mishra V, Nayak P, Yadav N, Singh M, Tambuwala MM, Aljabali AAA. Orally administered self-emulsifying drug delivery system in disease management: advancement and patents. Expert Opin Drug Deliv. 2021 Mar 4;18(3):315–32.
- Van Staden D, Du Plessis J, Viljoen J. Development of Topical/Transdermal Self-Emulsifying Drug Delivery Systems, Not as Simple as Expected. Sci Pharm. 2020 Mar 27;88(2):17.
- Nazzal S, Khan MA. Controlled release of a self-emulsifying formulation from a tablet dosage form: Stability assessment and optimization of some processing parameters. Int J Pharm. 2006 Jun;315(1–2):110–21.
- Sapra K, Sapra A, Singh SK, Kakkar S. Self Emulsifying Drug Delivery System: A Tool in Solubility Enhancement of Poorly Soluble Drugs. Indo Glob J Pharm Sci. 2012;02(03):313–32.
- Patel R, Kamble M, Katedeshmukh R, Zarikar N, Kulkarni A. A Review On Solid Self Emulsifying Drug Delivery System. 2013;2(4).
- Govindan I, Rama A, Kailas AA, Hebbar S, Naha A. Transformative solidification techniques for self-emulsifying drug delivery and its foresight in modern-day drug delivery. J Appl Pharm Sci [Internet]. 2024 [cited 2025 Jan 20];
- Crison JR, Amidon GL. Methods and formulation for increasing the bioavailability of poorly water soluble drugs. US Patent 5993858; 1999.
- Bose S, Kulkarni PK. Self emulsifying drug delivery systems: A review. Ind J Phar Edu 2002;36(4):184-190.
- Lee, S.J.; Choi, S.J.; Li, Y.; Decker, E.A.; McClements, D.J. Protein-Stabilized Nanoemulsions and Emulsions: Comparison of Physicochemical Stability, Lipid Oxidation, and Lipase Digestibility. J. Agric. Food Chem. 2010, 59, 415–427.
- Lee, S.J.; Choi, S.J.; Li, Y.; Decker, E.A.; McClements, D.J. Protein-Stabilized Nanoemulsions and Emulsions: Comparison of Physicochemical Stability, Lipid Oxidation, and Lipase Digestibility. J. Agric. Food Chem. 2010, 59, 415–427.
- Raman Kallakunta, V.; Dudhipala, N.; Nyavanandi, D.; Sarabu, S.; Yadav Janga, K.; Ajjarapu, S.; Bandari, S.; Repka, M.A. Formulation and Processing of Solid Self-Emulsifying Drug Delivery Systems (HME S-SEDDS): A Single-Step Manufacturing Process via Hot-Melt Extrusion Technology through Response Surface Methodology. Int. J. Pharm. 2023, 641, 123055.
- Prasad, D.; Chauhan, H.; Atef, E. Studying the Effect of Lipid Chain Length on the Precipitation of a Poorly Water Soluble Drug from Self-Emulsifying Drug Delivery System on Dispersion into Aqueous Medium. J. Pharm. Pharmacol. 2013, 65, 1134–1144..


 Pratik Korade*
Pratik Korade*
 Hrushikesh Joshi
Hrushikesh Joshi
 Amol Kharat
Amol Kharat




 10.5281/zenodo.14784958
10.5281/zenodo.14784958