Abstract
Transdermal drug delivery system is an essential part of novel drug distribution system. The topically administered medications in the form of patches which when applied to the skin deliver the drug .For operative TDDS the drug are easily able to penetrate the skin and easily reach the target site. TDDS avoids the first pass metabolism, less frequency of administration, reduction gastrointestinal side effects.The TDDS review articles provide valuable information regarding the transdermal drug delivery systems and its evaluation process details as a ready reference for the research scientist who is involved in TDDS . Transdermal patch is a transdermal delivery system that can overcome problems in conventional drug administration, such as oral drug administration. Patches can provide controlled drug release and have advantages over oral administration, such as avoiding first-pass metabolism, increasing drug bioavailability, avoiding adverse effects on the gastrointestinal (GI) tract, minimizing patient variability, maintaining a constant drug in plasma ,and providing a stable therapeutic effect. The effectiveness of a patch is determined by the drug’s ability to release fromthe patch matrix and penetrate the stratum corneum. The methods used to make the patches are divided into single layer, multi-layer, reservoir system, and matrix system patches. The basic components of a patch are polymer matrix membrane, drug, permeation enhancer, pressure-sensitive adhesives, backing film, release liner, and plasticizer.
Keywords
Human Skin, Permeation, transdermal drug delivery, patch, methods and Evaluation of transdermal patches
Introduction
The oral route is the most widely used method for drug delivery systems; however, it comes with certain drawbacks, such as first-pass metabolism and drug degradation within the gastrointestinal tract due to factors like enzymes and pH levels. To address these issues, a novel drug delivery system was developed by Chien in 1992, Banker in 1990, and Guy in 1996, known as transdermal patches or the transdermal delivery system. This system involves the creation of medicated adhesive patches that release therapeutically effective drug amounts when applied to the skin. These patches come in various sizes and may contain multiple ingredients. When applied to intact skin, they facilitate the delivery of active ingredients into systemic circulation by traversing the skin barriers. A transdermal patch designed to hold a high dosage of medication can remain on the skin for an extended period, allowing the drug to enter the bloodstream through diffusion.
Drug can penetrate through skin via three pathways
1)Through. sebaceous glands.
2) Through. hair follicals. 3) Through sweat duct
Transdermal drug delivery systems are used in various skin disorders, also in the management of angina pectoris, pains, smoking cessation & neurological disorders such as Parkinson’s disease [1][2]
Anatomy and physiology of skin
Human skin comprises of three distinct but mutually dependent tissues: The stratified, vascular, cellular called as “epidermis” Underlying dermis of connective tissues, Hypodermis
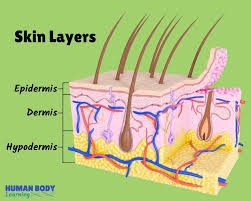
Fig.1 Structure of human skin
- Epidermis The epidermis is a multilayered structure with varying thickness, influenced by cell size and the number of cell layers. It ranges from 0.8 mm in thickness on the palms and soles to as thin as 0.06 mm on the eyelids. The outermost layer, known as the stratum corneum or horny layer, is roughly 10 mm thick when dry but expands to several times that thickness when fully hydrated. This layer consists of 10 to 25 layers of dead, keratinized cells called corneocytes. While it is flexible, it remains relatively impermeable. The stratum corneum acts as the primary barrier against drug penetration and can be conceptualized as a wall-like structure, where keratinized cells serve as protein "bricks" held together by lipid "mortar." The lipids within this layer are organized into multiple bilayers.
The lipid fraction contains adequate amounts of amphiphilic substances, such as polar free fatty acids and cholesterol, which help maintain the bilayer structure. Beneath the stratum corneum lies the viable epidermis, which varies in thickness from 0.06 mm on the eyelids to 0.8 mm on the palms. Moving inward, this layer is composed of several parts, including the stratum lucidum, stratum granulosum, stratum spinosum, and stratum basal. The basal layer is responsible for the continuous renewal of the epidermis through mitosis, compensating for the loss of dead horny cells from the skin's surface. As the newly formed cells in the basal layer migrate outward, they undergo morphological and histochemical changes, eventually keratinizing to form the outermost stratum corneum.
2) Dermis
The dermis, which is approximately 3 to 5 mm thick, is made up of a network of connective tissue that houses blood vessels, lymph vessels, and nerves. The blood supply to the skin plays a crucial role in maintaining the body's temperature. It also supplies essential nutrients and oxygen to the skin while eliminating toxins and waste products. Capillaries extend to a distance of approximately 0.2 mm from the skin's surface, creating an environment that facilitates the removal of most substances that penetrate the skin barrier. The blood circulation maintains a low concentration of the permeate in the dermal layer, creating a concentration difference across the epidermis that is crucial for transdermal absorption. .
Hypodermis The subcutaneous fat tissue, also known as the hypodermis, provides support to the dermis and epidermis. It functions as a storage site for excess fat. This layer plays a role in temperature regulation, offers nutritional support, and provides mechanical protection. It carries important blood vessels and nerves to the skin and may contain sensory pressure organs. In transdermal drug delivery, the drug needs to pass through the skin's three layers and enter the bloodstream. In topical drug delivery, only the outermost layer, the stratum corneum, needs to be penetrated, and the drug should stay in the skin layers. [3]
Transdermal Patch
Fig 2. Transdermal Patch
A transdermal patch, also known as a skin patch, is an adhesive medicated patch applied to the skin to deliver a precise dosage of medication into the bloodstream. The U.S. Food and Drug Administration approved the first commercially available prescription patch in December 1979, which contained scopolamine for the treatment of motion sickness. The most popular transdermal patch in the United States is the nicotine patch, designed to assist individuals in stop smoking by releasing nicotine. In 2007, Europe approved the first commercially available vapor patch aimed at helping people stop smoking. Additionally, various other patches are present in the market, including fentanyl, an analgesic for severe pain, nitroglycerin patches for angina, and lidocaine patches, branded as Lidoderm, which alleviate peripheral pain from shingles. Buprenorphine patches, marketed as BuTrans, serve as pain relief for moderate to severe chronic pain and are increasingly utilized for managing pain from both recent injuries and longstanding conditions. The Flector (diclofenac epolamine) patch is a topical NSAID used for acute pain treatment associated with minor strains, sprains, and contusions. It is also employed in managing pain and inflammation from chronic conditions that benefit from NSAIDs, such as fibromyalgia and arthritis. In 2005, the FDA announced an investigation into reports of fatalities and other serious adverse events related to narcotic overdoses in patients using Duragesic, the fentanyl transdermal patch designated for pain control, particularly in individuals dealing with Attention-Deficit/Hyperactivity Disorder (ADHD). [4]
Advantages
1) Drugs that cause irritation and absorption in the gastrointestinal tract can be administered through the skin.
2) The simplicity of operation allows patients to independently administer these systems.
3) In the event of an emergency, removing the patch at any given moment during therapy can immediately stop the administration of medication.
4) Due to the similar structure and biological makeup of the skin in almost all humans, there is minimal variation between individuals and within the same person.
5) Hepatic first-pass metabolism, salivary metabolism, and intestinal metabolism are bypassed.
Disadvantages
1) It is primarily intended for the management of long-term conditions such as hypertension, angina, and diabetes, where continuous drug therapy is necessary.
2) When the drug is applied to the skin, it can lead to an excessive amount of the drug being absorbed, causing potential side effects.
3) There is a chance of skin irritation caused by one or more of the ingredients in the formulation.
4) The duration of lag time can differ significantly and range from a few hours to several days for various drug candidates.
5) The way the skin metabolizes substances will impact how well the system works. [5]
Principles Of Transdermal Permeation5
Earlier skin was considered as an impermeable protective barrier, but later investigations were carried out which proved the utility of skin as a route for systemic administration Skin is the most intensive and readily accessible organ of the body as only a fraction of millimeter of tissue separates its surface from the underlying capillary network.
The various steps involved in transport of drug from patch to systemic circulation are as follows:
1) The absorption of medication through the capillary network in the dermal papillary layer.
2) The process of drug diffusion from the rate limiting membrane to the stratum corneum is a crucial step in its absorption.
3)The process of sorption by the stratum corneum and penetration through the viable epidermis.
4) The movement of a drug from its storage area to the membrane that controls its release.
5) Impact on the intended organ. [6]
Components of the transdermal patch:
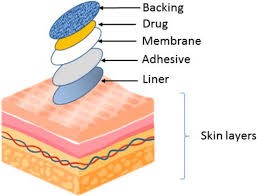
Fig 3 components of the Transdermal patch
Components of the Transdermal Patch: A transdermal patch is primarily composed of a polymer matrix or drug reservoir, the active ingredient (drug), permeation enhancers, pressure-sensitive adhesive (PSA), backing laminates, release liner, and various excipients such as plasticizers and solvents. (Refer to Figure No. 03).
1)Polymer Matrix:
Polymers form the essential base for transdermal drug delivery systems. These systems are typically constructed as multilayered polymeric laminates, where a drug reservoir or a drug-polymer matrix is positioned between two polymer layers. The outer layer is an impermeable backing that prevents drug loss, while the inner layer acts as an adhesive and/or rate-controlling membrane. To develop effective transdermal delivery systems tailored to specific needs, careful selection and design of polymers is essential. The primary challenge lies in the design of the polymer matrix, followed by the optimization of the drug-loaded matrix in terms of its release characteristics, adhesive properties, physicochemical attributes, compatibility, and stability with other system components and the skin.[7]
Polymers used in the preparation of transdermal patches [8][9][10]
Table no .1
|
Polymer
|
Category
|
|
Sodium alginate
|
Natural Polymer
|
|
e Natural Polymer Chitosan
|
|
Gelatine
|
|
Gum Arabic
|
|
Gum tragacanth
|
|
Hyaluronic acid
|
|
hydroxypropyl methylcellulose
|
Semi-synthetic Polymer
|
|
Methylcellulose
|
|
Carboxymethyl cellulose
|
|
Carmel lose
|
|
Polyvinylpyrrolidone (PVP)
|
Synthetic Polymer
|
|
Polyhydroxyethyl methacrylate (PHMA)
|
|
Polyvinyl alcohol
|
|
Polyvinyl chloride (PVC)‘
|
|
Polypropylene glycol
|
|
Polyethylene
|
2. Drug:
The critical factors for transdermal drug delivery systems (TDDS) are that the drug must have appropriate physicochemical and pharmacokinetic characteristics. Transdermal patches provide significant advantages for drugs that experience considerable first-pass metabolism, those with a narrow therapeutic window, or medications with a short half-life, which can lead to non-compliance due to the need for frequent dosing.[7]
Ideal Properties of Drugs[16] :-
Table no. 2
|
Sr no
|
Parameter
|
Properties
|
|
1.
|
Dose
|
Should be Low in weight (less than 20mg/day)
|
|
2.
|
Half- life
|
10/less (hrs).
|
|
3.
|
Skin permeability coefficient
|
>0.5*10-3cm/h.
|
|
4.
|
Molecular weight
|
<400da>
|
|
5.
|
Skin Reaction
|
Non irritating, Non sensitizing
|
|
6.
|
Oral bioavailability
|
Low
|
3. Permeation enhancers:
To increase the permeability of the stratum corneum and achieve higher therapeutic levels of drug delivery, permeation enhancers interact with the structural elements of the stratum corneum, such as proteins or lipids. The increased absorption of oil-soluble drugs is likely due to the partial extraction of epidermal lipids by these chemical enhancers, resulting in better conditions for wetting and facilitating both trans epidermal and trans follicular permeation. The miscibility and solubility characteristics of the enhancers employed may play a key role in improving the transdermal permeation of water-soluble drugs.
4. Pressure-sensitive adhesive (PSA):
A PSA ensures close contact between the patch and the skin. It should stick with only the pressure of a fingertip, be consistently sticky, and provide a strong holding force. Choosing an adhesive involves considering several factors, such as the design of the patch and the formulation of the drug. The PSA must be compatible both physiochemically and biologically and should not interfere with drug release. The PSA can be applied on the front face of the device or on the back, extending outward around the edges.
5. Backing laminate:
The main purpose of the backing laminate is to offer support. The backing layer must be resistant to chemicals and compatible with excipients, as extended exposure to the backing layer can result in the leaching of additives or the diffusion of excipients, drugs, or permeation enhancers through the layer. It is essential that they have a low rate of moisture vapor transmission. Additionally, they should possess optimal elasticity, flexibility, and tensile strength.
6. Release liner:
During storage, the release liner prevents against the loss of the drug that may have seeped into the adhesive layer and also prevents contamination. Therefore, it is considered part of the primary packaging material instead of being classified as part of the dosage form for administering the drug. The release liner consists of a base layer that can either be non?occlusive or occlusive, along with a release coating layer made from silicon or Teflon. Other materials utilized for the release liner in transdermal drug delivery systems (TDDS) include polyester film and metalized laminate.
7. Other excipients:
Various solvents like chloroform, methanol, acetone, isopropanol, and dichloromethane are employed to create drug reservoirs. Furthermore, plasticizers such as dibutyl phthalate, triethyl citrate, polyethylene glycol, and propylene glycol are incorporated to enhance the plastic [7].
Types patch
- Single-layer Drug in Adhesive A single-layer drug-in-adhesive patch utilizes the adhesive layer to stick together the various components and attach the entire system to the skin, while also facilitating the release of the drug. This adhesive layer is enclosed by a temporary liner and a backing. [11]
- Multi-layer Drug in Adhesive A multi-layer drug-in-adhesive patch shares similarities with a single-layer patch, as both adhesive layers contribute to drug release. However, this type includes an extra layer that adhere to the drug, typically separated by a membrane, although this is not always the case. This patch also features temporary and permanent liner layers. [11]
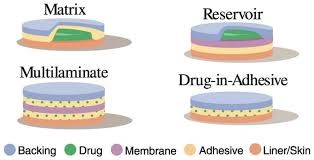
Fig 4 Types of Transdermal Patch
- Reservoir In this system,
drug reservoir is positioned between the supporting layer and the rate-control membrane, with the drug being released through the microporous rate-controlled membrane. The drug may exist as a solution, suspension, gel, or be dispersed within a solid polymer matrix in the reservoir compartment [12] .
- Matrix
The adhesive and backing material serve as the primary components of the matrix system, with the backing layer being the outermost part of the formulation. Initially, the drugs alongside other additives such as polymers and enhancers are combined to create an adhesive solution, which is then allowed to evaporate the solvent, resulting in a matrix film. Following this, the matrix film and the adhesive are then attached to the backing film. The matrix-type patch is the most widely utilized transdermal patch in the market. One benefit of this matrix system is that it results in a slim and aesthetically pleasing preparation, making it user-friendly, while the manufacturing process remains straightforward, soft, and cost-effective. [13]
Care taken while applying transdermal patch:
The area of skin must be thoroughly cleansed prior to the application of the patch. Altering the patch by cutting it compromises the drug delivery system, so it should remain intact. It is important to ensure that the previous patch is taken off the site before a new one is applied. Caution is necessary when applying or removing the patch, as anyone in contact with it may absorb the medication contained within. The patch needs to be positioned correctly at the designated application site. [14]
Factors Affecting Transdermal Patches
There are various factors which affects the action of transdermal patches. These are given below:
a). Physicochemical Properties
1. Partition coefficient
2. Molecular size
3. Solubility/melting point
4. Ionization
b). Physiological & Pathological Conditions of Skin
1. Reservoir effect of horny layer
2.Lipid film
3. Skin hydration
4. Skin temperature
5.Regional variation
6. Pathological injuries to the skin
7. Cutaneous self-metabolism
8. Skin barrier properties in the neonate and young infant
9. Skin barrier properties in aged skin
10. Race
11. Body site
12. Penetration enhancers used [15]
Evaluation of Transdermal Patches
a. Physical Characteristics
1. Organoleptic observations; -
Organoleptic patches can be observed visually, including colour, odour, flexibility and surface texture [17].
2. Thickness test
The thickness of the patch can be measured using a calliper by dividing the five areas to be measured. Then the thickness of each side of the section is measured, and the average is determined [18]
3. Weight uniformity test This test is performed by weighing each patch on a digital scale and then determining the average value [18]
4. Moisture content (%)
For 24 hours in a desiccator filled with calcium chloride, fabric patches have been produced, weighed, and stored at room temperature. The patch was weighed again after 24 hours in order to calculate its moisture content % using the formula:[19] . %moisture content= [Initial weight – final weight / Final weight] × 100[19] .
- Drug content
The drug content of the patch preparation was measured by dissolving a predefined amount of patch preparation in phosphate buffer saline (pH 7.4 0.05). A filter was placed over the solution, and the drug content was measured using UV or HPLC spectroscopy, respectively [20]
6. Test the uniformity of the active ingredient content This examination was conducted by cutting the patch preparation into three parts as much as 1 cm and dissolved in phosphate buffer saline pH 7.4 ± 0.05 as much as 25 ml. Then the results of the concentration values were observed using UV or HPLC spectrophotometry [21]
b. In vitro release test
The patch release test is important because it can determine the cumulative drug levels that can be released from the carrier matrix. This test is performed to determine whether a patch can maintain a consistent drug concentration in the skin’s stratum corneum and maintain it substantially higher than the drug concentration in the body to achieve a constant level of drug permeation. A transdermal patch release test can be performed using the paddle over disc method from USP apparatus V brand Parmeq using 500 ml of phosphate saline buffer pH 7.4 ± 0.5 as the dissolution medium. First, samples are included in the disc. Then the disc was placed in 500 ml of dissolution medium, and the paddle was placed at a distance of 2.5 cm from the container and then rotated at a speed of 50 rpm while maintaining the temperature at 37±0.5 °C. There are 5 ml samples taken at several time intervals for 24 hours and analyzed by UV or HPLC spectrophotometer, then replicated 3 times, and the average value could be calculated [22] [23] [24].
c. In vitro penetration test
A patch penetration test can be conducted using the Franz diffusion cell method, which can be used to test drug permeation through mouse skin in vitro. First, the hair from the rat’s stomach area had to be carefully removed and then the skin was thoroughly cleaned with distilled water to remove the adhering tissue or blood vessels, which would later become the membrane in the Franz diffusion cell method. This device consists of a receptor compartment, a donor compartment, and a water jacket. The water jacket maintains a constant temperature while the Franz diffusion cell operates. In the donor compartment and the receptor compartment, rat skin is placed with the epidermis facing upwards. The cell temperature was maintained at 37±0.5 °C using a thermostatically controlled heater, and the medium used was phosphate saline buffer pH 7.4 ±0.5. At a certain minute, the volume is taken from the receptor compartment periodically and replaced with new media with the same volume. Then the sample was filtered through a filtering medium and analyzed by spectrophotometry or HPLC [25][26].
d. Skin irritation test
Skin irritation and sensitisation tests can be performed on healthy rats, rabbits, and mice. Patches can be put on the backs of mice and rabbits. The patch mixture can then be applied to the skin of mice and rabbits after they have been thoroughly cleaned and dehaired. Before using the patch, mice and rabbits’ backs must be well cleaned. After 24 hours after patch application, mice and rabbits’ skin is checked for signs of oedema and/or erythema (redness and swelling). To classify a symptom into four severity levels (none, mild, moderate, and severe), this severity is compared to the standard 0.8% formalin irritation [23][27][28].
e. Stability test
Stability tests are performed to see and evaluate changes to the patch under any environmental conditions during storage and use. This test was carried out by following the guidelines of the International Conference on Harmonization (ICH). Patch samples were stored at 40±0.5°C and 75±5% RH for 6 months, and then samples were observed at 0, 30, 60, 90 and 180 days and analyzed for drug content [20][29] .
Recent Advances In The Field Of Transdermal Patches
Many research works have been and are few are going on in this field. Few of the latest research done in the field of transdermal patches are stated below:
1.Patch technology for protein delivery Transdermal administration of large proteins represents an innovative and promising delivery method. Currently, there is no commercially available technology that enables the incorporation of proteins into transdermal patches. TransPharma employs its distinctive printed patch technology for the transdermal delivery of proteins, which complements its ViaDerm delivery system. These printed patches deliver precise doses of proteins in a dry form. It is believed that highly water-soluble proteins are dissolved by interstitial fluid secreted from the skin through the RF-MicroChannels, creating a concentrated protein solution on-site. The uptake of the dissolved molecules is subsequently facilitated through the RF-Micro Channels into the viable skin tissues, diffusing across a steep concentration gradient. [30]
2. Painfree diabetic monitoring using transdermal patches
The initial prototype patch measures approximately 1cm in size and is constructed using polymers and thin metallic films. The 5× 5 sampling array is easily visible, along with the metallic connections between them. When the seal is breached, the interstitial fluid, along with the biomolecules it carries, becomes exposed on the skin's surface. By incorporating micro-heating elements into the structural layer of the patch positioned closest to the skin, a localized high-temperature heat pulse can be delivered, penetrating the stratum corneum. During the ablation process, the skin surface is subjected to temperatures of 130°c for a duration of 30ms. The temperature decreases significantly from the skin surface, and neither the living tissue nor the nerve endings are impacted. This painless and bloodless procedure involves the disruption of a 4050?m diameter region of the dead skin layer, which is approximately the size of a hair follicle. This disruption allows the interstitial fluid to come into contact with the patch's electrode sites. [31]
3. Testosterone transdermal patch system in young women with spontaneous premature ovarian failure
In women who have not yet reached menopause, the daily production of testosterone is around 300µg, with approximately half coming from the ovaries and the other half from the adrenal glands. Young women who experience premature ovarian failure without any prior warning may have lower levels of androgens compared to women who have regular menstrual cycles. The testosterone transdermal patch [ttp] was created to mimic the natural production of testosterone by the ovaries. The inclusion of ttp in cyclic e2/mpa therapy for women with spof resulted in mean free testosterone levels that are close to the upper limit of normal. [32]
4.Nanotechnology gaining hold
Another enhancer that is gaining advancement is microneedles. This technology combines the benefits of a needle and a transdermal patch. The devices are tiny, flexible pieces of polymer, each measuring only a few millimeters in length, with numerous hollow microneedles ranging from 100 to 1000 micrometers long. These tiny needles penetrate the outermost layers of the skin, facilitating the smooth passage of the medication. This technology can be integrated with an electronically controlled micropump that releases the drug at predetermined intervals or in response to specific triggers. Once approved by the FDA, these devices would enable the patient or physician to regulate the timing and dosage of the medication being administered. These devices have the potential to deliver drugs directly to the specific location where specialized immune cells are found, allowing for the modulation of the immune system with relative ease. Alza is employing a slightly different approach when it comes to the use of needles. The company has invented a unique technology called macroflux transdermal, which utilizes microprojections to establish pathways on the surface of the skin. The tips of the projections are filled with an active drug, which is released rapidly in a single dose. [33]
5.Pain relief
Transdermal patch technology consistently enhances pain relief. Many readers are familiar with the Duragesic patch, but there are numerous alternatives currently available on the market. One such option is the Lidoderm patch, which contains 5 percent lidocaine and is specifically designed for treating post-herpetic neuralgia. Additionally, there are notable innovations in pain management, including the E-Trans fentanyl (Cl) patch. This credit card-sized patch serves as an active delivery device and features a self-contained battery that releases pulses of fentanyl HCL, a powerful narcotic. This technology simulates intravenous self-controlled analgesic systems, which tend to be costly, unwieldy, and require substantial nursing assistance [33]
CONCLUSION: -
This article provide an valuable information regarding the transdermal drug delivery systems and its evaluation process details as a ready reference for the research scientist who are involved in TDDS . A transdermal patch has many advantages over conventional drug administration, such as increasing bioavailability, not undergoing first-pass metabolism, avoiding adverse gastrointestinal effects, maintaining drug in plasma, and increasing patient compliance. The characteristics of the patch formed from various polymers and in vitro release and penetration tests are the determining factors for the suitability of a patch for transdermal delivery.
REFERENCES
- Arti Kesarwani, Ajit Kumar Yadav, Sunil Singh, Hemendra Gautam, Haribansh N Singh, et al. (2013) A review-Theoretical aspects of Transdermal Drug Delivery System. Bulletin of Pharmaceutical Research 3(2): 78-89
- Sampath Kumar KP, Debjit Bhowmik, Chiranjib B, RM Chandira (2010) A review- Transdermal Drug Delivery System- A Novel Drug Delivery System and its market scope and opportunities. International Journal of Pharma and Bio Sciences 1(2).
- Ghosh B, Preethi Gb, Mishra R, Parcha V. Transdermal Delivery of Ibuprofen ond Its Prodrugs by Passive Diffusion and Iontophoresis. Int J Pharm Pharm Sci. 2010; 2(1):79-85.
- Ghosh, T.K. and Banga, A.K., Pharma. Tech., 1993, 75-78.
- Jain, N.K., In; Advances in Controlled and Novel Drug Delivery, 1st Edn., CBS publishers and distributors, 2002, 428-37.
- Aggarwal G. Development, Fabrication and Evaluation of Transdermal Drug Delivery? A Review. Pharmainfo .net .2099
- Soni, S. and Dixit, V.K., Indian Drugs, 1992, 29(11), 466-4675772/38156
- Shi Z, Gao X, Ullah MW, Li S, Wang Q, Yang G. Electroconductive natural polymer-based hydrogels. Biomaterials. 2016;40–54. doi: 10.1016/j.biomaterials.2016.09.020.
- Rahmanian-Devin P, Baradaran Rahimi V, Askari VR. Thermosensitive Chitosan- ? - Glycerophosphate Hydrogels as Targeted Drug Delivery Systems: An Overview on Preparation and Their Applications. Adv Pharmacol Pharm Sci. 2021;1–17. doi: 10.1155/2021/6640893
- Yadav PR, Munni MN, Campbell L, Mostofa G, Dobson L, Shittu M, et al. Translation of polymeric microneedles for treatment of human diseases: Recent trends, Progress, and Challenges. Pharmaceutics. 2021;13(8):1–45. doi: 10.3390/pharmaceutics13081132.
- Patel D, Chaudhary SA, Parmar B, Bhura N. Transdermal Drug Delivery System: A Review. Pharma Innov. 2012;1(4):66–75.
- Sharma N, Sharma S, Kaushik R. Formulation and evaluation of lornoxicam transdermal patches using various permeation enhancers. Int J Drug Deliv Technol. 2019;9(4):597–607. doi: 10.25258/ijddt.9.4.14.
- Gngr S, Erdal MS, Zsoy Y. Plasticizers in Transdermal Drug Delivery Systems. Recent Adv Plast. 2012;91–112. doi: 10. 7.
- Baichwal Mr. Polymer Films as Drug Delivery Systems Advances in Drug Delivery Systems. Bombay, Msr Foundation; 1985; 136-147.
- Archana K Gaikwad (2013) Reviewed Article, Transdermal Drug Delivery System: Formulation aspects and evaluation. Comprehensive Journal of Pharmaceutical Sciences 1(1): 1-10.
- Kamal Gandhi, Anu Dahiya, Monika, Taruna Karla, Khushboo Singh Transdermal drug delivery-A Review.
- Ginting E, Reveny J. Formulation And Evaluation Of In Vitro Transdermal Patch Diclofenac Sodium Using Chitosan Polymer And Polyvinyl Alcohol Cross-Linked Tripolyphosphate Sodium. Asian J Pharm Clin Res. 2018;11(8).
- Prabhu P, Prabhu P, Gundad S. Formulation development and investigation of domperidone transdermal patches. Int J Pharm Investig. 2011;1(4):240–6. doi: 10.4103/2230- 973x.93008.
- Prajapati ST, Patel CG, Patel CN. Formulation and Evaluation of Transdermal Patch of Repaglinide. ISRN Pharm. 2011;1–9. doi: 10.5402/2011/651909
- Kumar JA, Pullakandam N, Prabu SL, Gopal V. Transdermal drug delivery system: An overview. Int J Pharm Sci Rev Res. 2010;3(2):49–54. doi: 10.4103/0973-8398.104828.
- Rastogi V, Yadav P. Transdermal drug delivery system: An overview. Asian J Pharm. 2012;6(3):161–70. doi: 10.4103/0973-8398.104828
- Bagyalakshmi J, Vamsikrishna RP, Manavalan R, Ravi TK, Manna PK. Formulation Development and In Vitro and In Vivo Evaluation of Membrane-Moderated Transdermal Systems of Ampicillin Sodium in Ethanol: pH 4 . 7 Buffer Solvent System. AAPS PharmSciTech 2007. 2007;8(1):1–6.
- D NV, Shrestha N, Sharma J. Transdermal drug delivery system: An overview. Int J Res Pharm Sci. 2012;3(2):234–41.
- Jayaprakash S, Ramkanth S, Anitha P, Alagusundaram M, S Saleem MT, Chetty MC. Design and Evaluation of Monolithic Drug-in-Adhesive Transdermal Patches of Meloxicam. Malaysian J Pharm Sci. 2010;8(2):25–43.
- Patel A V., Shah BN. Transdermal Drug Delivery System: A Review. An Int J Pharm Sci. 2018;9(1):378–90
- Sharma K, Mittal A, Agrahari P. Skin Permeation of Candesartan Cilexetil from Transdermal Patch Containing Aloe Vera Gel as Penetration Enhancer. Asian J Pharm. 2016;10(2):124–31
- Aggarwal G, Dhawan S, Harikumar SL. Formulation, in vitro, and in vivo evaluation of matrix-type transdermal patches containing olanzapine. Pharm Dev Technol. 2013;18(4):916–25. doi: 10.3109/10837450.2011.609993
- Prabhakar D, Sreekanth J, Jayaveera KN. Transdermal Drug Delivery Patches: a Review. J Drug Deliv Ther. 2013;3(4):213–21. doi: 10.22270/jddt.v3i4.590.
- Prabhu P, Prabhu P, Gundad S. Formulation development and investigation of domperidone transdermal patches. Int J Pharm Investig. 2011;1(4):240–6. doi: 10.4103/2230- 973x.93008
- Levin G, Kornfeld J, Patel Y R, Damon S. Transdermal Delivery : Success Through A Deep Understanding Of The Skin. Corium.
- Shah S. Transdermal Drug Delivery Technology Revisited: Recent Advances. Pharmainfo.net 2008 6[5]
- Joseph S D. Transdermal Patches: An )nnovative Drug Delivery System That (as Raised Serious Safety Concerns. NewsInferno. [ serial online ]. 2006 [cited 2011 feb 22 ]. .
- Morrow T. Transdermal Patches Are MoreThan Skin Deep. Managed care. [ serial online] 2004 [cited 2011 feb 4]. Available online: URL: http://www.managedcaremag.com.


 Dhananjay Abhang *
Dhananjay Abhang *
 Priya Walke
Priya Walke




 10.5281/zenodo.14253927
10.5281/zenodo.14253927