Abstract
Along with the saveral application in research and clinical medicine as well as sevral other sciences,Solid Lipid Nanoparticles are rapidly growing field of recent nanotechnology.Solid Lipid Nano particles is very promising technique for site specific drug delivery systems.Solid Lipid Nanoparticles provides the possibility of developing new therapeutics.There is several type of nanocarriers like Solid Lipid Nanoparticles,lipid drug conjugates and nano structured lipid carriers.Several types of analytical or evaluation techniques and preparation methods are described.Along with these the advantages and disadvantages of Solid Lipid Nanoparticles were discussed. If properly focused on the Solid Lipid Nano Particles it is promissing techniques to overcome the limitations of conventional drug delivery systems.
Keywords
Colloidal drug carriers, Drug delivery system, Routes of administeration, Solid lipid nanoparticles
Introduction
Introduced in 1991, solid lipid nanoparticles (SLN) are an alternative delivery system to previous colloidal carriers such as emulsions, liposomes, and polymeric micro- and nanoparticles. In the last two decades, the particle size of materials has shifted from the microscale to the nanoscale with the development of technology. Colloidal particles between 10 and 1000 nm are called nanoparticles. Composed of synthetic/natural polymers, they are ideal for optimizing drug deliveryand reducing toxicity. Reducing the particle size of materials to the nanoscale increases their total surface area by several orders of magnitude.The word "nano" is easy to define, but covers many areas of application. The therapeutic efficacy of a drug depends on four basic modes of transport and transformation of the drug in the body: absorption, distribution, metabolism and excretion. Treatment failures include insufficient drug concentration due to poor absorption, rapid metabolism and elimination, poor drug solubility, and large fluctuations in plasma levels due to unpredictable bioavailability. SLN features a nanometer lipid core matrix stabilized with a surfactant layer. They have been used as ideal drug delivery systems for protein vaccines and other controlled release drugs compared to other colloidal drug delivery systems.Among colloidal delivery systems, solid lipid nanoparticles have many advantages and limited disadvantages compared to other colloidal delivery systems. Solid lipid nanoparticles are alternative materials to polymers, which are the same as the oil-in-water emulsion for parenteral nutrition, but the liquid lipid in the emulsion was immediately replaced with a solid lipid, as shown in Figures 1(A) and (B) shown.
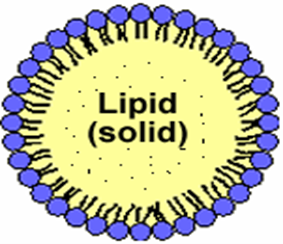
Fig 1(A): Structure of solid lipid nanoparticle (SLN)
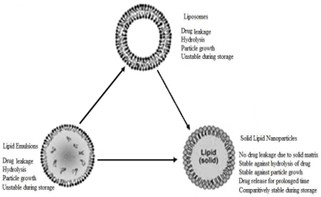
Fig. 1(B) : A diagrammatic representation on SLN over emulsions and liposomes
ADVANTAGES AND PROBLEMS OF SLNS AND OTHER NANOPARTICLES
SLNs combine the advantages and avoid the disadvantages of several colloidal carriers in this class. Potential disadvantages have been observed, such as B. a low drug loading capacity, drug excretion after polymerization during storage and a relatively high water content in the dispersions (70-99.9%). The drug loading capacity of a conventional SLN is limited by the drug solubility in the lipid melt, the structure of the lipid matrix, and the polymer state of the lipid matrix. Does the lipid matrix ofconsist of particularly similar molecules (e.g.Volume. Tristearin or Tripalmithin) creates a perfect crystal with few imperfections. Because incorporated drugs reside between fatty acid chains, between lipid layers, and in crystalline imperfections, a highly ordered crystal lattice is unable to accommodate large amounts of drug. Therefore, the use of more complex lipids makes more sense with higher drug loads.2
ADVANTAGES
- Control and/or target drug release.
- Improve stability of pharmaceuticals.
- High and enhanced drug content (compared to other carriers).
- Feasibilities of carrying both lipophilic and hydrophilic drugs.
- Most lipids being biodegradable, SLNs have excellent biocompatibility. Water based technology
- (Avoid organic solvents).
- Easy to scale-up and sterilize.
- • More affordable (less expensive than polymeric/surfactant based carriers) .
- Easier to validate and gain regulatory approval.
- No special solvent required.2,7
DISADVANTAGES OF SLNP
- Drug loading capacity is limited.
- High water content.
- High pressures induce drug degradation.
- Coexistences of several colloidal species.
- Lipid crystallization and drug incorporation.
- Drug expulsion.
- Particle growth
- Unpredictable gelation tendency.
- Unexpected dynamics of polymeric transitions.5,7
ADVANTAGES OF SLNs OVER POLYMERIC NPs
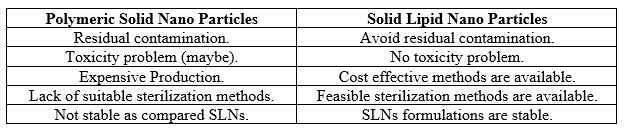
Aims of solid lipid nanoparticles
-
- Possibility of controlled drug release.
- Increased drug stability.
- High drug pay load.
- No bio-toxicity of the carrier.
- Avoidance of organic solvents.
- Incorporation of lipophilic and hydrophilic drugs.5,9
APPLICATIONS OF SLNP
- Used in CNS-related disorders.
- Used in CV-related diseases.
- Used in Diabetes.
- Used in Osteoporosis.
- Used in cosmetic and dermatoligical preparations.11
SLN in cosmetic and dermatological preparation
Solid lipid nanoparticles are also used in cosmetic and dermatological preparations. SLNs are considered to be the next-generation transport system after liposomes. Due to the lower risk of systemic side effects, topical treatment of skin diseases seems to be beneficial, however, the stratum corneum prevents the penetration of xenobiotics into living skin. Particle transport systems could be an option to improve skin penetration. Since epidermal lipids are abundant in thepermeation barrier, lipid transporters that attach to the skin surface and allow lipid exchange between the outermost layers of the stratum corneum and the transporter seem promising.Besides liposomes, solid lipid nanoparticles (SLNs) and nanostructured lipid transporters (NLCs) have also been extensively studied42. After the water has evaporated from the lipid nanodispersion applied to the skin's surface, the lipid molecules form an adhesive layer that seals the skin's surface. Subsequently, the hydration of the stratum corneum may increase, thereby reducing the accumulation of corneocytes and widening the gaps between the corneocytes, which may facilitate penetration of the drug into deeper layers of the skin. Occlusal effects appear to be strongly related to particle size. Nanoparticles have been found to be 15 times more occlusive than microparticles, with particles smaller than 400nm being strongest in a dispersion containing at least 35% highly crystalline lipids.8
SLN PREPARATION
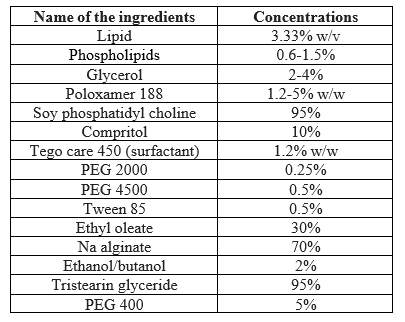
SLNs are made up of solid lipid, emulsifier and water/solvent. The lipids used may be triglycerides (tri-stearin), partial glycerides (Imwitor), fatty acids (stearic acid, palmitic acid), and steroids (cholesterol) and waxes (cetyl palmitate). Various emulsifiers and their combination have been used to stabilize the lipid dispersion. The combination of emulsifiers might prevent particle agglomeration more efficiently. A clear advantage of SLN is the fact that the lipid matrix is made from physiological lipids which decreases the danger of acute and chronic toxicity. The choice of the emulsifier depends on the administration route with a suitable number of emulsifier suitable for parenteral administration.
Table no 2 INGREDIENTS USED IN THE PREPARATION OF NANOPARTI
METHOD OF SLN PREPARATION
- High shear homogenization:
- Hot homogenization
- ?Cold homogenization
- Ultra sonication/high spe ed homogenization:
- Probe ultrasonication
- Bath ultrasonication
- Solvent emulsification/evaporation
- Micro emulsion based SLN preparations
- SLN preparation by using supercritical fluid
- Spray drying method
- Double emulsion method
METHOD OF SLN PREPARATION
High energy approaches
High-pressure homogenization (HPH):-
HPH includes two types of methods: hot homogenization and cold homogenization. Both hot and cold methods involve a first step of dissolving or dispersing the drug in the molten solid lipid.12
The HPH method is a piston with a high-pressure chamber and a small space. The pressure piston can generate a pressure of 10 to 500 mPa. The limited space in the HPH is where the primary emulsion must flow through the valve and in the restricted area of the valve the emulsion droplets are reduced to small sizes.13,14
Hot homogenization method:- On a laboratory scale, this is the most accepted method for SLN formulation. By adding the molten lipid containing the drug to the aqueous phase containing the emulsifier, adding high shear homogenization energy at a pressure of 500-1500 bar, a small-sized pre-emulsion is formed. A hot colloidal O/W emulsion is formed, which leads to the formation of SLN by cooling the melted lipid dispersion in the blood cells.15,16
Cold homogenization method:-
To solve problems related to hot homogenization processes, such as B. (a) heat-labile drugs can not withstand high temperatures, (b) drug loss during its distribution in the aqueous phase and (c) complex crystalline lipid structure 54-56, the homogenization mechanism was introduced cold .15,16
Low energy approaches
Microemulsion method:
The word microemulsion was originally coined by Schulman et al. suggested. Microemulsions are two-phase systems. emulsifier (e.g. polysorbate 80), co-emulsifier (e.g.G. butanol) and water are important components of typical microemulsions. They are an optically transparent mixture.17
Membrane contractor method:-
An effective module was identified for this process, consisting of a layer of Kerasp clay (estimated pore size 0.1, 0.2, 0.45 µm) that isolates the aqueous phase, allowing it to penetrate the surface of the layer, and the lipid that allows their movement down to the surface of the layer.The lipid phase is heated above its melting point in a pressure vessel, passed through the module through a cylinder and forced through the membrane pores to allow the formation of smaller particles. Upon cooling, SLNs form in the aqueous phase.18,19
Phase inversion temperature (PIT) method:-
The essential elements of the phase inversion temperature method are mechanical emulsion at the phase inversion temperature and subsequent rapid cooling to room temperature, producing an emulsion with a large number of fine droplets. Two main components are used in this method: one is an oil phase containing solid lipids and a nonionic surfactant, and the other is an aqueous phase containing NaCl. Both phases are heated to ~90°C (above the phase transition temperature). With constant stirring and constant temperature, the aqueous phase is added dropwise to the oil phase to form a w/o emulsion. The mixture is then allowed to cool to room temperature with constant stirring.At the phase inversion temperature, the cloudy mixture becomes clear and a nanoemulsion forms below the PIT O/W. The stability of lipid nanoparticles after preparation depends on the storage temperature relative to the PIT and the melting/crystallization points.20
Coacervation method:-
It is a solvent-free technique for preparing SLN by acidifying micellar salts. When the pH is low, fatty acids begin to precipitate due to proton transfer between the acidic solution and the soap. This method is widely used to formulate polymer nanoparticles. This method produces nanoparticles with a size between 250 and 500 nm and a spherical shape.20
Double emulsion method:-
This method is mainly used for hydrophilic drugs. The active ingredient is dissolved in the aqueous phase and emulsified in the melted lipid. A primary emulsion is formed, which is stabilized with suitable surfactants and co-surfactants. The primary emulsion is then dispersed in an aqueous phase containing an aqueous emulsifier.21
ROUTES OF ADMINISTRATION
- Parenteral
- Oral
- Transdermal
- Nasal
- Ocular
- Pulmonary
- Rectal
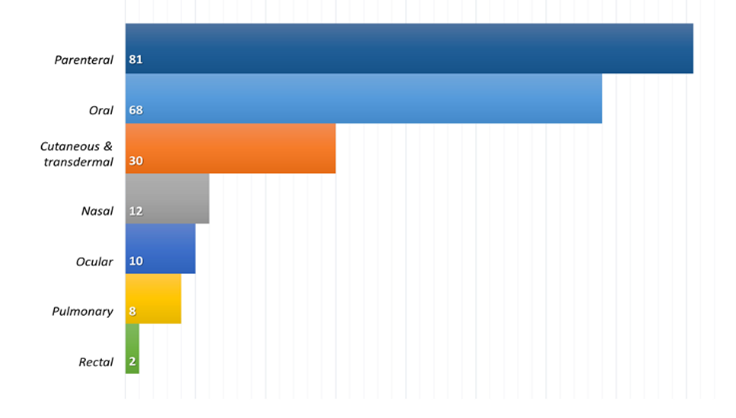
Fig 2: The most commonly proposed administration route for SLNPs
As shown in Figure 2, the parenteral route is the most commonly proposed route of administration for lipid-based nanosystems, closely followed by the oral route. Both routes of administration aim to achieve the systemic effects of encapsulated drugs. However, the trend described is opposite to the current drug distribution in the market where the oral route is the preferred and most commonly used route of drug administration.The parenteral route, on the other hand, allows drugs to be administered directly into the systemic circulation without having to overcome absorption barriers or with minimal limitations as in the case of the intramuscular and/or subcutaneous routes. More than 50% of parenterally evaluated lipid nanosystems (46 out of 81) correspond to anticancer drugs, a therapeutic area that is still dominated by intravenous administration, despite significant negative aspects such as invasiveness, associated risks, inability to self-manage and technology that claims to be Production of a product with sufficient microbiological quality.
PARENTERAL ROUTE OF ADMINISTRATION
The parenteral route of administration is widely used compared to other routes of administration and is also the most appropriate and studied route of administration of SLN, especially in the targeted treatment of cancer.23 Because of the enzymatic degradation of SLNPs in the gastrointestinal tract, the parenteral route is often preferred for the efficient delivery of bioengineered products such as proteins and peptides. The injectable GLS studied so far have been accompanied by various therapeutic drug classes such as: B. anti-cancer drugs, imaging agents, anti-Parkinson drugs, antibiotics, etc.24
ORAL ROUTE OF ADMINISTERATION:-
The lipid structure of SLN makes oral administration convenient and attractive to increase bioavailability by protecting the drug from chemical and enzymatic degradation, thus delaying metabolism in vivo.22 Conditions in certain parts of the stomach lead to the accumulation of particles due to the high acid concentration and ionic strength in the stomach. Along with this fact, the question of the effect of gastric and pancreatic lipases on SLN degradation remains.Sarmento et al. developed insulin-loaded SLNs for oral drug delivery by modified solvent evaporation emulsion. The investigator observed that hypoglycemic effect was observed in diabetic rats after oral administration of insulin-loaded SLNs, and it can also be stated that SLNs can promote oral insulin absorption.20
Transdermal route of administration
Since the skin's epidermis (the top layer of skin) contains the majority of lipids, the support looks promising as all lipid nanoparticles quickly attach to the skin's surface and facilitate lipid exchange between the outer layers of the skin. cool; and for topical and transdermal administration. The level of lipids must be kept low for efficient delivery of the SLN transporter to the skin.2
PATENTS FOR SOLID LIPID NANO PARTICLES
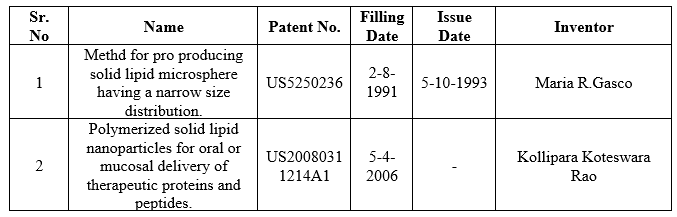
Table no 3 Patents for SLNPs
EVALUATION OF SLNPs
Determination of ? max of pantoprazole:-
Pantoprazole, 100 mg, was carefully weighed into a 100 mL volumetric flask, dissolved in 0.1 M HCl and made up to volume with 0.1 M HCl. Pipet 1 mL of this solution into a 10 mL volumetric flask containing 0.1 M HCl (vol) and mark as stock solution.Prepare the appropriate dilution to bring the concentration back to 2-10 ?g/mL. The resulting solution is scanned with a UV spectrophotometer (UV-1700 Shimadzu Corporation, Japan) in the range (200-400 nm) to determine the absorption maximum (?max).27
Drug content
1 ml of the prepared SLN preparation suspension is dissolved in 10 ml of a combination of PBS 7.4 buffer and ethanol. A UV spectrophotometer at 274 nm was used to determine the amount of pantoprazole. A placebo formulation prepared in the same manner as drug-loaded SLNs served as a control. The total amount of drug was determined.27
In vitro drug release:-
In vitro drug delivery was tested with the prepared SLN delivery system. A USP XXII paddle-type dissolution device was used for the drug release experiments. The dissolution experiment was performed in 900 ml of dissolution medium (PBS pH 7.4) stirred at 100 rpm and maintained at 370.2°C.Samples were taken at different time intervals and filled up with an equal amount of fresh dissolving medium. PBS was used to dilute the sample to a maximum of 10 ml (pH 7.4). The collected samples were tested spectrophotometrically with a UV-visible spectrophotometer for the presence of pantoprazole at 274 nm. Pantoprazole release was calculated using the pantoprazole standard curve.27
Stability studies:-
The drug-loaded SLNs were tested for three months at three different temperatures: refrigerated (4.0 ± 0.2 °C), room temperature (25-28 ± 2 °C), and 45 ± 1 °C. To avoid any interaction between the formulation and the glass container, the stability formulation was stored in a borosilicate container. The physical changes and the active ingredient content of the preparations were examined.27
CONCLUSION
SLNs have the potential to maintain high stability during storage. A wide range of lipids (oils) and fatty acids are available to regulate the release kinetics. Sentinel lymph nodes are very flexible lipid carriers that can be easily tailored to fixed lipid endgroups to achieve effective potentiation for a given therapy. Problems with clearance and drug targeting can be effectively addressed by surface modification. Sentinel lymph nodes are not only used in therapy, the potential as an imaging or diagnostic tool is also being explored.researchers have already filed and received several SLN-related patents, and young researchers can expect to see more patented Surface Modified SLN (SLN)-based systems soon for the treatment and diagnosis of various diseases, especially targeting by Customization of surfaces. Well-studied and very well-designed, SLNs appear to be a promising vector that could set new standards in treatment, diagnosis, and as a vector for biologics.
REFERENCE
- Yongtao Duana, Abhishek Dharb, Chetan Patelc, Mehul Khimanic, Swarnali Neogib, Prolay Sharmab, Nadavala Siva Kumar d and Rohit L. Vekariya. A brief review on solid lipid nanoparticles.
- S.Mukherjee, S.Ray, and R.S.Thakur. Solid Lipid Nanoparticles: A Modern Formulation Approach in Drug Delivery System.
- Sebastián Scioli Montoto1,2 , Giuliana Muraca1,3 and María Esperanza Ruiz. Solid Lipid Nanoparticles for Drug Delivery: Pharmacological and Biopharmaceutical Aspects.30 October 2020doi: 10.3389/fmolb.2020.587997.
- Houli Li, Xiaobin Zhao, Yukun Ma and Guangxi Zhai, Ling Bing Li and Hong Xiang, Lou. J. Cont. Release, 133, 238-244 (2009).
- Melike Uner, Gulgun Yener, Int. J. Nanomedicine, 2(3), 289-300 (2007).
- Indu Pal Kaur, Rohit Bhandari, Swati Bhandari and Kakkur. J. Cont. Rel., 127, 97-109 (2008).
- Wolfgang Mehnart and Karsten Mader, Adv. Drug. Deliv. Rev., 47, 165-196 (2001).
- L. Harivardhan Reddy and R. S. R. Murthy, AAPS Pharm. Sci. Tech., 6(2), 24 (2005).
- Indu Pal Kaur, Rohit Bhandari, Swati Bhandari and Kakkur. J. Cont. Rel., 127, 97-109 (2008).
- P. Ekambaram, A. Abdul Hasan Sathali And K. Priyanka. Solid Lipid Nanoparticles: A Review. Sci. Revs. Chem. Commun.: 2(1), 2012, 80-102.
- Chih-Hung Lin, , Chun-Han Chen, Zih-Chan Lin, Jia-You Fang. Recent advances in oral delivery of drugs and bioactive natural products using solid lipid nanoparticles as the carriers. www.sciencedirect.com.
- Yong AP, Islam MA, Hasan N. The effect of pH and high-pressure homogenization on droplet size. Int J Eng Mater Manuf. 2017;2(4):110–22. doi: 10.26776/ijemm.02.04.2017.05. [CrossRef] [Google Scholar]
- Teja VC, Chowdary VH, Raju YP, Surendra N, Vardhan RV, Reddy BK. A glimpse on solid lipid nanoparticles as drug delivery systems. J Glob Trends Pharm Sci. 2014;5(2):1649–57. [Google Scholar]
- Shylaja P, Mathew M. Preparation and characterization of alpha tocopherol loaded solid lipid nanoparticles by hot homogenization method. Int J Pharm Pharm Res. 2016;7(1):437–48. [Google Scholar]
- Pallerla SM, Prabhak B. A review on solid lipid nanoparticles. ChemInform. 2014;45(21):196–206. [Google Scholar]
- Lingayat VJ, Zarekar NS, Shendge RS. Solid lipid nanoparticles: a review. Nanoscience and Nanotechnology Research. 2017;4(2):67–72. doi: 10.12691/nnr-4-2-5. [CrossRef] [Google Scholar]
- Prieto C, Calvo L. Performance of the biocompatible surfactant Tween 80, for the formation of microemulsions suitable for new pharmaceutical processing. J Appl Chem. 2013;2013:930356. doi: 10.1155/2013/930356. [CrossRef] [Google Scholar]
- Battaglia L, Gallarate M, Panciani PP, Ugazio E, Sapino S, Peira E. et al. Techniques for the preparation of solid lipid nano and microparticles In: Application of Nanotechnology in Drug Delivery. IntechOpen. 2014 doi: 10.5772/58405. [CrossRef] [Google Scholar]
- Charcosset C, El-Harati A, Fessi H. Preparation of solid lipid nanoparticles using a membrane contactor. J Control Release. 2005;108(1):112–20. doi: 10.1016/j.jconrel.2005.07.023. [PubMed] [CrossRef] [Google Scholar]
- Sonia pandey et al. A Recent Update: Solid Lipid Nanoparticles for E!ective Drug Delivery.
- Ramteke KH, Joshi SA, Dhole SN. Solid lipid nanoparticle: a review. IOSR J Pharm. 2012;2(6):34–44. [Google Scholar]
- Müller RH, Runge S, Ravelli V, Mehnert W, Thünemann AF, Souto EB. Oral bioavailability of cyclosporine: solid lipid nanoparticles (SLN) versus drug nanocrystals. Int J Pharm. 2006;317(1):82–9. doi: 10.1016/j.ijpharm.2006.02.045. [PubMed] [CrossRef][Google Scholar]
- Üner M, Yener G. Importance of solid lipid nanoparticles (SLN) in various administration routes and future perspectives. Int J Nanomedicine. 2007;2(3):289–300. [PMC free article] [PubMed] [Google Scholar]
- Harms M, Müller-Goymann CC. Solid lipid nanoparticles for drug delivery. J Drug Deliv Sci Technol. 2011;21(1):89–99. doi: 10.1016/S1773-2247(11)50008-5. [CrossRef] [Google Scholar]
- Mühlen A, Schwarz C, Mehnert W. Solid lipid nanoparticles (SLN) for controlled drug delivery--drug release and release mechanism. Eur J Pharm Biopharm. 1998;45(2):149–55. doi: 10.1016/s0939-6411(97)00150-1. [PubMed] [CrossRef] [Google Scholar]
- Schäfer-Korting M, Mehnert W, Korting HC. Lipid nanoparticles for improved topical application of drugs for skin diseases. Adv Drug Deliv Rev. 2007;59(6):427–43. doi: 10.1016/j.addr.2007.04.006. [PubMed] [CrossRef] [Google Scholar]
- Atiya Sheikh, Sandesh Asati. Preparation, evaluation and optimization of solid lipid nanoparticles composed of pantoprazole. Journal of Drug Delivery and Therapeutics.


 Pinjari Ahmed Akbar*
Pinjari Ahmed Akbar*
 Shaikh Imran
Shaikh Imran






 10.5281/zenodo.11080484
10.5281/zenodo.11080484