Abstract
Second wave of Covid-19 in India led to rise in cases of Mucormycosis. Reviewed causes, symptoms, and treatment of Mucormycosis Immunosuppressants increase risk of Mucormycosis in Covid-19 patients. Factors increasing susceptibility to Mucormycosis: hyperglycemia, ketoacidosis, transplants, liver cirrhosis, neutropenia. Early diagnosis, removing predisposing factors, timely antifungal treatment, and surgical removal of infected tissue key in fighting Mucormycosis.
Keywords
Covid-19 , Mucormycosis , Immunosupresent , hyperglycemia
Introduction
India has never faced such a high prevalence of black fungus infection. So, the recent spike of India affects a lot with the worldwide pandemic Covid-19 caused by “Severe acute respiratory syndrome Corona virus-2 (SARS-CoV-2)”. First case of COVID-19 was reported in Kerala, India on 30th January 2020, afterwards highest cases i.e. 1 lakhs per day were reported for the year by May 2020. After mid-June recovery of patient’s increases successively with decrease in infection rate, further active case dropped to less than 15000 in January 2021. Afterwards second wave was begun in March 2021 with a larger blow of active cases then first wave with deficiency of hospital beds, vaccines, medicines, oxygen cylinders and oxygen. The daily reported cases were reached to around 4.5 lakhs in starting of May 2021.The effect of Covid-19 ranges from mild to moderate to life threatening with some associated disorders such as diabetes mellitus, cardiac diseases and immune compromised conditions. Research articles also reported about the development of severe opportunistic infectious diseases like pneumonia, candidiasis, pulmonary aspergillosis etc. in Covid-19 affected patients. There are also reports of development of mysterious fungal infection known as Mucormycosis or Black fungus in Covid-19 patients. Covid-19 patients in India also suffer with this epidemic disease (mucormycosis) with a reported case of 8848 till May 22, 2021. Here the current article reports signs, symptoms, diagnosis, treatment, prevention against black fungus.[1] This fungal infection in India was unanticipated. Black fungus is an opportunistic pathogen that affects immune-compromised patients due to comorbidities, excessive administration of steroids, organ transplantation, exposure to ventilation, oxygen therapy, poor hospital hygiene, etc. A recent summary of the Indian black fungus infection upsurge indicates that 94% of patients had diabetes. In India, the black fungus victims are mainly covid-19 and post-covid-19 patients. The airways of covid patients are favorable for black fungus due to the exposure to humidity and moisture during ventilation in ICU. Besides, the indiscriminate use of steroids and antibiotics for treating covid-19 patients might create chance for this opportunistic fungus. Therefore, health experts primarily suspected overmedication, hospital hygiene, and comorbid diseases as possible contributing factors for this sharp spike of black fungus infection during the catastrophic second wave of the covid-19 pandemic in India . Besides, people in India have a tendency to alternative therapy for covid-19 like cow dung and urine to cure covid. Many people in India consume cow dung and urine under branded “cow dung therapy” for covid cure . There is no scientific evidence supporting this behavior of using byproducts of cows to boost immunity against diseases. Moreover, the behavior can spread other diseases from animals to the human body, for example, mucormycosis, because animal dung is a potential host for black fungus. Therefore, we assume the wide use of cow dung and urine might contribute to this epidemic.[3]
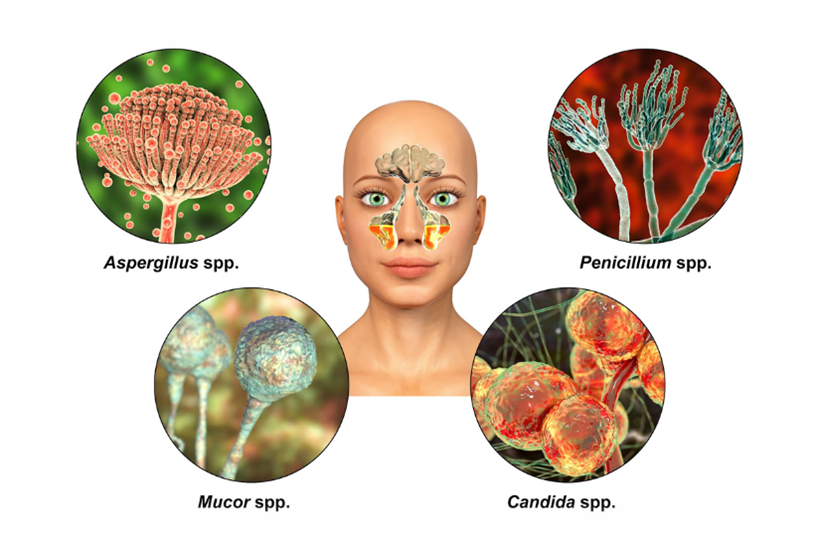
Mucormycosis or black fungus
Fig no-1 Mucormycosis
Mucormycosis, also known as black fungus, is a rare but dangerous infection. It's caused by a group of molds called mucormycetes and often affects the sinuses, lungs, skin, and brain.[2] It caused by Mucormycosis belong to the class Zygomycetes having order Mucorales. The mucormycosis could mainly occurs in soil, leaves, decayed wood, manure etc. Species of Mucoraceae family i.e. Rhizopus arrhizus, Rhizopus pusillus, Apophysomyces elegans, Absidia elegans and Mucor racemosus are most common cause of the disease.[1]
Mucormycosis[3]
Other names-
Zygomycosis and black fungus
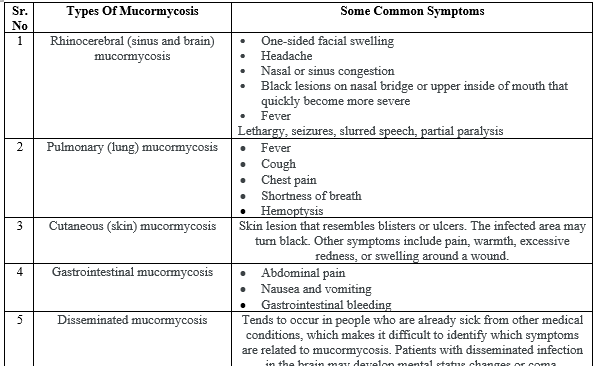
Fig no – 2 Periorbital mucormycosis
Specialty –
oral and maxillofacial surgery, infectious disease, emergency medicine
Symptoms –
depending up on location: runny nose, black area of skin, facial swelling, headache, fever, cough, blurred vision
Complication –
Blindness, thrombosis
Usual onset –
rapid sinuses and brain, lungs, stomach, intestine and skin
Types –
disseminated, miscellaneous
Cause –
fungi of the muckrakes type
Risk factors –
diabetes, iron overload, low white cells, cancer, and organ transplant kidney problems, immune suppressants, and long term steroids
Diagnostic method –
Biopsy, culture, medical imaging
Differential diagnosis –
Orbital cellulitis, cavernous sinus thrombosis, aspergillosis
Prevention –
face mask, avoiding contact with soil water-damaged buildings, good diabetic control
Treatment –
Antifungal, surgical debridement, treat underlying medical conditions
Medication –
Amphotericin B , isavuconazole Posaconazole
Prognosis –
Poor
Frequency –
Rare
CLASSIFICATION[4]
Generally, mucormycosis is classified into five main types according to the part of the body affected. A sixth type has been described as mucormycosis of the kidney, or miscellaneous, i.e., mucormycosis at other sites, although less commonly affected.
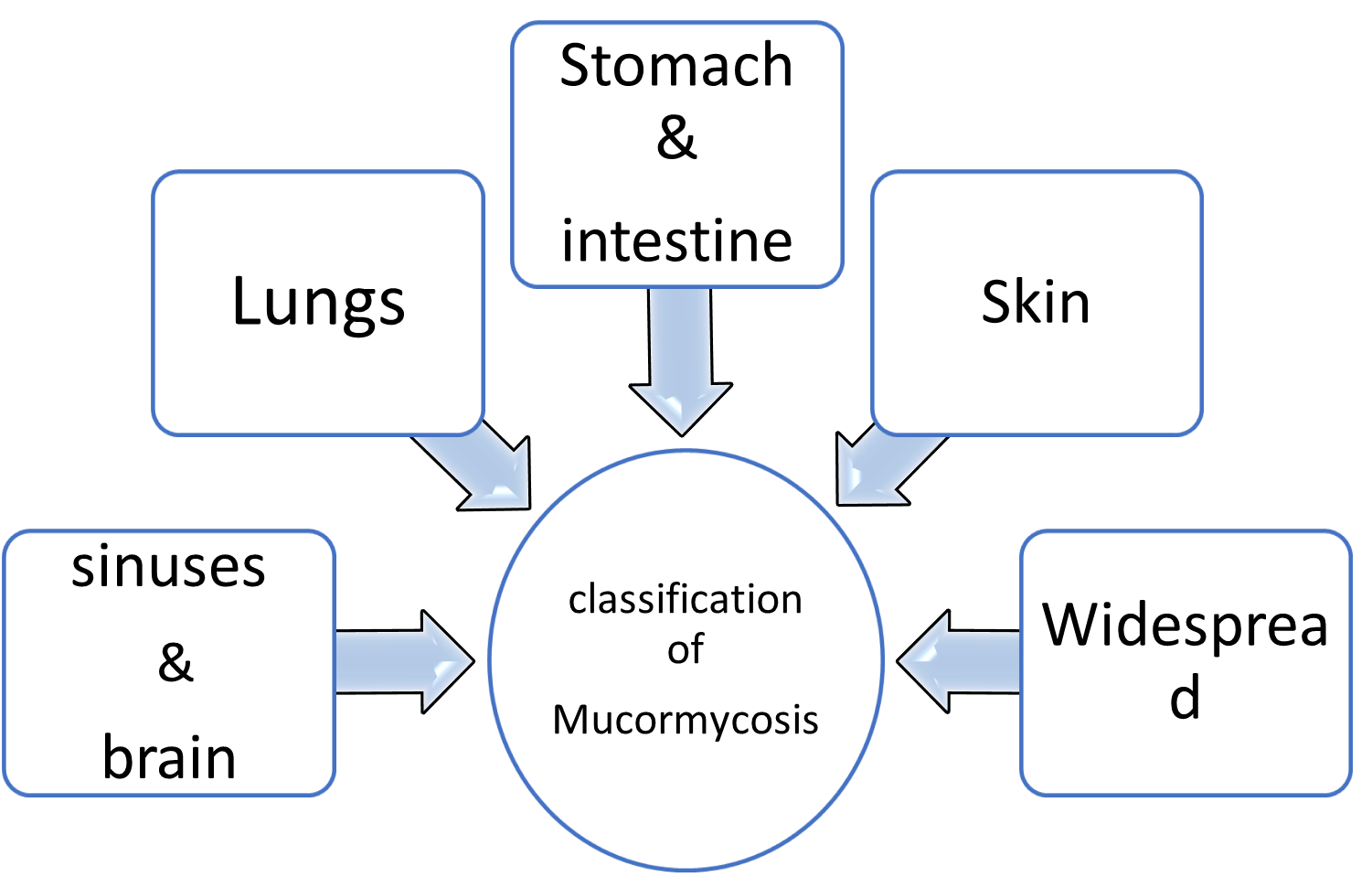
Table & Chart no – 1 Classification
- Sinuses and brain (rhino cerebral);
most common in people with poorly controlled diabetes and in people who have had a kidney transplant.
- Lungs (pulmonary);
the most common type of mucormycosis in people with cancer and in people who have had an organ transplant or a stem cell transplant.
- Stomach and intestine (gastrointestinal);
more common among young, premature, and low birth weight infants, who have had antibiotics, surgery, or medications that lower the body's ability to fight infection.
- Skin (cutaneous);
after a burn, or other skin injury, in people with leukemia, poorly controlled diabetes, graft-versus-host disease, HIV and intravenous drug use.
- Widespread (disseminated);
when the infection spreads to other organs via the blood
Clinical pathogenesis[1]
Mucormycosis could can invade in the susceptible host via nostrils, mouth or burned/disrupted skin which results in rhino-orbit-cerebral, gastrointestinal or cutaneous wound infections. Mucormycosis also results in vascular thrombus and may lead to tissue necrosis. Studies suggested that Rhino cerebral Mucormycosis is most common among all other cases of Mucormycosis. It is most common in the patients with uncontrolled diabetes and leukemia. Sometimes progression of rhino-cerebral Mucormycosis may leads to central nervous system and it becomes fatal. The second most preferred site of infection could be lungs and sinuses. Mortality rate associated with lungs infection may be over 60%.
In severe Covid-19 situation patient could develop dysfunction of immune system with decrease in lymphocyte counts and exponentially rise in inflammatory cytokines such as IL-6, IL-1?, IFN- ?, MCP-1, IP-10, IL-4, IL-10 and Tumor necrosis factor (TNF) that leads to hyper inflammation in the lungs and some patients may leads to death. Due to the severity of hyper inflammation or viral load physicians preferred use of immunosuppressant or steroids as a lifesaving treatment in critically severe patients. A steroid reduces inflammation in the lungs besides these steroids also reduce immunity of the body and increases blood sugar level in both diabetic and normal patients.18 According to the physicians immunosuppressed patients are more likely to be affected with Mucormycosis or Black fungus. (Fig.3)
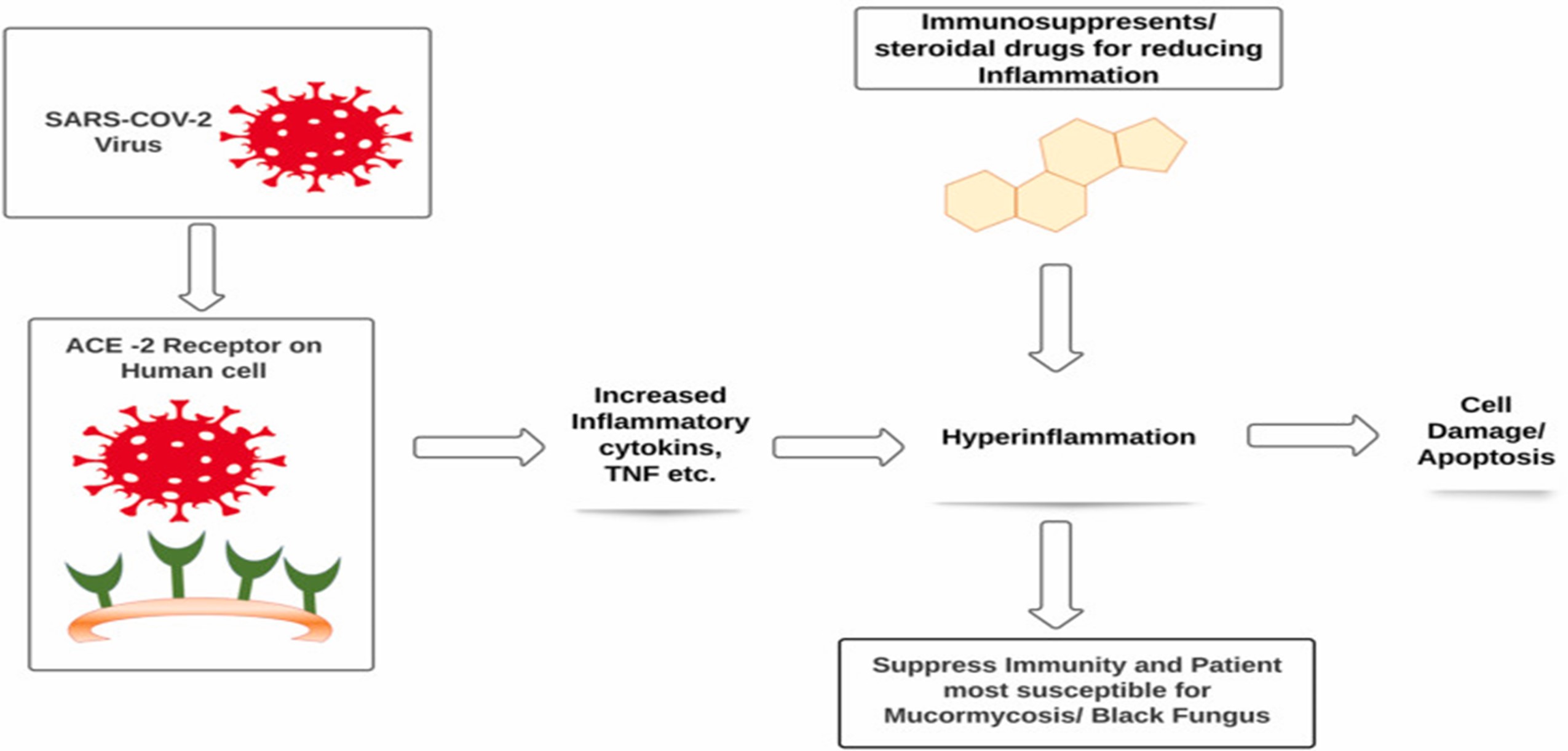
Fig. 3 A deadly black fungus infection among Covid -19
The entry of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is possible via spike protein available on the envelope which binds with angiotensin converting enzyme 2 (ACE 2), which are available at pancreatic beta cells, lungs, kidney and small intestine. It is possible that entry of virus into pancreatic cells may damage beta cells and leads to insulin deficiency. Patients with hyperglycemia and ketoacidosis are more susceptible to get attacked by Mucormycosis moulds. Treatment of Covid-19 patient with immunosuppressant having uncontrolled diabetes mellitus and ketoacidosis are also at major risk for Mucormycosis as it leads to dysfunctional phagocytes causes impaired intracellular killing by oxidative and non-oxidative mechanism. (Fig 4)
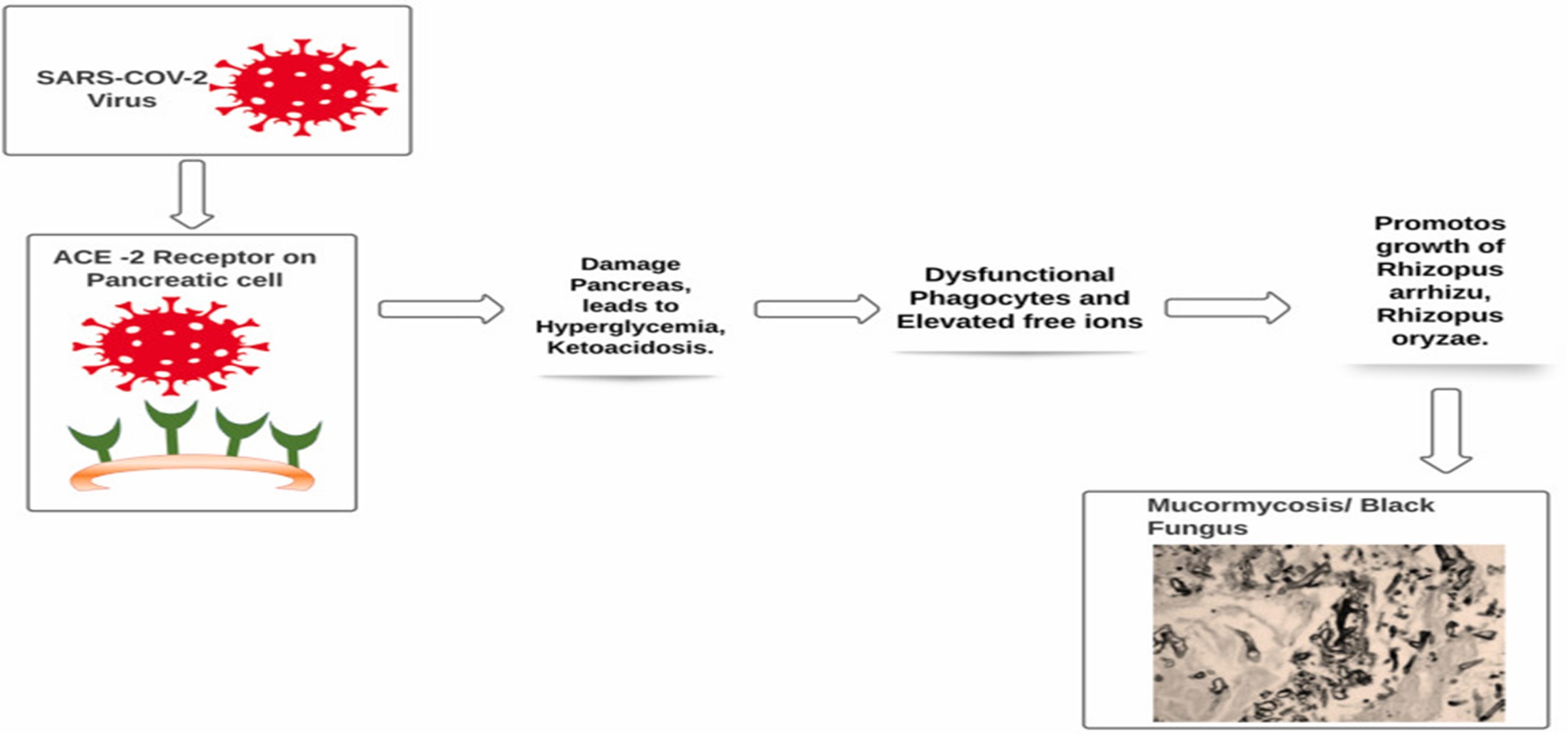
Fig. 4The entry of SARS-CoV-2
The pathogenesis of patients having diabetes mellitus in ketoacidosis also suggested that hyperglycemia and acidic pH (7.3–6.88) also leads to elevated free iron in the serum which is due to release of iron from the binding proteins. This free iron also promotes growth of Mucormycosis could such as Rhizopus arrhizu, Rhizopus oryzae. Moreover patients receiving deferoxamine are also more susceptible to get attacked by Rhizopus species while deferoxamine act as iron chelator. Studies suggested that Xenosiderophore, Siderophores of fungus has higher affinity for Iron than deferoxamine, so they easily detach iron from deferoxamine and provide it to fungus. It was also pointed out that obese adipose tissues releases adipokines that modulate glucose metabolism by excessive release of inflammatory cytokines (IL-6, IL-8, TNF-?) and causes hyperinflammation. Adipose tissues in obese patient also induce mitochondrial production of reactive oxygen species (ROS). In hyperglycemic state, higher level of ROS will cause increased glycosylation and activation of protein kinase C. Therefore, Covid-19 patient with obesity are also more prone to get attacked by Mucormycosis. Patients with solid organ or bone marrow transplantation, liver cirrhosis, neutropenia are also more susceptible to get infected with Mucormycosis. As these patients have lesser number of monocytes and neutrophils which has ability to inhibit mucormycosis mould. so here the inference is that Covid patient with lesser number of monocytes and neutrophils has higher probability to get infected by mucormycosis. As discussed above, the probability of the development of Mucormycosis could is mainly associated with patients suffering from diabetes mellitus, ketoacidosis, decreased immunity and patient's receiving immunosuppressant/corticosteroids as in case of Covid-19. The source of developing or inoculation of Mucormycosis molds is mainly accompanied by contamination with water and soil. In case of Covid-19 probably the source could be water for humidifier during oxygen therapy before inhaling inside by the patients. The infection can be life threatening and has a mortality rate of 38–80%.
EPIDEMIOLOGY[5]
Most human infections result from inhalation of fungal sporangiospores that have been released in the air or direct inoculation of organisms into disrupted skin or mucosa . The Mucorales are ubiquitous in nature, but their precise ecology remains to be determined; they are thermotolerant and are usually found in decaying organic matter. Cases with mucormycosis have been reported from all over the world. Seasonal variation in Mucorales infection is possible. In a study from Israel, 16 of 19 reported cases of rhino-orbitocerebral mucormycosis (ROCM) occurred in autumn . In another study from Japan, a similar seasonal variation among hematology patients was noted, with 6 of 7 cases of pulmonary mucormycosis having developed from August to September Differences in the epidemiology of mucormycosis seem to exist between developed and developing countries. In developed countries, the disease remains uncommon and, at present, is mostly seen in patients with diabetes mellitus and hematological malignancies (HMs) undergoing chemotherapy and those who have received allogeneic stem cell transplants . In contrast, in developing countries, especially in India, mucormycosis cases, although sporadic, occur mainly in patients with uncontrolled diabetes or trauma [2, 16]. There are several factors that limit our ability to accurately determine the exact incidence of mucormycosis. Autopsy rates, the ‘‘gold standard’’ approach, have been in continuous decline globally during the last decades. Nevertheless, mucormycosis remains an uncommon disease, even in high-risk patients, and represents 8.3%–13% of all fungal infections encountered at autopsy in such patients . Postmortem prevalence evaluation shows that mucormycosis is 10–50-fold less frequent than candidiasis or aspergillosis with a frequency of 1–5 cases per 10 000 autopsies
COVID-19–associated mucormycosis

Fig no – 5 Global distribution of covid-19
In India, the major mucormycosis epidemiologic change during the COVID-19 pandemic was an increase in the percentage of patients treated with corticosteroids who developed CAM. Compared with the rest of the world, India reported a higher mucormycosis incidence even before the COVID-19 pandemic[6] A post-mortem study conducted between March 2020 and April 2020 from the UK revealed pathological findings in a patient, which upon biopsy, PCR, and DNA extraction confirmed the presence of disseminated mucormycosis . Since then, multiple cases of mucormycosis co-infection in ongoing or post COVID-19 have emerged. Countries amidst the second and the third COVID-19 waves are now overlooking a syndemic sweeping lives globally. According to a review of published and unpublished studies, CAM has affected 18 countries, including but not limited to India, Pakistan France, Iran, Mexico, Russia, Bangladesh, Brazil, Chile, Czech Republic, Germany, Italy, Kuwait, Lebanon, and Turkey . During the first week of June 2021, India with over 20,000 cases of CAM, remains the hardest-hit country in the world[7].
Recurrence of mucormycosis during COVID-19 second wave in India[3]
Pre-COVID mucormycosis was a very rare infection, even in India. It is so rare that an ENT (ear, nose, and throat) doctor would not witness often a case during their university time. So, the documentation available on the treatment of mucormycosis is limited. In fact, there used to be a couple of mucormycosis expert ENT surgeons for millions of people pre-pandemic. The sudden rise in mucormycosis cases has left a majority of the ENT doctors with no option but to accept mucormycosis cases, as the expert doctors were very much occupied and the patient would die if left untreated. The majority of the ENT doctors had to manage with minimal or no experience on mucormycosis, this has led to the recurrence of mucormycosis in the patients they treated. When a highly experienced doctor in mucormycosis treats a patient even he cannot guarantee that the individual is completely cured and will not have a relapse of mucormycosis; an inexperienced ENT surgeon will definitely have a high number of patients with recurrence due to which there were many recurrent cases of mucormycosis although it did not get the limelight of media or the Indian Government.
Prognosis[8]
Rhino-orbital mucormycosis is a grave disease with significant mortality rates, with some published studies reporting mortality rates of up to 80% or more if untreated.[3] Even with prompt treatment, the prognosis remains guarded. In a series of 929 patients published in 2005, the survival rates for patients treated with amphotericin alone, surgical debridement alone, and both amphotericin and surgical debridement were 61%, 57%, and 70%, respectively.[2] Delay of treatment has been well correlated to poor outcomes, with one study showing a significant difference in survival between patients with a lag time to treatment of 7-12 days (63%) vs patients with lag to treatment time of 13-30 days (44%). Another study reports survival rates of 85% in patients with a lag time of 3-9 days versus just 55% in patients with a lag time of 10-45 days. Development of hemiplegia, facial/eyelid gangrene, and cerebral invasion were all associated with a poor prognosis.
Pathogenesis[9]
Usually, the spores of the fungi disperse through the air and enter within humans via inhalation, inoculation in some regions like skin lesions, the oral way in the gastrointestinal tract . Whatever may be the entry point the fungus propagation involves crucial steps like spore inoculation, escaping phagocytosis from macrophages and neutrophils, hyphae germination, growing using the condition of the host like iron overloads, ketoacidosis, attaching with the endothelium using receptors and via endocytosis damages the endothelium resulting in necrosis of tissue, thrombus formation or haemorrhages as shown in figure The omnipresent fungal spores enter in the body through naso-oral pathway or skin injury, followed by absconding from the host immune cells due to pathophysiological or immunocompromised conditions in the body. After passing the cellular barricade the fungal spores produce hyphae in the blood vessels causing dissemination in various organs and leading to fungal infection at the site of entry.
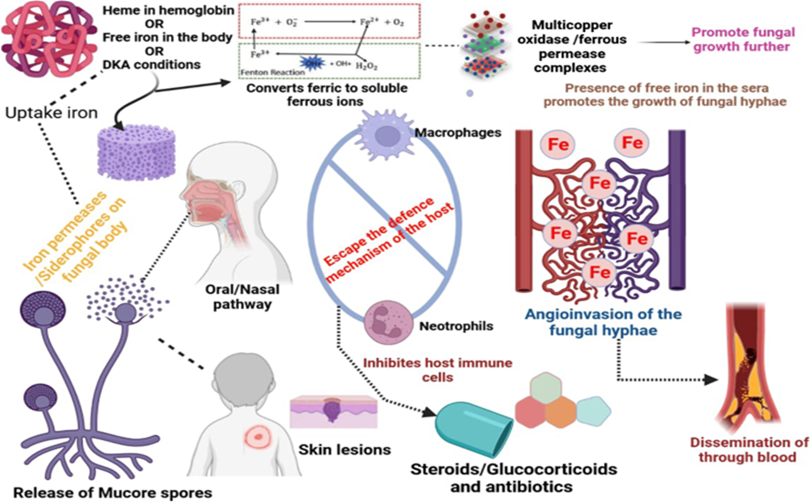
Fig no 6 Pathogenesis of fungal infection mucormycosis
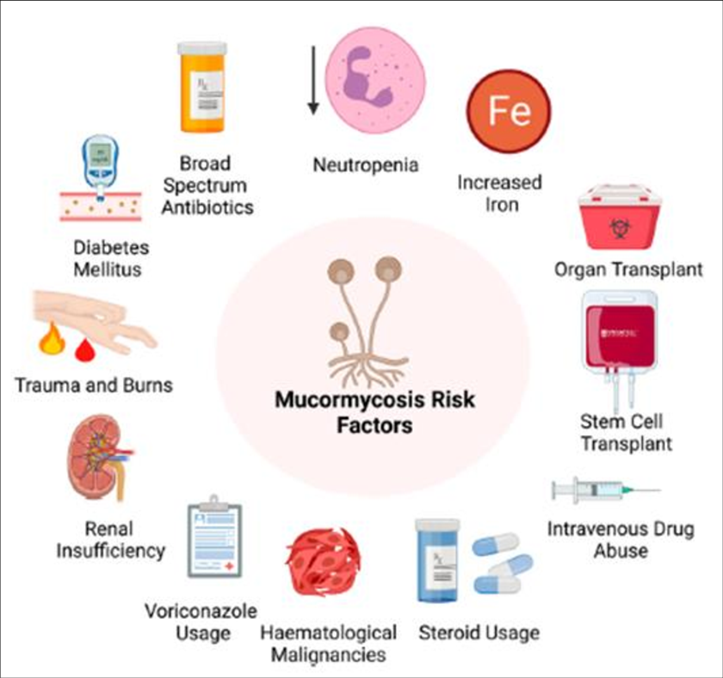
Fig no -7 signs & symptoms
During treatment or post Covid-19 patient's complaint for fever, headache, and reddish swollen skin over nose and around eyes all are the major signs and symptoms of Mucormycosis. Patients also reported visual abnormalities, eye swelling, ocular pain, facial edema and breathe shortening. Diabetic patients also reported for the symptoms of diplopia which is also the sign of infection. In scientific terminology sinus pain, proptosis, periorbital swelling, orbital apex syndrome and ulcer of the palate and cranial nerve palsy are the major symptoms of Mucormycosis infection.
TABLE & CHART NO – 2 SIGHN & SYMPTOMS
CAUSES[11]
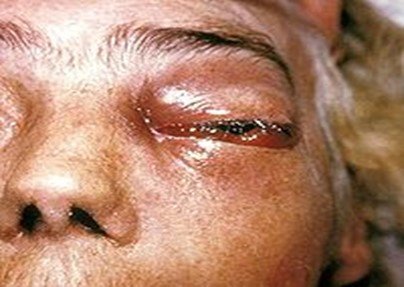
Fig no-8 Mucormycosis causes
Mucoraceae family are the most commonly identified cause of mucormycosis in humans. Other fungal causes may include Mucor species, Cunninghamella Bertholletia, Apophysomyces elegans, Absidia species, Saksenaea species, Rhizomucor pusillus, Entomophthora species, Conidiobolus species, and Basidiobolus species.
- Mucoraceae are found worldwide and in the ecosystem are responsible for initiating and decaying most organic material in the environment.
- Most fungi are identified by their unique morphological appearance viewed microscopically and determined by a professional practicing in fungal identification (microbiologist or pathologist).
- In general, mucormycosis is an infection not often seen by many doctors because the fungal causes are not readily infectious.
- Usually, an infection develops because of some unusual circumstance that places the fungi in contact with compromised or injured animal or human tissue.
- However, once established, the fungi can rapidly multiply in blood vessel walls where it effectively reduces and cuts off blood to tissues, thereby creating its own decaying organic food source resulting in widespread tissue destruction.
- If this fulminant spread of fungi is not stopped, death is the outcome.
Risk factors[12]

Fig no 9- Risk factor
Predisposing factors for mucormycosis include immune deficiencies, a low neutrophil count, and metabolic acidosis. Risk factors include poorly controlled diabetes mellitus (particularly DKA), organ transplant, iron overload, such cancers as lymphomas, kidney failure, liver disease, severe malnutrition, and long term corticosteroid and immunosuppressive therapy. Other risk factors include tuberculosis (TB), deferoxamine and to a lesser extent HIV/AIDS. Cases of mucormycosis in fit and healthy people are less common. Corticosteroids are commonly used in the treatment of COVID-19 and reduce damage caused by the body's own immune response to the virus. They are immunosuppressant and increase blood sugar levels in both diabetic and non-diabetic patients. It is thought that both these effects may contribute to cases of mucormycosis.
MECHANISM OF MUCORMYCOSIS[13]
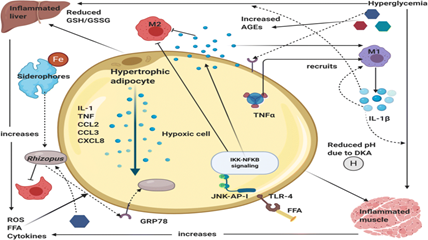
Fig no-10 Mechanism
COVID-19 virus increases the ferritin levels thereby increasing intracellular iron (growth factor for mucor). In systemic ketoacidosis there is temporary disruption of transferring to bind iron hence concentration of free iron increases in serum as seen in Figure. The acidic background results in oxidative environment which affects glutathione renovation through GSH/GSSG enzyme cycle and more free iron production by reducing its binding to transferrin High levels of glucose cause endoplasmic reticulum (ER) stress and increased release of reactive oxygen species (ROS), free fatty acids (FFA) and cytokines in the liver, muscle, and adipose tissue. There is an upregulation of GRP 78 receptors and their translocation to plasma membrane for binding with hyphal elements receptors cot H. Hypertrophic cells in adipose tissue release pro-inflammatory cytokines like interleukin-1?, TNF-? and chemokines like CCL2, CCL3 and CXCL8 (Fig. 4). M1 macrophages are recruited by the action of TNF-? and its activation releases more pro-inflammatory cytokines (mainly IL-1?) that generate persistent inflammation and the recruitment of more M1 macrophages (cytokine storm). In addition, at the cytoplasm level of tissue cells, FFA are recognized by Toll-like receptors 4 (TLR-4) activating JNK-AP-I and IKK-NF?B signaling
DIAGNOSIS[14]
The tissue necrosis resulting from angio-invasion is a classical feature of mucormycosis but, the other fungi ,like Aspergillus, Lomentospora (Scedosporium), and Fusarium spp, also result in tissue necrosis. In such cases, a joint approach is made by clinicians, histopathologist, microbiologist and radiologist. A high index of suspicion is needed for early diagnosis of mucormycosis in patients having multiple predisposing risk factors.Endoscopy mediated nasal cavity examination, especially the middle turbinate and sinuses, may provide the initial evidence for mucormycosis. NCCT and MRI of the paranasal sinuses and orbit help to diagnose mucormycosis where the hyperinflamed densed sinus mucosa with bone erosion, including the periosteum, is observed Figure. These imaging techniques are essential to define the extent of the lesion. These clinic-radiological features provide a high index of suspicion for mucormycosis, whereas the diagnosis is confirmed by microbiological and histopathological examination. In cases of respiratory mucormycosis, reverse halo sign (RHS), multiple (?10) nodules, pleural effusion, central necrosis, and air-crescent sign are observed on HRCT of the thorax.
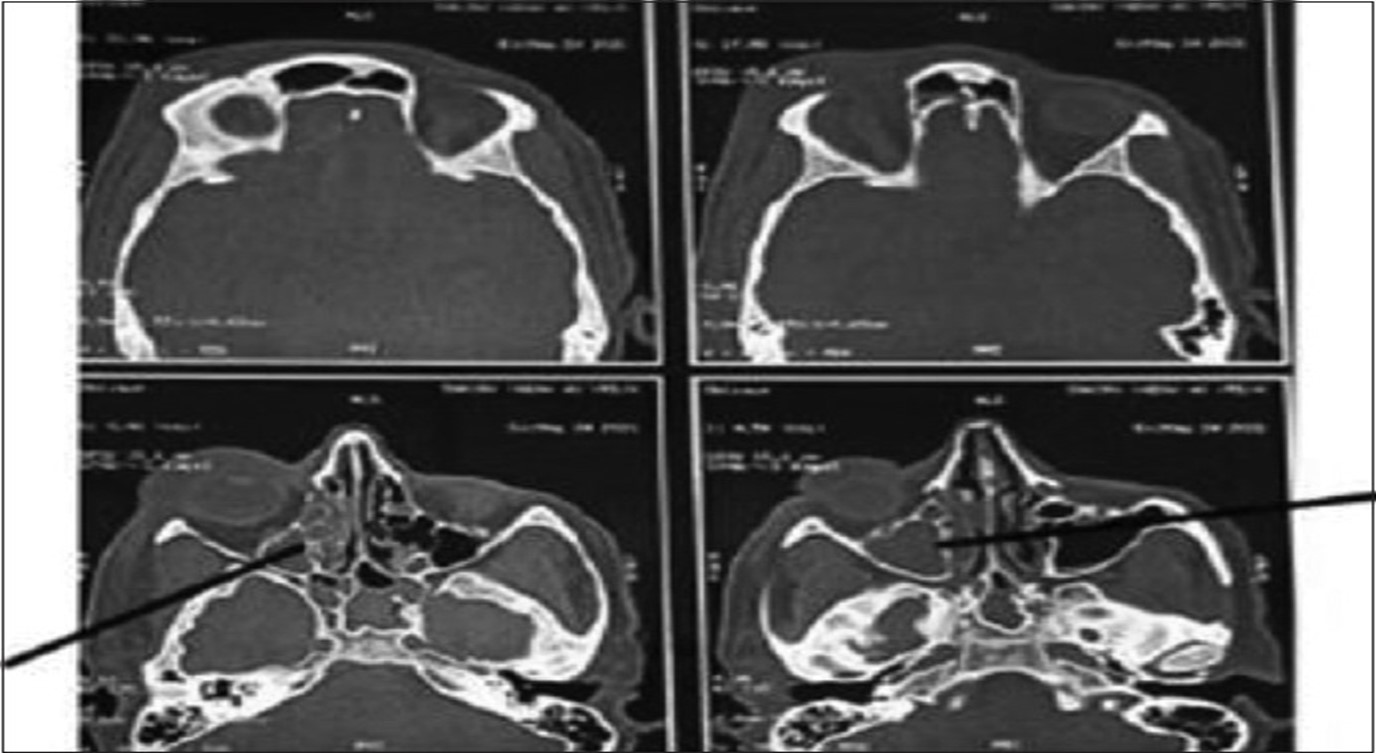
Fig no 11 - Diagnosis
CT axial images of skull showing ill defined soft tissue density noted filling right maxillary, ethmoid and sphenoid sinus with rarefaction of anterior and medial wall of maxillary sinus with mild right orbital bulge and periorbital swelling
Differential diagnosis[15]
In a case of mucormycosis in its rhinocerebral form, one must consider different pathologies such as orbital cellulitis and cavernous sinus thrombosis. When we consider the diagnosis of pulmonary mucormycosis, we should think about aspergillosis, nocardiosis, and Wegener's granulomatosis
Other[3]
Matrix-assisted laser desorption/ionization may be used to identify the species. A blood sample from an artery may be useful to assess for metabolic acidosis
Imaging[3]
Imaging is often performed, such as CT scan of lungs and sinuses. Signs on chest CT scans, such as nodules, cavities, halo signs, pleural effusion and wedge-shaped shadows, showing invasion of blood vessels, may suggest a fungal infection, but do not confirm mucormycosis. A reverse halo sign in a person with a blood cancer and low neutrophil count is highly suggestive of mucormycosis. CT scan images of mucormycosis can be useful to distinguish mucormycosis of the orbit and cellulitis of the orbit, but images may appear identical To those of aspergillosis. MRI may also be useful. Currently, MRI with gadolinium contrast is the investigation of choice in rhinoorbito-cerebral mucormycosis.
Culture and biopsy[3]
To confirm the diagnosis, biopsy samples can be cultured. Culture from biopsy samples does not always give a result as the organism is very fragile. Microscopy can usually determine the genus and sometimes the species, but may require an expert mycologist. The appearance of the fungus under the microscope can vary but generally shows wide (10–20 micron), ribbon-like filaments that generally do not have septa and that—unlike in aspergillosis—branch at right angles, resembling antlers of a moose, which may be seen to be invading blood vessels.
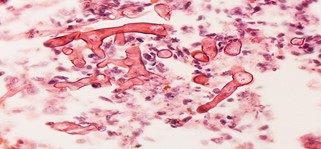
Fig no- 12 Ribbon-like hyphae which branch at 90°
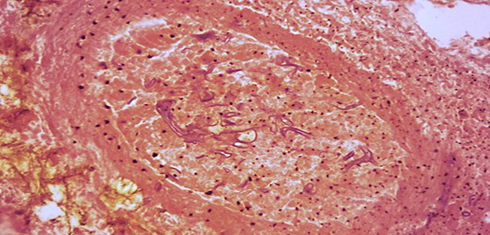
Fig no- 13 Hyphae in blood vessel
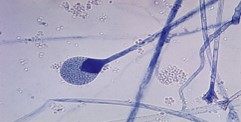
Fig no-14 Mature sporangium of a Mucor
TREATMENT[15]
Early diagnosis and treatment of mucormycosis are important to prevent death or to avoid extensive surgery, which often causes disfigurement. Thus, treatment is started as soon as this infection is diagnosed or suspected. Most people with mucormycosis or suspected mucormycosis are treated with high doses of the antifungal medication amphotericin B given intravenously. Isavuconazonium, taken by mouth or given intravenously, is an alternative. Controlling the underlying condition is very important. For example, people with a low white blood cell count are given injections to increase their white blood cell count. People with uncontrolled diabetes are given insulin to lower blood sugar levels. Infected tissue and especially dead tissue must be removed by surgery. If possible, removal of infected tissues is the best possible treatment for the mucormycosis. However this is easier in some cases such as rhino-cerebral or cutaneous infection but it is impossible to operate in many cases such as pulmonary disease or if the virus invades cerebra. A study reported that early surgical excision of infected sinuses in rhino-cerebral mucormycosis prevent the infection from invasion in eyes which results in higher cure rates of 85%. In a study, it was also reported that mortality was reduced to 14% from 70% if surgery was performed with antifungal agents In several studies it was found that use of Amphotericin B is preferred antifungal drug of choice for the treatment of mucormycosis infection. Liposomal amphotericin B with a low dose of 5 mg/g/day to higher dose of 10 mg/kg/day to cerebral infection patient is most preferred as of low toxicity and higher CNS penetration. however, the duration of treatment with Amphotericin B is still not properly reported and it was decided by the physician on the basis of underlying condition of the patient. Some reports proposed at least three weeks treatment with Amphotericin B and if radiological and clinical improvement was observed then further treatment is clubbed with triazoles such as posaconazole (POSA), isavuconazole, voriconazole (VORI) etc.
In an experimental murine model, Caspofungin alone showed minimal activity against mucorales when tested in-vitro however in combination with amphotericin B it shows synergistic effect. It has very less toxicity. In an in-vitro activity, low dose of Caspofungin found effective by inhibiting (1–3)-?-D-glucan synthetase enzyme expressed by Rhizopus oryzae. Other adjunctive therapies include iron chelator other than deferoxamine. Iron chelators did not allow the fungus to take iron and not support its growth whereas deferoxamine promotes growth of moulds. Use of hyperbaric oxygen also suppresses the growth of Mucormycosis could as higher pressure of oxygen improves the ability of neutrophils to the kill the moulds.
Treatment involves a combination of antifungal drugs, surgically removing infecting tissue and correcting underlying medical problems, such as diabetic ketoacidosis.
Medication[13]
Once mucormycosis is suspected, amphotericin B at an initial dose of 1 mg is initially given slowly over 10–15 minutes into a vein, then given as a once daily dose according to body weight for the next 14 days. It may need to be continued for longer. Isavuconazole and Posaconazole are alternatives.
- Amhotericin B
Structure –
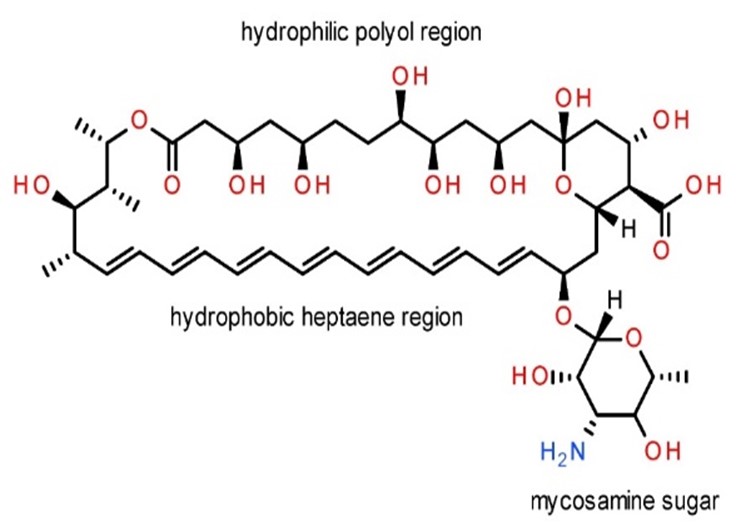

Dose –
1-1.5mg/kg/d for 3-6 weeks
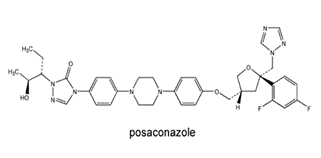


Fig no – 15 Amphotericin - B
-
Posaconazole
Structure -
Posaconazole tab –
300mg bid on a day 1and then 300mg/d for 3-6 months
Posaconazole inj –
300mg bid on a day and then 300mg/d for 3-6 weeks
Fig no – 16 Posaconazole
-
Isavuconazole
Structure -
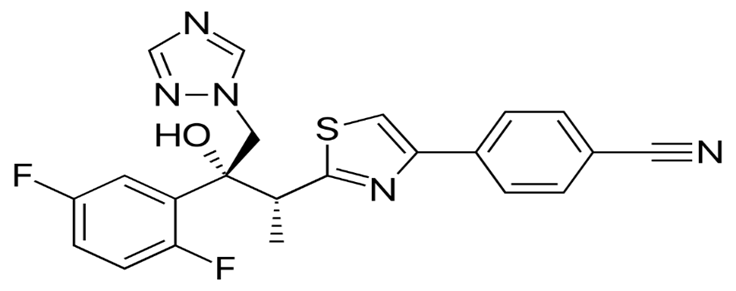
Isavuconazole tab –
200mg tid on day 1-2 and then 200mg/d for 3-6 month
Isavuconazole inj –
200mg tid on a day 1-2 and 200mg/d from day 3 for 3-6 weeks
Fig no -17 Isavuconazole
Surgery[3]
Surgery can be very drastic, and, in some cases of disease involving the nasal cavity and the brain, removal of infected brain tissue may be required. Removal of the palate, nasal cavity, or eye structures can be very disfiguring. Sometimes more than one operation is required.
Other considerations[3]
The disease must be monitored carefully for any signs of reemergence. Treatment also requires correcting sugar levels and improving neutrophil counts. Hyperbaric oxygen may be considered as an adjunctive therapy, because higher oxygen pressure increases the ability of neutrophils to kill the fungus. The efficacy of this therapy is uncertain.
OTHER CONSIDERATIONS[3]
The disease must be monitored carefully for any signs of reemergence. Treatment also requires correcting sugar levels and improving neutrophil counts. Hyperbaric oxygen may be considered as an adjunctive therapy, because higher oxygen pressure increases the ability of neutrophils to kill the fungus. The efficacy of this therapy is uncertain.
LITERATURE REVIEW
- Pandey & Kaur –
Invasive fungal infections caused by the members of Mucoromycotina (mucormycosis) are relatively rare but have increased in the last years. These aggressive and highly destructive infections fail to induce disease in most immunocompetent persons but can do so in those with impaired host defenses. Compared to other fungal pathogens, such as Aspergillus fumigatus or Candida albicans, only little is known so far on the fungal properties leading to successful infection and host immune response to the various representatives of the Mucorales. This article explains the new nomenclature, clinical manifestations, risk factors and focuses on virulence traits associated with mucormycosis. Early diagnosis and prompt treatment can reduce themortality and morbidity of this lethal fungal infection[16]
- Suganya et al –
Mucormycosis is an angio invasive infection that occurs due to the fungi mucorales. It is a rare disease but increasingly recognized in immunocompromised patients. It can be categorized into rhino-orbito- cerebral, cutaneous, disseminated, gastrointestinal, and pulmonary types. Overall increased mortality rate is reported, even though the aggressive treatment is given. The main aim and purpose of this review related to overview and Etiopathogenesis of Mucormycosis, fatality of rhinocerebral Mucormycosis, recent advances in diagnostic and treatment methods Mucormycosis is an angio invasive infection that occurs due to the fungi mucorales. It is a rare disease but increasingly recognized in immunocompromised patients. It can be categorized into rhino-orbitocerebral, cutaneous, disseminated, gastrointestinal, and pulmonary types. Overall increased mortality rate is reported, even though the aggressive treatment is given. The main aim and purpose of this review related to overview and Etiopathogenesis of Mucormycosis, fatality of rhinocerebral Mucormycosis, recent advances in diagnostic and treatment methods[17]
- Sunita & Kamlesh sharma –
Mucormycosis is a rare but serious life threatening, angioinvasive fungal infection with high mortality rate. In 2nd wave of pandemic it has been increasing as an opportunistic infection in covid 19 recovered patients. The most common organism is Rhizopus oryzae and is responsible for 70% of all cases of mucormycosis. India is dealing a double blow as rapid increase in Covid-19 cases and also with this nasty rare fungal infection. More than 70?ses of mucormycosis are from India. The fatality rate in mucormycosis if patient goes untreated is as high as 80%, If treated, it is still 40-50%. Mucormycosis was predominantly seen in males (79%), both in people who were active (59%) or recovered (41%) from COVID-19. Diabetes was present in 80% of cases, while concomitant diabetic ketoacidosis (DKA) was present in 15% and corticosteroid treatment was given for COVID-19 in 76%. DM has been the most common risk factor linked with mucormycosis and India is the diabetes capital of the world although hematological malignancies and organ transplant takes the lead in Europe and the USA. Treatments need to be fast and aggressive, because by the time even the presumptive diagnosis is made, often the patient has suffered significant tissue damage that cannot be reversed. Liposomal Amphotericin B (?5mg/kg) combined with surgery is the first line therapy for mucormycosis. Due to rapidly growing number of cases many Indian state Governments have declared it an epidemic. Himachal government declared mucormycosis as an epidemic for one year on 21st May 2021 after detection of first case on 20th May 2021[18]
- Alom et al-.
Mucormycosis is an infection caused by a group of filamentous molds belong to order Mucorales and class Zygomycetes. Mucormycosis is commonly known as black fungus disease. This infection mainly targets diabetic and immunecompromised patients. As COVID-19 infection declines the immunity of patients, so mucormycosis cases are also increasing due to inhalation of molds containing industrial oxygen[19] The comparative analysis highlights significant differences in the drug labeling requirements between the USA and India. While the USA follows a comprehensive approach with detailed content requirements and specific formatting guidelines, India's regulations are comparatively more flexible, focusing on essential information without prescribing specific formats.
CONCLUSION:
Immunosuppressants are used to treat severe inflammation or high viral loads in Covid-19 patients but can increase the risk of mucormycosis infection. This risk is higher in patients with uncontrolled diabetes, leukemia, and ketoacidosis. Healthcare providers should consider a patient's medical history, as those with solid organ or bone marrow transplants, liver cirrhosis, or neutropenia are at increased risk of mucormycosis. Patients should promptly report any signs or symptoms of Mucormycosis like fever, headache, and swollen skin near the nose and eyes. Early diagnosis, removal of infected tissue with antifungal treatment are key for eradicating the infection. More research is required to improve prevention and control of Mucormycosis in Covid-19 patients. Further developments are needed for the use of immunosuppressants in Covid-19 treatment.
ABBRIVATION
REFERENCES:
- Naveen kumar Chaudhary, Amit. K . Jain, Rupesh Soni & Neha Gahlat , Mucormycosis : A deadly black fungus infection among covid-19 patient in india , received 2021 jan 14 , revised 2021 Aug 4 Accepted 2021 Oct 28
- Medically received by Melinda Ratani , Do on May 26 2023 Written By Shishira Sreenivas
- https://en.wikipedia.org/wiki/Mucormycosis#
- Mucormycosis [black fungus]: Rare fungal infection Covid-19 Types & fungal spores on youtube video https://www.youtube.com/watch?v=jOjHz1P71dA
- George Petrikkos, Anna Skiada, Olivier Lortholary, Emmanuel Roilides, Thomas J. Walsh, and Dimitrios P. Kontoyiannis Epidemiology and Clinical Manifestations of Mucormycosis 1 june 2024
- Gregoire Pasquire : Covid-19 Associated Mucormycosis in India:why such an Outbreak on 2023may 9 by J.Mycol Med
- Farah Yasmin, Hala Najeeb ,Aisha Naeem ,Kartik Dapke , Rachana Phadke , Muhammad shoaib Asghar, Syed Muhammad Ismail Shah ,Doooomenico De Berardis &Irfan Ullah . Covid-19 Associated Mucormycosis : A Systemic Review from Dignostic challenges to management . 22September 2021
- https://www.mdpi.com/2079-9721/9/4/65
- Adam R Sweney md :A Mucormycosis on April14 2024 https://eyewiki.aao.org/Mucormycosis
- Karthika Pushparaj, Haripriya Kuchi Bhotla, Vijaya Anand Arumugam, Murugesh Easwaran , wen-Chao Liu, Utthapon Issara , Kannan R.R Rengasamy, Balamuralikrishna Balasubramaniam , Mucormycosis [black fungus] ensuring covid -19 & comorbidity meets- magnifying global pandemic grives & Catastrophe begins 20 january 2022
- World health organization WHO https://www.who.int/india/home/emergencies/coronavirus-disease-(covid-19)/mucormycosis
- Medical Author- Charles Patrick Davis, Md Phd , Medical Editor Mary. D . Nettleman Md,Ms ,Macp Medicine Net ; Mucormycosis (Zygomycosis)
- Aayushi Sharma & Anjana Goel , Mucormycosis : Risk Factors, Diagnosis , treatment and challenges during Covid-19 pandemic https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8881997/
- Ragaya Bharadwaj, S. Thilagavathy , Mucormycosis in Covid-19: A Clinico-MicrobiologicalDilemmahttps://www.kauveryhospital.com/kauverian-scientific-journal/mucormycosis-in-covid-19-a-clinico-microbiological-dilemma/ Munesh K Gupta , Nilesh Kumar , Neeraj Dhameja, Arti Sharma & Ragini Tilak ; Laboratory diagnosis of Mucormycosis : Present perspective , 2021 dec 21
- Jorge. L. Hernandez , Clifford. J, Buckley , Mucormycosis June 12 ,2023
- Aarushi Pandey & Gurkiran kaur , Mucormycosis revisited : Case report with review of literature, November 2020. https://www.researchgate.net/publication/348952487_Mucormycosis_revisited_Case_report_with_review_of_literature
- Ramalingam suganya , Narsimha Malathi , Vinithra Karthikeyan , and Vyshnavi Devi Janagaraj , Mucormycosis : A Brief Review . March 2019 https://www.researchgate.net/publication/332137866_Mucormycosis_A_Brief_Review
- Sunita & Dr Kamlesh Sharma ; Mucormycosis in Covid-19 Pandemic , New Challenge A review article, July 2021 https://www.researchgate.net/publication/358376313_Mucormycosis_in_COVID_19_Pandemic_New_Challenge_A_Review_Article
- Shahnaz Alom, Farak Ali , Md.KAmaraz Zaman, A Comprehensive Review on Mucormycosis [black fungus] & Its Associated with covid-19 https://dibru.ac.in/wp-content/uploads/2021/09/03-CTPR-Review-SA-01.pdf


 Pahilwan Pradnya* 1
Pahilwan Pradnya* 1
 Kunal Padwal 2
Kunal Padwal 2
























 10.5281/zenodo.12148120
10.5281/zenodo.12148120