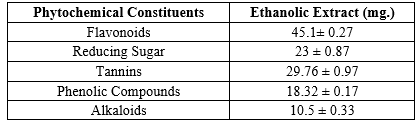
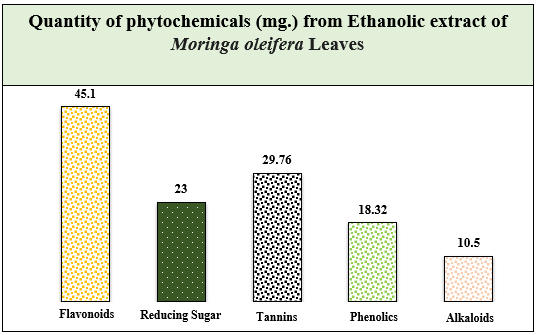
Moringa oleifera is a plant traditionally used for its medicinal properties. This study investigated the phytochemical profile and quantified the bioactive compounds present in Moringa oleifera leaves. Qualitative analysis of various solvent extracts revealed the presence of alkaloids, flavonoids, tannins, reducing sugars, and phenolic compounds. The highest number of positive tests were observed in the ethanol extract. Quantitative analysis of the ethanolic extract demonstrated the presence of flavonoids (45.1 mg/g), tannins (29.76 mg/g), phenolics (18.32 mg/g), alkaloids (10.5 mg/g), and reducing sugars (23 mg/g). These findings suggest that Moringa oleifera leaves are a rich source of various bioactive compounds with potential health benefits. Further research is needed to optimize extraction techniques, investigate synergistic effects, and elucidate mechanisms of action for the development of Moringa oleifera as a therapeutic agent.
Moringa oleifera, phytochemical analysis, flavonoids, tannins, phenolics, alkaloids, reducing sugars, medicinal properties
Moringa oleifera, often referred to as the "horseradish tree," is an angiosperm plant renowned for its significant nutritional and medicinal properties. This versatile tree is valued for its multiple uses and the important materials it provides [1]. Moringa oleifera, commonly known as the "drumstick tree" or "horseradish tree" in India, is widely distributed across tropical and subtropical regions globally. This species is part of the Moringaceae family, which includes other notable species such as Moringa peregrina and Moringa stenopetala. The plant is known by various names in different countries, such as "Shiferaw" in Ethiopia [2]. Moringa oleifera, also known as the "drumstick tree" or "horseradish tree" in India, is widely grown in tropical and subtropical regions around the world. This species is a member of the Moringaceae family, which includes other species such as Moringa peregrina and Moringa stenopetala. It is called "Shiferaw" in Ethiopia and has different names in various countries. [3, 4, 5]. Moringa oleifera is known for its antitumor, anti-inflammatory, antioxidant, and antimicrobial properties. Originally a small tree native to the sub-Himalayan regions of Northwest India, it has become indigenous to various parts of Africa, Arabia, Southeast Asia, the Pacific and Caribbean islands, and South America. [6, 7, 8, 9]. Traditionally, Moringa oleifera is not only a staple vegetable in the diets of people from various regions but is also well-regarded for its health benefits. Known among the populace as the "miracle tree," it is celebrated for its remarkable ability to aid in the treatment of various ailments and even some chronic diseases. [10, 11, 12].
Cultivation Condition
Moringa oleifera is a small to medium-sized tree that can be either deciduous or evergreen. It is commonly found in tropical regions, foothills, and mid-hill areas of Nepal and Chotnagpur plateau. The tree thrives in direct sunlight and can grow at elevations ranging from sea level up to 1000 meters. [13].
Harvesting
In high-density cultivation, Moringa trees can be harvested once they attain a height of 1.5 to 2 meters. The leaves should be collected by either cutting the leaf stems with a sharp knife or snapping them off from branches at a height of 20 to 45 centimeters above the ground. [14].
Medicinal Properties [15, 16, 17, 18, 19]
Moringa oleifera possesses numerous medicinal properties, including anti-inflammatory, anti-diabetic, cardiovascular protection, brain health support, and antimicrobial and antibacterial activities. It is effective in treating chronic and acute inflammation by suppressing inflammatory enzymes and proteins in the body. Moringa leaf powder significantly reduces lipid and glucose levels while regulating oxidative stress in diabetic patients, thereby lowering blood sugar and cholesterol levels, and protecting against cell damage. Additionally, Moringa leaf juice plays a crucial role in stabilizing blood pressure, supporting cardiovascular health. The plant also enhances cognitive function due to its antioxidant and neuro-enhancing properties and has antibacterial and antifungal properties that combat infections.

Fig.1: Aerial part of Moringa oleifera
EXPERIMENTAL SECTION
The research was carried out at the Medicinal Chemistry Laboratory located in the Faculty of Medical Science and Research at Sai Nath University, Ranchi. The experiments were conducted daily from 4:00 P.M. to 5:00 P.M. over a period of one and a half months.
PLANTS MATERIALS AND REAGENT
The fresh aerial parts of Moringa oleifera were collected from the grounds of Sai Nath University in Ranchi, Jharkhand, India (coordinates: 23° 29? 18.47? N, 85° 24? 28.73? E). Botanical identification and authentication were conducted at Shibpur Botanical Garden in West Bengal, India. To ensure purity, the leaves were washed gently with tap water and air-dried in shaded areas to remove any dirt particles. For the extraction process, various chemical solvents, including ethanol and benzene distilled water, were utilized. Sai Nath University provided all necessary chemicals, such as Sodium Hydroxide, concentrated Hydrochloric acid, Magnesium powder, Ferric chloride solution, Distilled water, Sodium bicarbonate, Potassium Iodide, Mercuric chloride, and Benedict reagent, all meeting analytical grade standards. Working standards and samples were prepared by diluting the stock solution (1 mg/ml) in ethanol and double-distilled water to achieve the required concentrations for the experiments. All solvents used in the study adhered to analytical grade standards.
Extraction Methodology [20]
Freshly harvested aerial parts of Moringa oleifera were first washed with distilled water to remove any contaminants. They were then air-dried in a shaded area until fully desiccated. The dried leaves were finely ground into powder using a blender. Following this, 60 grams of the powdered material were extracted through maceration using solvents such as ethanol and distilled water over a period of 5 days. After the extraction process, the resulting extracts were concentrated and stored in hermetically sealed containers for future use.
Phytochemical Assessment
The qualitative analysis of phytoconstituents in Moringa oleifera leaves was conducted using established protocols to identify various chemical components. The study revealed the presence of flavonoids, tannins, saponins, cardiac glycosides, phenolic compounds, terpenoids, reducing sugars, and alkaloids in the plant material.
Quantification of Total Flavonoids [21]
The quantification of total flavonoid content in plant extracts can be achieved using a well-established aluminum chloride complex formation assay. This assay exploits the complexation between flavonoids and aluminum trichloride (AlCl3), resulting in the formation of a colored product with peak absorbance at 415 nanometers (nm). The protocol entails preparing plant extracts (10 mg/ml in methanol) and a separate 20% AlCl3 solution in methanol. To create the reaction mixture, a 100 µl aliquot of the extract is combined with 100 µl of the AlCl3 solution and a drop of acetic acid, followed by dilution to a final volume of 5 ml with methanol. A blank solution, prepared identically but omitting the AlCl3 solution, is used for background subtraction. Furthermore, a standard solution of rutin (a well-characterized flavonoid) at a concentration of 0.5 mg/ml in methanol is incorporated for calibration purposes. After a 40-minute incubation period, the absorbance of the reaction mixture, blank, and standard solution is measured at 415 nm. Each measurement is performed in triplicate to enhance accuracy. By comparing the sample absorbance to the blank and referencing a standard rutin calibration curve, the total flavonoid content in the plant extract can be estimated. It is crucial to recognize that this method provides an approximation of total flavonoid content, as the complexation efficiency can vary between different flavonoid subclasses.
Quantification of Total Tannins [22]
The tannin quantification protocol begins with precise sample preparation, where 500 mg of the sample is accurately weighed and transferred to a 50 mL polypropylene container. For extraction, 50 mL of deionized water is added to the sample, and the mixture undergoes mechanical agitation for 60 minutes to ensure thorough extraction. The resulting suspension is then filtered into a 50 mL volumetric flask and brought to the calibration mark with deionized water. In the reaction setup phase, a 5 mL aliquot of the filtrate is transferred to a test tube using a precision pipette. A reagent mixture containing 0.1 M FeCl3 solution, 0.1 N HCl, and 0.008 M potassium ferrocyanide is prepared, and 2 mL of this mixture is added to the sample aliquot. The final step involves spectrophotometric analysis, where the absorbance of the reacted solution is measured at 120 nm and monitored over a 10-minute period to assess reaction kinetics and determine the sample's tannin content. This method effectively quantifies tannins through aqueous extraction followed by colorimetric analysis, utilizing iron (III) chloride and potassium ferrocyanide as chromogenic agents.
Quantification of Total Reducing Sugar [23]
To determine the total reducing sugar content, materials required include DNS reagent, glucose standard solution, the sample containing reducing sugars, distilled water, sodium potassium tartrate solution (Rochelle salt), a spectrophotometer, test tubes, pipettes, micropipettes, and a water bath. For reagent preparation, dissolve 1 g of 3,5-dinitrosalicylic acid, 200 mg crystalline phenol, and 50 mg sodium sulfite in 100 mL of 1% NaOH to make the DNS reagent. Prepare Rochelle salt by dissolving 40 g of sodium potassium tartrate in 100 mL of distilled water. To create a glucose standard curve, prepare glucose solutions at concentrations of 0.2, 0.4, 0.6, 0.8, and 1.0 mg/mL. Pipette 1 mL of each solution into separate test tubes, add 1 mL of DNS reagent, mix well, and heat in a boiling water bath for 5 minutes. After cooling to room temperature, add 1 mL of Rochelle salt and measure the absorbance at 540 nm using a spectrophotometer. Plot absorbance against glucose concentration to generate the standard curve. For sample analysis, pipette 1 mL of the sample solution into a test tube, add 1 mL of DNS reagent, mix well, and heat in a boiling water bath for 5 minutes. Cool to room temperature, add 1 mL of Rochelle salt, and measure the absorbance at 540 nm. Calculate the concentration of reducing sugars in the sample using the standard curve. If necessary, dilute the sample to ensure the absorbance falls within the range of the standard curve.
Quantification of Total Phenolic compounds [24]
A 100-milligram sample extract was dissolved in triple distilled water, and a portion of this solution was subjected to reaction with ferric chloride reagent, alkaline reagent, benedict’s reagent, 0.5N chloroform, 0.4N sulfuric acid, Mayer’s reagent and 20% Na2CO3 solution. After standing for 2 hours, the absorbance at 765 nanometre was measured, and the total phenolic content was calculated using a calibration curve established with various concentrations of gallic acid.
Quantification of total alkaloid [25]
A 5-gram sample was treated with a 10?etic acid solution in ethanol and allowed to stand for 4 hours. The resulting extract was then concentrated and precipitated using concentrated ammonium hydroxide. The precipitate was collected, washed, filtered, and dried, and the weight of the alkaloid residue was measured for subsequent analysis.
RESULT
The qualitative phytochemical analysis of Moringa oleifera extracts involved the use of distinct solvents: aqueous, ethanol. Through a series of tests outlined in Table 1, researchers investigated the phytochemical properties of these extracts. The findings revealed that each solvent produced positive results in at least one of the six phytochemical tests. Notably, the ethanol, petroleum ether, aqueous, and chloroform extracts exhibited positive outcomes across all six tests, indicating a broad spectrum of chemical compounds present. Conversely, the benzene and aqueous extract showed positive results in five tests, suggesting a slightly lower diversity of phytochemicals compared to ethanol. Overall, the ethanol extract demonstrated the highest number of positive outcomes, followed by benzene, chloroform, aqueous, and petroleum ether extracts. The primary focus of the investigation was to screen for various phytochemical compounds within the five solvent extracts. These compounds encompassed alkaloids, phenolic compounds, flavonoids, saponins, tannins, and cardiac glycosides, all of which are significant secondary metabolites renowned for their medicinal attributes in plants. Additionally, researchers conducted supplementary analytical tests to quantitatively assess the presence of these phytochemical compounds in the extracts.
TABLE.1 Qualitative Phytochemical Analysis of Moringa oleifera Leaf Extract
The identified bioactive compounds within ethanolic extract of Moringa oleifera leaves possess significant medicinal promise attributed to their diverse phytochemical composition, including alkaloids, flavonoids, tannins, reducing sugar and phenolic compounds [26]. Alkaloids like morphine are renowned for their analgesic and anti-inflammatory properties, while quinine and its derivatives exhibit notable antimalarial and antibacterial efficacy [27]. Vincristine and vinblastine, other alkaloids present, find utility in cancer chemotherapy [28, 29]. Flavonoids offer antioxidant protection, anti-inflammatory effects, and cardioprotective benefits [30, 31]. Tannins serve as astringents with antimicrobial and antioxidant activities [32, 33], Phenolic compounds contribute to antioxidant defence, anti-inflammatory effects, and broad-spectrum antimicrobial activities. The collective presence of these phytochemicals underscores the medicinal potential of Moringa oleifera providing a broad spectrum of therapeutic benefits [34]. Reducing sugars like glucose and fructose can act as antioxidants by donating electrons and neutralizing free radicals. They are involved in the Maillard reaction, a series of reactions that occurs when foods are cooked at high temperatures and is important for determining food flavour. Reducing sugars serve as precursors for the synthesis of various organic compounds in the chemical industry, including alcohols, organic acids, and other chemicals. In plants, reducing and nonreducing sugars play an important role in central metabolic pathways and help produce secondary metabolites that enhance medicinal properties. [35, 36, 37]Future research avenues should prioritize optimizing extraction techniques to enhance the yield of specific bioactive compounds tailored to their intended therapeutic applications. Due to solvent-dependent variability in content, identifying the most efficacious solvent is paramount [38], particularly for targeting compounds such as cardiac glycosides, alkaloids, or flavonoids. Additionally, investigations into the synergistic effects of these compounds within complex biological systems are warranted to elucidate their full pharmacological potential. Further exploration of mechanisms of action, bioavailability, and toxicity will provide a more comprehensive understanding of their medicinal value [39]. Advanced methodologies including metabolomics and molecular docking studies hold promise for identifying novel compounds and predicting their biological activities. Ultimately, standardizing extraction protocols and conducting detailed pharmacokinetic studies are imperative for potential commercialization of Moringa oleifera extracts as therapeutic agents. [40]
The current study successfully employed various qualitative and quantitative methods to establish the presence and quantify the content of several key bioactive compounds within Moringa oleifera leaves. The findings demonstrate that the ethanolic extract holds the richest profile of these bioactive compounds, including flavonoids, tannins, phenolics, alkaloids, and reducing sugars. These results support the traditional medicinal applications of Moringa oleifera and provide a foundation for further exploration of its therapeutic potential. Future research efforts should focus on optimizing extraction techniques to target specific bioactive compounds for tailored therapeutic applications. Additionally, investigating the synergistic effects of these compounds and elucidating their mechanisms of action are crucial steps towards developing Moringa oleifera into a viable therapeutic agent.
We would like to express our sincere gratitude to the esteemed Vice Chancellor of Sai Nath University, Prof. Dr. SP Agarwal, and the respected Dean of Academics, Prof. Dr. K. Rajeswar Dutt, for their invaluable support throughout this project. Their guidance, encouragement, and provision of resources were instrumental in allowing us to delve deeper into our chosen field of research. Their unwavering belief in the importance of research has inspired us to strive for excellence and explore new avenues of inquiry. We are truly grateful for their leadership and dedication to fostering a research-intensive environment at Sai Nath University. Their support has not only enriched our academic experience but has also empowered us to contribute meaningfully to the advancement of knowledge.


 Arnab Roy*
Arnab Roy*
 Ankita Singh
Ankita Singh
 Nahida Khatun
Nahida Khatun
 Divya Roshni Panna
Divya Roshni Panna
 Priyanka Kumari
Priyanka Kumari
 Suman Kumari
Suman Kumari
 Bina Kumari
Bina Kumari
 Shweta Kumari
Shweta Kumari


 10.5281/zenodo.12669695
10.5281/zenodo.12669695