Abstract
Polymers are everywhere in our daily lives, from workplaces to homes. Biopolymers play a crucial role in biomedical applications. These organic materials found in natural sources are known as biopolymers. They are gaining increased attention due to environmental concerns and the awareness that global petroleum resources are limited. Biopolymer nanocomposites have been a focus of much research due to their unique properties, including improved structural and functional features, non-toxicity, biocompatibility, and biodegradability. Many naturally occurring polymers such as chitin, starch, collagen, alginate, and cellulose are attractive options as they can reduce dependency on manufactured items while being environmentally friendly. Biopolymers have been used for years as excipients in conventional immediate-release forms for oral drugs, aiding in the manufacturing process and protecting the drug from degradation during storage. This review focuses on the main properties of biopolymers as well as the recent trends in biopolymer-based Drug Delivery Systems, forecasting their broad future clinical applications.
Keywords
Biodegradability, Immediate-release, Natural sources, Biocompatibility, biopolymer-based DDS
Introduction
Biopolymers are biodegradable polymers produced by living organisms. (1) Due to the increasing interest in promoting environmental sustainability, biopolymers can potentially replace synthetic plastics. (2) Their structural backbone of nitrogen, oxygen, and carbon atoms makes biopolymers readily biodegradable. These materials are naturally recycled through biological processes, forming humic matter, water, carbon dioxide, biomass, and other natural substances during biodegradation. (3)
Biopolymers are commonly found in the life cycles of fungi, bacteria, plants, and animals, and include polysaccharides produced by bacteria and fungi, such as cellulose and starch. Additionally, they encompass non-polymeric molecules like lipids and macrocycles, as well as macromolecules such as nucleic acids, proteins, carbohydrates, and lipids. Microbial biopolymers have the potential to replace natural water-soluble polymers or synthetic materials in various applications related to food, medicine, and other sectors. These biopolymers are created by different microorganisms and consist of exopolysaccharides, endopolysaccharides, and polyhydroxyalkanoates. Most microbial polysaccharides consist of carbohydrate moieties such as glucose, mannose, and rhamnose, along with non-carbohydrate moieties and uronic acid.
Biopolymers-based nanoparticles show promise for delivering therapeutic medications to tumour cells, such as ovarian cancer cell lines, due to their good biodegradation and biodistribution in biological systems. (4,5) Biopolymers have a wide range of applications, including tissue engineering, medication delivery, gene delivery, and other biomedical uses, making them an important interface between chemistry and biology. (6–8) While natural polymers are biocompatible and biodegradable, their poor solubilities and low mechanical and thermal qualities limit their applicability.
Classification Of Biomaterials
Chitosan, Nanocellulose, Polylactic acid, and Lipids are some of the most popular biomaterials in biomedicine. They are widely used due to their availability and inherent qualities including biocompatibility and antibacterial activity.
Chitosan: Chitosan is considered a "magical" polysaccharide with various therapeutic applications. Chitosan characterized as a non-toxic biopolymer, ranks as the second most abundant compound composed of glucosamine and N-acetyl glucosamine units, holding significance in biomedicine. The exoskeletons of fungus and crustaceans contain it in nature. (9)
Its qualities, including biocompatibility, biodegradability, muco-adhesiveness, and renewable sourcing, are its primary advantages. Chitosan is often combined with either natural or synthetic polymers, which enhances its physicochemical and mechanical properties. Despite its numerous advantages, the main disadvantage of chitosan is its poor solubility, as it is minimally soluble at the physiological pH of 7.4. (10,11)
Nanocellulose: Cellulose is a type of linear homopolymer that consists of D-glucose units linked together by ?-1,4 glycosidic bonds. It is made up of microfibrils with nanoscale diameter and is surrounded by lignin and hemicellulose. Cellulose is the most abundant biopolymer found on Earth. There are three forms of nano-structured cellulose referred to as "nanocellulose." These forms include cellulose nanofibers, nanocrystals, and bacterial nanocellulose (BNC) which originates from Gluconacetobacterxylinus.(12,13) Nanocellulose possesses several characteristics that make it suitable for medical usage, including abundance, elasticity, low thermal expansion, solubility, substantial surface area, aspect ratio, low cost, and low density. Nanocellulose can undergo several chemical modifications such as oxidation, amination, epoxidation, esterification, silylation, carboxymethylation, sulfonation, and can also have thiol- and azido-functional properties. Additionally, they can be charged or hydrophobic.(14,15)
Polylactic Acid: Polylactic Acid is produced through the fermentation of carbohydrates. In contrast to traditional petroleum-based polymers, Polylactic Acid is the most popular polymer for use in biomedical applications. The raw material for Polylactic Acid is produced through the fermentation of sugars. (16,17) In the manufacturing and medical industries, Poly lactic acid has several advantages. When it comes into contact with water, it breaks down into L-lactic acid monomers, which are used to modify its mechanical properties. (18)
Lipids: Lipids and fats are essential for the body's homeostatic processes. It is vital to the human body's most important processes, namely energy storage and cell integrity maintenance. Organic solvents can dissolve lipids since they are nonpolar and greasy by nature. Phospholipids, fats and oils, waxes, and steroids are examples of lipids. (19,20)
Lipid-based drug delivery systems are one of the main kinds of drug delivery systems that are comprised of these biomolecules because of their enormous potential. Due to their ability to deliver water-insoluble medications, boost drug bioavailability, exhibit low antagonistic potential, high bioactivity, natural biocompatibility, and facilitate drug transport and release by fusion with the celiac membrane, lipid-based drug delivery systems have drawn a lot of interest.(21)
Collagen: The human body contains a significant amount of collagen, with 29 different types known. Type I collagen is mainly found in bones, tendons, and skin. Collagen types I, II, III, V, and XI naturally produce fibrils that support the extracellular matrix in animals mechanically and structurally. Collagen-based materials have become increasingly popular in tissue engineering due to their advantages, including improved cell-material interaction and essential cell adhesion and proliferation. (22)
Polyphenols: Plant-based meals contain different types of polyphenols, each with unique amounts, physicochemical properties, and nutritional benefits. These polyphenolic substances are easily oxidized because they have numerous hydroxyl groups. However, since they are naturally strong antioxidants, many of them can be added to food to provide additional health benefits. The different types of polyphenols found in plant-based meals include flavonoids, phenolic acids, stilbenoids, curcuminoids, and tannins.(23)
polymers From Renewable Resources
Poly Lactic Acid: The first step involves the creation of lactic acid, which can be done chemically or biologically. Depending on the method employed, lactic acid polycondensation produces PLA with a low or high molar mass. It took 45 to 60 days for polylactic acid to break down into mono and oligomers in the compost's high humidity and 50 to 60 °C temperatures.
Thermoplastic Starch: Blends of polymers and starches are used to make hard injection-molded packaging, foamed loose-fill to fill in voids in transport packaging, flexible and rigid films, trays, and containers, as well as cardboard.
Polyesters of Microbiological Origin: 3-hydroxybutyrate, poly(3-hydroxybutyrate-co-3-hydroxy valerate), and poly(3-hydroxybutyrate-co-3-hydroxy hexanoate. Both agriculture and medicine use these kinds of biopolyesters. Polyester decomposes in soil and ocean in the form of fibers, films, bottles, and containers. They are also utilized in tissue engineering and medicine to create coatings for bone implants and medications with controlled release.
Renewable Polyolefins: Biopolyethylene is the primary representative of this class of bioplastics. Petrochemical raw materials are substituted with raw materials derived from renewable resources in the manufacturing of this biopolymer.
Polymers Derived From Fossil Raw Materials
Synthetic Aliphatic Polyesters: Biodegradable surgical sutures are made of a polymer called polycaprolactone. Copolymers are utilized to accelerate the deterioration process because the material takes several months to degrade. It has additionally been utilized in the creation of stiffening dressings because of its low softening point.
Synthetic Aliphatic–Aromatic Copolymers:To enhance these characteristics, biodegradable aliphatic polyester chains are extended with additional monomers, either aromatic or aliphatic. Due to one of the monomers' initial restricted availability, Poly trimethylene terephthalate flexibility and mechanical qualities make it an excellent polymer for use in the manufacturing of fibers.
Water-Soluble Polymers: A polymer known as poly(vinyl alcohol) (PVA) is soluble in water but insoluble in the majority of organic solvents. At times, the definition of "biodegradation" is not entirely clear.
Role Of Biopolymers And Importance In Nature
Biopolymers are essential for the basic cellular and metabolic functions of living organisms. They can be found in plants and algae, which are abundant in organic ecosystems. Gelidium seaweeds are a great source of nutritional agar and alginin, while brown algae can also be used to extract these biopolymers. In addition, companies can obtain biopolymers like starch and dextran through fermentation processes. (24)
Specific Features Of Biopolymer:
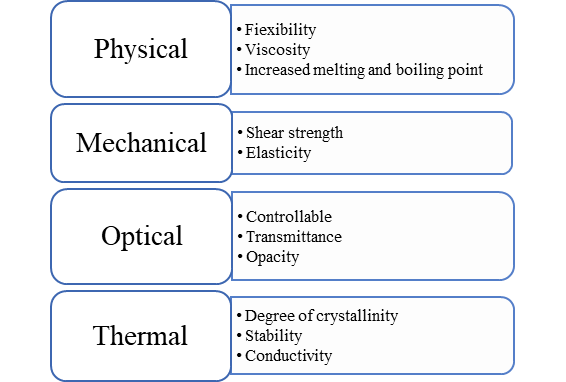
Biopolymers Vs Conventional Synthetic Materials
Living organisms produce a diverse range of polymers, which are an essential component of their cells, morphology, and dry matter. These polymers are also non-carcinogenic, non-immunogenic, non-thrombogenic, carbon-neutral, and less toxic, making them easily extractable. However, recent research has suggested that these materials may have adverse health impacts, particularly on pregnant women and newborns.(25–27)
Fortunately, biopolymers are an eco-friendly alternative to traditional plastics. They are biodegradable, so they can be disposed of in the environment and broken down by microorganisms' enzymes. Additionally, biopolymers are extremely hydrophilic, sustainable, biocompatible, and low-toxicity, giving them an edge over traditional plastics (Table 1).(28)
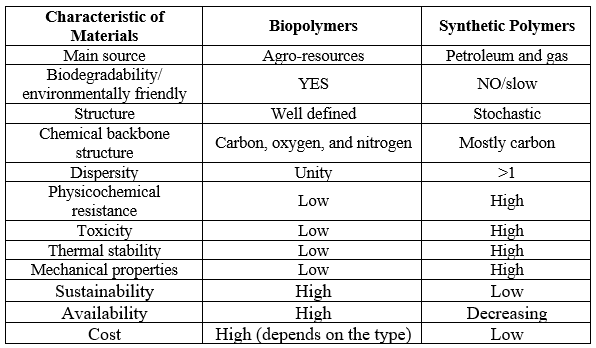
Table 1. Characteristics of biopolymers vs. synthetic polymers
Applications Of Biopolymers
The properties that are most suitable for proposing biomaterials include surface energy, hydrophilicity/hydrophobicity erosion process, shape and structure, molecular weight, lubricity, material chemistry, water absorption degradation, and erosion. The packaging of pharmaceutical medicines plays a crucial role in ensuring their convenience, safety, and identification. Additionally, the packaging also affects isolation. Biopolymers are widely used to deliver probiotics and other bioactive substances that are prone to breakdown during preparation, storage, or in unfavorable environmental conditions of the human gut. The biopolymer components inhibit aggregation and are characterized by a large electrical charge (Fig-1). (29–31)
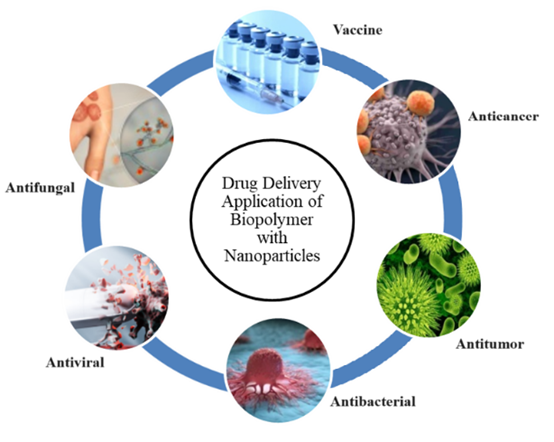
Figure 1. The use of biopolymers with modified nanoparticles for drug delivery
Biopolymers Use In Controlled Drug Release: Encapsulation plays a crucial role in shielding living cells from harm by ensconcing them in biopolymer membranes. This technique is utilized for the formation of microcapsules and macrocapsules. The process entails enclosing or covering one or more materials, referred to as the active core material, with another material or system, which acts as the shell, casing, carrier, or encapsulant. (32,33) In macrocapsules, living cells are trapped in large chambers that allow for diffusion. These chambers can be made from flat sheets, hollow fibers, and disks with semi-permeable properties. Macro capsules can be used as intra or extra-vascular devices. However, the main disadvantage of this technique is the potential for developing thrombosis. Microcapsules are used in encapsulation to rapidly transfer beneficial substances such as glucose or insulin. Most studies focus on developing microcapsules with low inflammatory responses, successfully used in treating endocrine diseases.(34) Numerous biocompatible polymers have been utilized as encapsulation materials. The controlled-release methods may vary depending on the mechanism that governs the release of the active agent from the delivery system. The primary mechanisms that control the release of the active agent are biopolymer erosion, diffusion, and swelling, followed by diffusion, or degradation. The diffusion process takes place as an enclosed medication or another active substance moves across the outer membrane of the capsule via the biopolymer used in the controlled-release device. In diffusion-controlled systems, the drug delivery mechanism needs to remain stable in the body and retain its size and form through swelling or breakdown.(35,36)
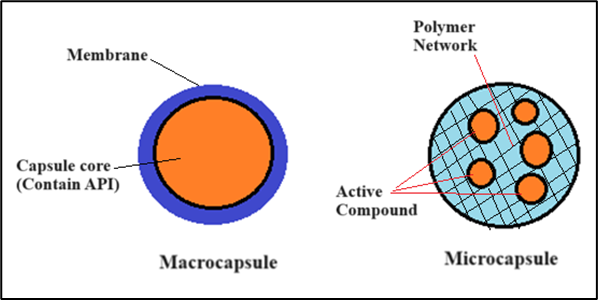
Figure 2. Graphical representation of drug encapsulation
Alginate Use For Drug Delivery: The substance alginate is derived from brown algae and is present in the form of alginic acid sodium, calcium, and magnesium salts. Macrocystis pyrifera, Laminaria hyperborea, Saccharina japonica, and Ascophyllum nodosum are the algae species used for extracting alginate. While alginate can be produced by various bacteria species such as Azotobacter vinelandii and various Pseudomonas species, it is not commercially available. (37). Alginate is used as an encapsulating agent in diabetes treatment, bioartificial organs, and for the protection of hepatocytes or parathyroids. However, alginate gels cannot provide immunoprotection as they are too porous, so they must be coated with cationic polymers of synthetic origin in most applications (Table 2).(38)
Chitosan Use For Drug Delivery: It exhibits a significant affinity for polyanions, is soluble in acidic aqueous solutions, and contains reactive NH3+ and OH? groups. Chitosan-based compounds are a good fit for controlled-release technologies because of their pH-sensitive characteristics. Acidic drugs have more permeability when chitosan is utilized in membrane formation. Chitosan is crucial in creating biocomposites for biomedical uses like tissue engineering and drug delivery, allowing for various modifications. Chitosan is widely used in pharmaceutical and dental professions due to its anti-tumor, antibacterial, and fungal properties (Table 3). It also plays a key role in dental material synthesis. (39,40)

Table 2 Alginate use for drug delivery
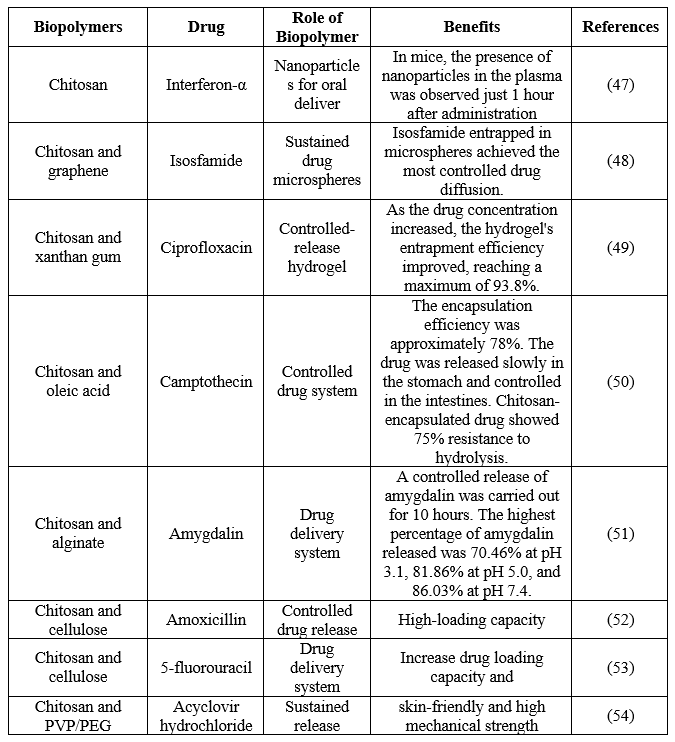
Table 3. Chitosan use for drug delivery
Agar Use For Drug Delivery: Agar is a biopolymer obtained from specific types of algae, mainly Gelidium sp. and Gracilaria sp., which make up the supporting structure of algae. Agar consists of two fractions: agarose and agaropectin, which are responsible for gelling and non-gelling properties, respectively. During processing, agaropectin is usually removed to produce agar powder with higher gel strength. Chemical modification, such as hydroxypropylation, acetylation, etherification, and oxidation, is commonly used to improve agar quality. Among these methods, oxidation is the most commonly used. Agar is widely used in the food industry due to its gelling capacity, gel reversibility, and high hysteresis. It is odorless and can form a gel. Agar can be used as an encapsulating agent; however, when only agar is used, drug release occurs in two phases. The first phase results in the release of 10-20% of the drug, based on the agar content of the beads, followed by a slower and more prolonged second phase. Agar is a natural, inert, non-toxic, renewable, biocompatible, and inexpensive material, making it an excellent candidate for the development of sustained-release dosage systems.(55,56)
Starch Use For Drug Delivery: The constituents of starch granules consist of water, proteins, phosphate esters, free fatty acids, and lysophopholipids. The capacity of native starch to be digested by pancreatic enzymes following oral consumption and then absorbed from the small intestine into the systemic circulation has been the subject of research. For instance, starch has been modified to create resistant starch, which has led to its application in enhancing the gut microbiota population and influencing signalling pathways linked to the prevention of inflammation, the management of diabetes, and the prevention of overweight. (57)
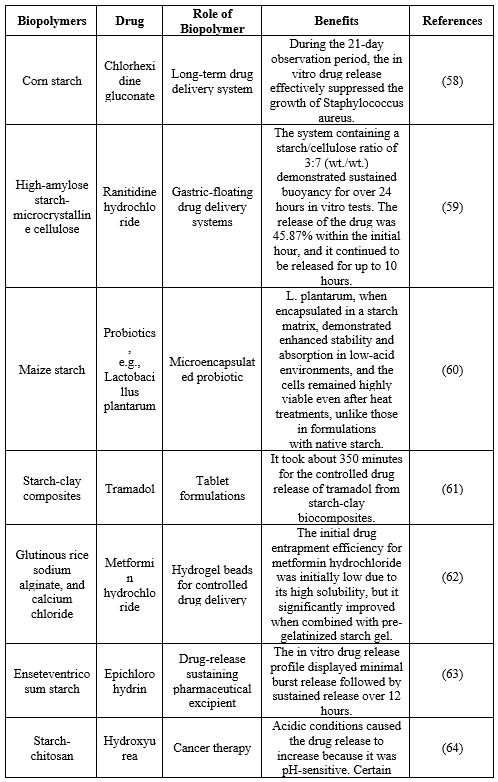
Table 4. Applications of starch for drug delivery.
Cellulose Use For Drug Delivery: The structural component of green plants, algae, and oomycetes' cell walls is called cellulose. Nanocellulose is a type of biocompatible nanomaterials that are generally safe to use in various biomedical applications. Building nanocellulose-based biocomposites has gained a lot of popularity in the last several years. Furthermore, during fabrication, Nanocellulose plays a part in the adhesion and brittleness properties and assists in incorporating desired qualities such as antibacterial and antioxidant activities, barrier properties, super-hydrophobicity, or super-hydrophilicity. Some nanoparticles are also employed with the alginate and gelatin matrix in addition to these two biopolymers. But now since it has a naturally occurring matrix-like structure and is biocompatible, Nanocellulose is thought to be the best biomaterial for tissue engineering.(66)
Hydroxypropyl methyl cellulose ethyl cellulose, bacterial cellulose, cellulose esters, and cellulose nanocrystals are some of the cellulose systems that have been reported for transdermal medication administration.
Polylactic Acid And Its Use For Drug Delivery: Polylactic acid is increasingly employed in diverse biomedical applications such as medication delivery, prostheses, vascular grafts, bone screws, anchors, spinal cages, tissue engineering scaffolds, and implants for both short- and long-term use. Chen et al. utilized calcium phosphate, magnesium powder, and Poly lactic acid to form a biocomposite. This composite, characterized by lower cytotoxicity, improved mechanical properties, and biocompatible byproducts upon degradation, augmented bone tissue healing.(67)
Probiotic Encapsulation And Delivery Using Biopolymers: Probiotics are live organisms that, when consumed in sufficient quantities, can improve the health of their hosts. Strains such as Lactobacillus plantarum, Lactobacillus casei, and Lactobacillus paracasei exhibited antifungal, antibacterial, and antioxidant properties. Probiotics encapsulated in food matrices retained viability when stored in the refrigerator for two months. (68)
Probiotic Delivery Using Chitosan: Chitosan possesses an uncommon characteristic as a positively charged polysaccharide with antibacterial properties. Due to its role as an encapsulating agent that supports cell survival, it cannot be used independently.
Probiotic Delivery Using Agar: The cause of this is the increased capacity to produce films and the decreased capacity to aid in the creation of coatings. To use the film-forming solution as an encapsulating material, it must be cooled to 40 °C to integrate essential oils. (69,70)
Probiotic Delivery Using Starch: Starch is mostly utilized in the pharmaceutical industry to encapsulate medications or active ingredients when they are formed into tablets or oral formulations. For instance, the addition of alginate to starch produced microcapsules that were more resistant to probiotics in gastric simulation circumstances.
Probiotic Delivery Using Cellulose: Its degradation in the digestive system restricts its use as an encapsulating material for probiotics. When other materials like gelatin or carrageenan are added, the microencapsulation properties of carboxymethyl cellulose are enhanced.
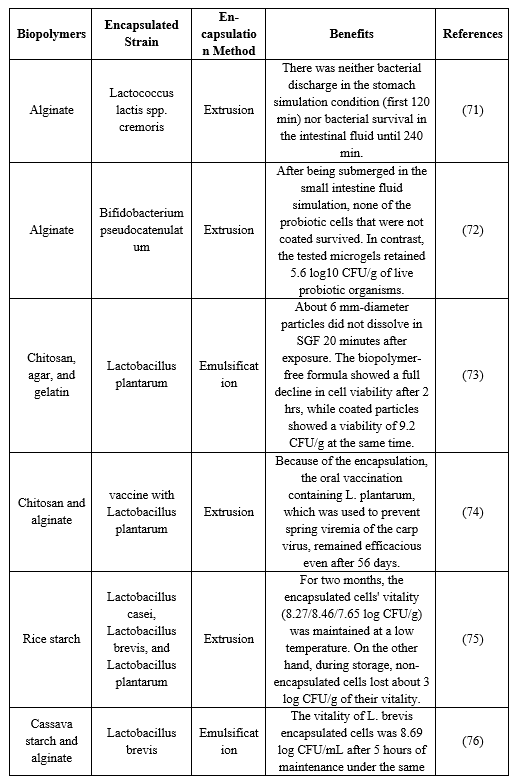
Table 5. Recent application of Biopolymer
Microorganism sources of natural biopolymers:
Dextran: Dextransucrase is an enzyme found in lactic acid bacteria that plays a crucial role in the creation of dextran in nature. This enzyme transfers D-glucopyranosyl residues from sucrose to dextran, which is the primary extracellular pathway for dextran production. Various reactions, such as etherification, oxidation, and esterification, have been used to modify a wide range of glycoconjugates. However, clinical trials have shown that dextran can have some unexpected side effects, such as hepatotoxicity and thrombocytopenia.(77,78)
Plant Sources Of Natural Biopolymers
Pectin In Drug Delivery Applications: Pectin's hydrophilicity, which results in its good biocompatibility, low toxicity, and biodegradability, is mostly due to the widespread occurrence of hydroxyl and carboxyl groups in the substance. Furthermore, empirical evidence indicates that the incidence of colon cancer is significantly elevated in individuals with intestinal media pH of 7.0, while the average pH of the colon in healthy individuals is 6.5.(79)
Smart Biopolymers for Special Applications:
The distinction between natural and synthetic polymers comes from the fact that while these molecules can be produced chemically from fats, proteins, amino acids, or vegetable oils, they also exist naturally in our bodies. Biodegradable polymers have numerous applications in different areas of life. Examples of these include lignin, natural rubber, and polysaccharides such as sodium alginate, cellulose, and chitosan. The more significant polysaccharide is sodium alginate, which has been extensively utilized in medicine as an alginate fiber for hemostatic gauze or as a hydrogel preparation.. (80)
Table 6 displays the recent patents and ongoing clinical trial stages of major drug delivery systems (DDS) for various treatment applications. Lipid-based nano drug delivery systems, such as liposomal and extracellular vesicles (EVs), are the most researched, followed by hydrogels and nanofibers.
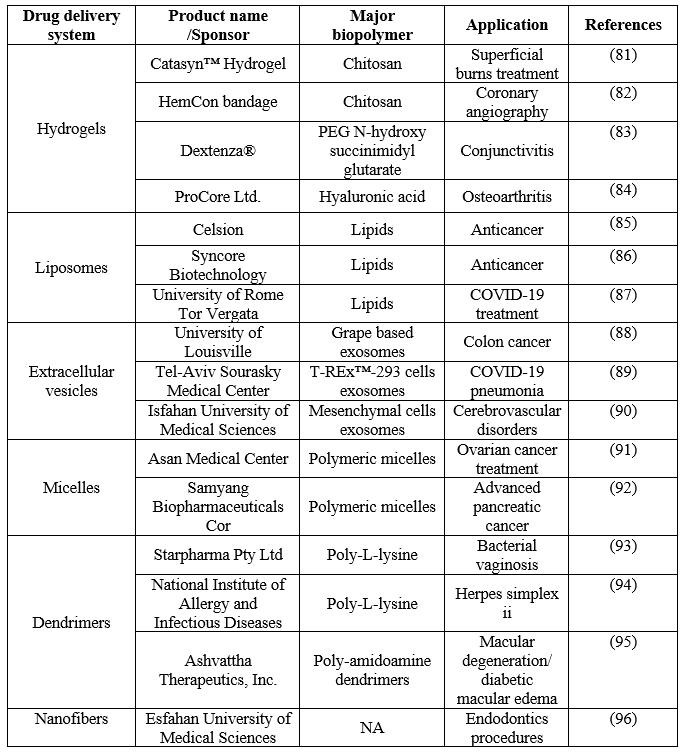
Table 6 Ongoing clinical trials and patents of major drug delivery systems
Biomaterials for Special Applications Based on Natural Polymers:
It provides dressing materials made mostly of natural polymers, which are created in the form of films and hydrogels among other things. Chitin and its derivative chitosan, which are the fundamental components of marine crustacean shells (e.g., crabs, shrimp, and krill), are examples of natural polymers with intriguing biological features that are obtained from renewable sources and additionally generated by certain bacterial or fungal species' microbial fermentation. The criteria of contemporary dressing materials and implants are mostly satisfied by biomaterials based on the polysaccharides above. The usage of biodegradable materials responds to worldwide trends in the creation of mostly disposable items while also assisting in lowering the expenses related to trash storage and disposal.(97,98)
Evaluation Parameters For Biopolymeric Nanoformulations
The determination of the mean and distributional sizes of the particles is a crucial factor in determining the bionanosuspension'sstability.In both nanosuspension and bionanosuspension, the amorphous drug-loaded nanoparticles can be described. The determination of zeta potential is an additional metric utilized in the assessment of particle surface charge, which determines the stability of both nano- and bionanosuspension. Therefore, both nanosuspension and bionanosuspension stability are attributed to consistent particle size distribution.(99–101)
Biopolymers A Novel Bioretardant In Nano-Formulations Development
After extracting the biodispersant from Cicer arietinum seeds using double-distilled water and ethanol, the biodispersant was collected and subjected to additional analysis for physicochemical properties such as colour, odor, particle size, shape, solubility, and IR spectral studies. Major biopolymers found in cereal grains include lipids, non-starch polysaccharides, protein, and starch (Table 7). The IR spectrum of the isolated biomaterial revealed the presence of tertiary alcohol groups, aromatic ring secondary, and saturated hydrocarbons. (102)
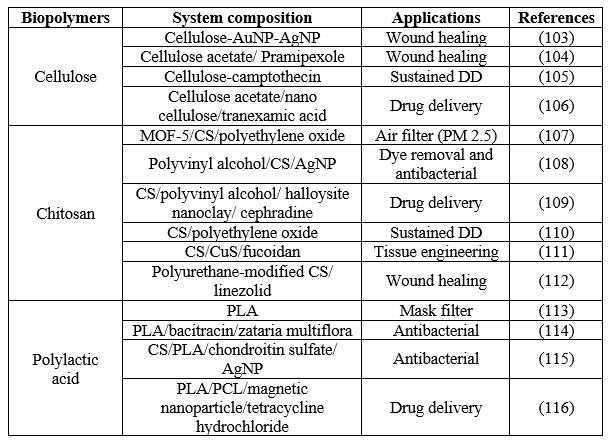
Table 7 Recent biomedical applications of major biopolymers-based nanofibers fabricated by electrospinning.
CHALLENGES AND LIMITATIONS
Numerous problems continue to impede the quick acceptance of biopolymer utilization. Certain items, particularly in humid or unfavorable settings, have low mechanical properties, quick disintegration, and high hydrophilic capacity, making their use unfeasible. Furthermore, by taking into account the inherent qualities and susceptibility of the chosen strain, this will optimize the entire process and reveal methods for creating the ideal formulation using preclinical, in vitro, and in vitro methods that take into account capsule release, production, packaging, shipping, and storage. First, the majority of research done to date has been done in vitro; as a result, additional in vivo and clinical studies are required to prove the health advantages of biopolymers and their biocompatibility in a range of biomedical applications, particularly when they are utilized as drug delivery encapsulation materials. More research is required to determine the right molecule to utilize in combination or alone to treat different diseases and achieve the necessary payload at therapeutically relevant concentrations in a carefully regulated manner. Second, enhancing end-use mechanical qualities, kinetics and release, heat resistance, and barrier properties to produce materials that are as good as or better than synthetic products. Certain items, particularly in humid or unfavorable settings, have low mechanical properties, accelerate degradation, and have a high hydrophilic capacity, making their use unfeasible. A thorough multidisciplinary study involving microbiologists, physicians, and engineers specializing in biomaterials, food, agronomy, and chemistry is required, even though the application of encapsulated probiotics has been extensively studied. Probiotic encapsulating formulation prototypes will become more refined and effective as a result, and the most appropriate polymeric carriers will be used in product manufacture to identify the most targeted and potent probiotic strains.
Third, in this quickly developing subject, it is necessary to address the issues pertaining to expenses, economic factors, and the discrepancy between policy and global adoption of the new technology.
CONCLUSION
The use of biopolymers such as PLA, silk, and chitosan is the subject of growing research interest in medical applications. Biopolymer nanocomposites are particularly intriguing due to their unique properties, including biocompatibility, biodegradability, nontoxicity, and improved structural and functional features. These new materials are highly significant in medicine, as synthetic materials do not fully meet the requirements of living systems. This review succinctly highlights the significant benefits of specific biopolymers, particularly chitosan and cellulose, in biomedical applications, such as antimicrobial agents, drug delivery systems, implant materials, and tissue engineering. Recent studies have demonstrated that the use of biopolymers, in combination with synthetic materials, has the potential to revolutionize the field of medicine.
ABBREVIATION
DDS-Drug delivery system.
CFU-Colony forming unit.
BVC- Bacterial nanocellulose
PVA- Poly vinyl alcohol
BNC -Bacterial nanocellulose
CNC- Cellulose nanocrystal
CNF- Cellulose nanofibers
CS -Chitosan
CM- Convergent method
CMC- Critical micellar concentration
DM -Divergent method
DDS- Drug delivery system
ES -Electrospinning
ECM- Extracellular matrix
EVs- Extracellular vesicles
FDA -Food and Drug Administration
GPC- Global production capacity
GTA -Glycerol triacetate
HMPA -2,2-bis(hydroxymethyl)propanoic acid
HNT- Halloysite nanotubes
MTX-- Methotrexate
NC- Nanocellulose
NF- Nanofiber
PEG- Polyethylene glycol
PVP- Polyvinylpyrrolidone
PCL- poly-caprolactone
PLA -Poly lactic acid
PMS -Polymeric micelles
AuNP –Gold nanopartical
REFERENCE
- Pattanashetti NA, Heggannavar GB, Kariduraganavar MY. Smart biopolymers and their biomedical applications. Procedia Manuf. 2017;12:263–79.
- Rendón-Villalobos R, Ortíz-Sánchez A, Tovar-Sánchez E, Flores-Huicochea E. The role of biopolymers in obtaining environmentally friendly materials. Composites from renewable and sustainable materials. 2016;151.
- Yadav P, Yadav H, Shah VG, Shah G, Dhaka G. Biomedical biopolymers, their origin and evolution in biomedical sciences: a systematic review. J Clin Diagn Res. 2015;9(9):ZE21.
- Stryer L, Berg JM, Tymoczko JL. Biochemistry And Study Guide. WH Freeman: New York, NY, USA; 2002.
- Gullapalli S, Wong MS. Nanotechnology: a guide to nano-objects. Chem Eng Prog. 2011;107(5):28–32.
- Rebitski EP, Darder M, Carraro R, Aranda P, Ruiz-Hitzky E. Chitosan and pectin core–shell beads encapsulating metformin–clay intercalation compounds for controlled delivery. New Journal of Chemistry. 2020;44(24):10102–10.
- Cheikh D, García-Villén F, Majdoub H, Zayani MB, Viseras C. Complex of chitosan pectin and clay as diclofenac carrier. Appl Clay Sci. 2019;172:155–64.
- de Oliveira Pedro R, Goycoolea FM, Pereira S, Schmitt CC, Neumann MG. Synergistic effect of quercetin and pH-responsive DEAE-chitosan carriers as drug delivery system for breast cancer treatment. Int J Biol Macromol. 2018;106:579–86.
- Yen MT, Yang JH, Mau JL. Physicochemical characterization of chitin and chitosan from crab shells. Carbohydr Polym. 2009;75(1):15–21.
- Szyma?ska E, Winnicka K. Stability of chitosan—a challenge for pharmaceutical and biomedical applications. Mar Drugs. 2015;13(4):1819–46.
- Seidi F, Yazdi MK, Jouyandeh M, Dominic M, Naeim H, Nezhad MN, et al. Chitosan-based blends for biomedical applications. Int J Biol Macromol. 2021;183:1818–50.
- Gopi S, Balakrishnan P, Chandradhara D, Poovathankandy D, Thomas S. General scenarios of cellulose and its use in the biomedical field. Mater Today Chem. 2019;13:59–78.
- Kargarzadeh H, Mariano M, Gopakumar D, Ahmad I, Thomas S, Dufresne A, et al. Advances in cellulose nanomaterials. Cellulose. 2018;25:2151–89.
- Liu D, Chen X, Yue Y, Chen M, Wu Q. Structure and rheology of nanocrystalline cellulose. Carbohydr Polym. 2011;84(1):316–22.
- Tortorella S, Vetri Buratti V, Maturi M, Sambri L, Comes Franchini M, Locatelli E. Surface-modified nanocellulose for application in biomedical engineering and nanomedicine: A review. Int J Nanomedicine. 2020;9909–37.
- Drumright RE, Gruber PR, Henton DE. Polylactic acid technology. Advanced materials. 2000;12(23):1841–6.
- Garlotta D. A literature review of poly (lactic acid). J Polym Environ. 2001;9:63–84.
- Milovanovic S, Markovic D, Mrakovic A, Kuska R, Zizovic I, Frerich S, et al. Supercritical CO2-assisted production of PLA and PLGA foams for controlled thymol release. Materials Science and Engineering: C. 2019;99:394–404.
- Panickar KS, Bhathena SJ. Control of fatty acid intake and the role of essential fatty acids in cognitive function and neurological disorders. Fat detection: taste, texture, and post ingestive effects. 2010;470–4.
- Watson H. Biological membranes. Essays Biochem. 2015;59:43–69.
- Boyd BJ, Bergström CAS, Vinarov Z, Kuentz M, Brouwers J, Augustijns P, et al. Successful oral delivery of poorly water-soluble drugs both depends on the intraluminal behavior of drugs and of appropriate advanced drug delivery systems. European Journal of Pharmaceutical Sciences. 2019;137:104967.
- Avila Rodríguez MI, Rodríguez Barroso LG, Sánchez ML. Collagen: A review on its sources and potential cosmetic applications. J Cosmet Dermatol. 2018;17(1):20–6.
- Bellion P, Digles J, Will F, Dietrich H, Baum M, Eisenbrand G, et al. Polyphenolic apple extracts: Effects of raw material and production method on antioxidant effectiveness and reduction of DNA damage in Caco-2 cells. J Agric Food Chem. 2010;58(11):6636–42.
- Rao MG, Bharathi P, Akila RM. A comprehensive review on biopolymers. Sci Revs Chem Commun. 2014;4(2):61–8.
- Parker G. Measuring the environmental performance of food packaging: Life cycle assessment. In: Environmentally compatible food packaging. Elsevier; 2008. p. 211–37.
- Hassan MES, Bai J, Dou DQ. Biopolymers; definition, classification and applications. Egypt J Chem. 2019;62(9):1725–37.
- Soldo A, Mileti? M, Auad ML. Biopolymers as a sustainable solution for the enhancement of soil mechanical properties. Sci Rep. 2020;10(1):267.
- Jayanth D, Kumar PS, Nayak GC, Kumar JS, Pal SK, Rajasekar R. A review on biodegradable polymeric materials striving towards the attainment of green environment. J Polym Environ. 2018;26:838–65.
- Ferraris S, Spriano S, Scalia AC, Cochis A, Rimondini L, Cruz-Maya I, et al. Topographical and biomechanical guidance of electrospun fibers for biomedical applications. Polymers (Basel). 2020;12(12):2896.
- Dhandayuthapani B, Yoshida Y, Maekawa T, Kumar DS. Polymeric scaffolds in tissue engineering application: a review. Int J Polym Sci. 2011;2011.
- Ogueri KS, Jafari T, Escobar Ivirico JL, Laurencin CT. Polymeric biomaterials for scaffold-based bone regenerative engineering. Regen Eng Transl Med. 2019;5:128–54.
- de Vos P, Lazarjani HA, Poncelet D, Faas MM. Polymers in cell encapsulation from an enveloped cell perspective. Adv Drug Deliv Rev. 2014;67:15–34.
- Madene A, Jacquot M, Scher J, Desobry S. Flavour encapsulation and controlled release–a review. Int J Food Sci Technol. 2006;41(1):1–21.
- Almutairi FM. Biopolymer Nanoparticles: A Review of Prospects for Application as Carrier for Therapeutics and Diagnostics. International Journal of Pharmaceutical Research & Allied Sciences. 2019;8(1).
- Sharma K, Singh V, Arora A. Natural biodegradable polymers as matrices in transdermal drug delivery. Int J Drug Dev Res. 2011;3(2):85–103.
- Julian MD. Designing biopolymer microgels to encapsulate, protect and deliver bioactive components: Physicochemical aspects. 2017;
- Mart?u GA, Mihai M, Vodnar DC. The use of chitosan, alginate, and pectin in the biomedical and food sector—biocompatibility, bioadhesiveness, and biodegradability. Polymers (Basel). 2019;11(11):1837.
- Lim F, Sun AM. Microencapsulated islets as bioartificial endocrine pancreas. Science (1979). 1980;210(4472):908–10.
- Pakdel PM, Peighambardoust SJ. Review on recent progress in chitosan-based hydrogels for wastewater treatment application. Carbohydr Polym. 2018;201:264–79.
- Naz S, Ali JS, Zia M. Nanocellulose isolation characterization and applications: a journey from non-remedial to biomedical claims. Biodes Manuf. 2019;2:187–212.
- Bose PS, Nagaraju R, Saritha D, Padmasri B, Reddy PS. Preparation and evaluation of indomethacin loaded alginate microspheres. Ceska Slov Farm. 2016;65(3):104–10.
- Patel N, Lalwani D, Gollmer S, Injeti E, Sari Y, Nesamony J. Development and evaluation of a calcium alginate based oral ceftriaxone sodium formulation. Prog Biomater. 2016;5:117–33.
- Hariyadi DM, Hendradi E, Erawati T, Jannah EN, Febrina W. Influence of drug-polymer ratio on physical characteristics and release of metformin hydrochloride from metforminalginate microspheres. Tropical Journal of Pharmaceutical Research. 2018;17(7):1229–33.
- Thomas D, KurienThomas K, Latha MS. Preparation and evaluation of alginate nanoparticles prepared by green method for drug delivery applications. Int J Biol Macromol. 2020;154:888–95.
- Raha A, Bhattacharjee S, Mukherjee P, Paul M, Bagchi A. Design and characterization of ibuprofen loaded alginate microspheres prepared by ionic gelation method. Int J Pharma Res Health Sci. 2018;6:2713–29.
- Rajalekshmy GP, Rekha MR. Synthesis and evaluation of an alginate-methacrylate xerogel for insulin delivery towards wound healing applications. Ther Deliv. 2021;12(3):215–34.
- Cánepa C, Imperiale JC, Berini CA, Lewicki M, Sosnik A, Biglione MM. Development of a drug delivery system based on chitosan nanoparticles for oral administration of interferon-?. Biomacromolecules. 2017;18(10):3302–9.
- Shariatinia Z, Mazloom-Jalali A. Chitosan nanocomposite drug delivery systems designed for the ifosfamide anticancer drug using molecular dynamics simulations. J Mol Liq. 2019;273:346–67.
- Hanna DH, Saad GR. Encapsulation of ciprofloxacin within modified xanthan gum-chitosan based hydrogel for drug delivery. Bioorg Chem. 2019;84:115–24.
- Almeida A, Araújo M, Novoa-Carballal R, Andrade F, Gonçalves H, Reis RL, et al. Novel amphiphilic chitosan micelles as carriers for hydrophobic anticancer drugs. Materials Science and Engineering: C. 2020;112:110920.
- Sohail R, Abbas SR. Evaluation of amygdalin-loaded alginate-chitosan nanoparticles as biocompatible drug delivery carriers for anticancerous efficacy. Int J Biol Macromol. 2020;153:36–45.
- Ngwabebhoh FA, Zandraa O, Patwa R, Saha N, Capáková Z, Saha P. Self-crosslinked chitosan/dialdehyde xanthan gum blended hypromellose hydrogel for the controlled delivery of ampicillin, minocycline and rifampicin. Int J Biol Macromol. 2021;167:1468–78.
- Metwally MM, Muñoz-Espi R, Youssef I, Abdelaal M. Synthesis of 3-dimensional chitosan/carboxymethyl cellulose/zno biopolymer hybrids by ionotropic gelation for application in drug delivery. Egypt J Chem. 2022;65(1):299–307.
- Han AS, Kim J, Park JW, Jin SG. Novel acyclovir-loaded film-forming gel with enhanced mechanical properties and skin permeability. J Drug Deliv Sci Technol. 2022;70:103213.
- Mostafavi FS, Zaeim D. Agar-based edible films for food packaging applications-A review. Int J Biol Macromol. 2020;159:1165–76.
- Zhang C, An D, Xiao Q, Weng H, Zhang Y, Yang Q, et al. Preparation, characterization, and modification mechanism of agar treated with hydrogen peroxide at different temperatures. Food Hydrocoll. 2020;101:105527.
- Santana ÁL, Meireles MAA. New starches are the trend for industry applications: a review. 2014;
- Qi X, Tester RF. Starch granules as active guest molecules or microorganism delivery systems. Food Chem. 2019;271:182–6.
- Xu J, Tan X, Chen L, Li X, Xie F. Starch/microcrystalline cellulose hybrid gels as gastric-floating drug delivery systems. Carbohydr Polym. 2019;215:151–9.
- Li W, Liu L, Tian H, Luo X, Liu S. Encapsulation of Lactobacillus plantarum in cellulose based microgel with controlled release behavior and increased long-term storage stability. Carbohydr Polym. 2019;223:115065.
- Alabi CO, Singh I, Odeku OA. Evaluation of starch-clay composites as a pharmaceutical excipient in tramadol tablet formulations. Polymers in Medicine. 2020;50(1):33–40.
- Sachan N, Bhattacharya A. Modeling and characterization of drug release from glutinous rice starch based hydrogel beads for controlled drug delivery. International Journal of Health Research. 2009;2(1).
- Tesfay D, Abrha S, Yilma Z, Woldu G, Molla F. Preparation, optimization, and evaluation of epichlorohydrin cross-linked enset (Ensete ventricosum (Welw.) Cheeseman) starch as drug release sustaining excipient in microsphere formulation. Biomed Res Int. 2020;2020.
- Alwaan IM, Ahmed M, Al-Kelaby KKA, Allebban ZSM. Starch-chitosan modified blend as long-term controlled drug release for cancer therapy. Pakistan Journal of Biotechnology. 2018;15(4):947–55.
- Fathollahipour S, Maziarfar S, Mehrizi AA, Tavakoli J. Synthesis and characterization of Erythromycin loaded PVA-corn starch hydrogel wound dressing.
- Cherian RM, Tharayil A, Varghese RT, Antony T, Kargarzadeh H, Chirayil CJ, et al. A review on the emerging applications of nano-cellulose as advanced coatings. Carbohydr Polym. 2022;282:119123.
- Singhvi MS, Zinjarde SS, Gokhale D V. Polylactic acid: Synthesis and biomedical applications. J Appl Microbiol. 2019;127(6):1612–26.
- Díaz-Montesa E, Cerón-Montesa GI, Vargas-Leóna EA. Encapsulación de compuestos bioactivos: una revisión sistemática Encapsulation of bioactive compounds: a systematic review.
- Çakmak H, Özselek Y, Turan OY, F?ratl?gil E, Karbancio?lu-Güler F. Whey protein isolate edible films incorporated with essential oils: Antimicrobial activity and barrier properties. Polym Degrad Stab. 2020;179:109285.
- Zhang B, Liu Y, Wang H, Liu W, Cheong K leong, Teng B. Characterization of seaweed polysaccharide-based bilayer films containing essential oils with antibacterial activity. LWT. 2021;150:111961.
- Ramos PE, Silva P, Alario MM, Pastrana LM, Teixeira JA, Cerqueira MA, et al. Effect of alginate molecular weight and M/G ratio in beads properties foreseeing the protection of probiotics. Food Hydrocoll. 2018;77:8–16.
- Zhang Z, Gu M, You X, Sela DA, Xiao H, McClements DJ. Encapsulation of bifidobacterium in alginate microgels improves viability and targeted gut release. Food Hydrocoll. 2021;116:106634.
- Albadran HA, Monteagudo-Mera A, Khutoryanskiy V V, Charalampopoulos D. Development of chitosan-coated agar-gelatin particles for probiotic delivery and targeted release in the gastrointestinal tract. Appl Microbiol Biotechnol. 2020;104:5749–57.
- Jia S, Zhou K, Pan R, Wei J, Liu Z, Xu Y. Oral immunization of carps with chitosan–alginate microcapsule containing probiotic expressing spring viremia of carp virus (SVCV) G protein provides effective protection against SVCV infection. Fish Shellfish Immunol. 2020;105:327–9.
- Ashwar BA, Gani A, Gani A, Shah A, Masoodi FA. Production of RS4 from rice starch and its utilization as an encapsulating agent for targeted delivery of probiotics. Food Chem. 2018;239:287–94.
- Thangrongthong S, Puttarat N, Ladda B, Itthisoponkul T, Pinket W, Kasemwong K, et al. Microencapsulation of probiotic Lactobacillus brevis ST-69 producing GABA using alginate supplemented with nanocrystalline starch. Food Sci Biotechnol. 2020;29:1475–82.
- Dhaneshwar SS, Kandpal M, Gairola N, Kadam SS. Dextran: A Promising Macromolecular Drug Carrier. Indian J Pharm Sci. 2006;68(6).
- Varshosaz J. Dextran conjugates in drug delivery. Expert Opin Drug Deliv. 2012;9(5):509–23.
- Juloski JT, Rakic A, ?uk V V, ?uk VM, Stefanovi? S, Nikoli? D, et al. Colorectal cancer and trace elements alteration. Journal of Trace Elements in Medicine and Biology. 2020;59:126451.
- Yu Y, Shang L, Guo J, Wang J, Zhao Y. Design of capillary microfluidics for spinning cell-laden microfibers. Nat Protoc. 2018;13(11):2557–79.
- Saeedi M, Vahidi O, Moghbeli MR, Ahmadi S, Asadnia M, Akhavan O, et al. Customizing nano-chitosan for sustainable drug delivery. Journal of Controlled Release. 2022;350:175–92.
- Fuste?-D?moc I, M?lu?an T, Mija A. High content chitosan-based materials with high performance properties. Int J Biol Macromol. 2022;223:263–72.
- Ibach MJ, Zimprich L, Wallin DD, Olevson C, Puls-Boever K, Thompson V. In clinic optometrist insertion of dextenza (dexamethasone ophthalmic insert 0.4 mg) prior to cataract surgery: the PREPARE Study. Clin Ophthalmol. 2022;16:2609.
- Øvrebø Ø, Perale G, Wojciechowski JP, Echalier C, Jeffers JRT, Stevens MM, et al. Design and clinical application of injectable hydrogels for musculoskeletal therapy. Bioeng Transl Med. 2022;7(2):e10295.
- Andra VVSNL, Pammi SVN, Bhatraju LVKP, Ruddaraju LK. A comprehensive review on novel liposomal methodologies, commercial formulations, clinical trials and patents. Bionanoscience. 2022;12(1):274–91.
- Akhtar N, Mohammed SAA, Singh V, Abdellatif AAH, Mohammad HA, Ahad A, et al. Liposome-based drug delivery of various anticancer agents of synthetic and natural product origin: a patent overview. Pharm Pat Anal. 2020;9(3):87–116.
- Campione E, Lanna C, Cosio T, Rosa L, Conte MP, Iacovelli F, et al. Lactoferrin as antiviral treatment in COVID-19 management: preliminary evidence. Int J Environ Res Public Health. 2021;18(20):10985.
- Karamanidou T, Tsouknidas A. Plant-derived extracellular vesicles as therapeutic nanocarriers. Int J Mol Sci. 2021;23(1):191.
- Rezaie J, Feghhi M, Etemadi T. A review on exosomes application in clinical trials: perspective, questions, and challenges. Cell Communication and Signaling. 2022;20(1):145.
- Yuan Y, Sun J, You T, Shen W, Xu W, Dong Q, et al. Extracellular vesicle-based therapeutics in neurological disorders. Pharmaceutics. 2022;14(12):2652.
- Kaur J, Gulati M, Jha NK, Disouza J, Patravale V, Dua K, et al. Recent advances in developing polymeric micelles for treating cancer: Breakthroughs and bottlenecks in their clinical translation. Drug Discov Today. 2022;27(5):1495–512.
- Cai Y, Qi J, Lu Y, He H, Wu W. The in vivo fate of polymeric micelles. Adv Drug Deliv Rev. 2022;188:114463.
- Mahant S, Sharma AK, Gandhi H, Wadhwa R, Dua K, Kapoor DN. Emerging trends and potential prospects in vaginal drug delivery. Curr Drug Deliv. 2023;20(6):730–51.
- Caminade AM. Dendrimers in personalized medicine. Encyclopedia Platform. 2022;entry-27135.
- Caminade AM. Dendrimers, an emerging opportunity in personalized medicine? J Pers Med. 2022;12(8):1334.
- Golestannejad Z, Khozeimeh F, Mehrasa M, Mirzaeei S, Sarfaraz D. A novel drug delivery system using acyclovir nanofiber patch for topical treatment of recurrent herpes labialis: A randomized clinical trial. Clin Exp Dent Res. 2022;8(1):184–90.
- Tolaimate A, Desbrieres J, Rhazi M, Alagui A, Vincendon M, Vottero P. On the influence of deacetylation process on the physicochemical characteristics of chitosan from squid chitin. Polymer (Guildf). 2000;41(7):2463–9.
- Struszczyk H. Chitin and chitosan. Part I. Properties and production. Polimery. 2002;47(5):316–25.
- Madhav NVS, Jaiswal V, Ojha A. Development and evaluation of nanosized aripiprazole-loaded bioflexy films using a biopolymer from Lagenaria siceraria for brain delivery through orosoft palatal mucosal platform. Egyptian Pharmaceutical Journal. 2017;16(1):62–8.
- Tyagi Y, Madhav NVS. Inbuilt novel bioretardant feature of biopolymer isolated from cucumis sativa for designing drug loaded bionanosuspension. J Drug Assess. 2020;9(1):72–81.
- Grau MJ, Müller RH. Increase of dissolution velocity and solubility of poorly soluble drugs by formulation as nanosuspension. In: Proceedings 2nd World Meeting APGI/APV, Paris. 1998. p. 623–4.
- Madhav NVS, Pranshu Tangri PT. Formulation and evaluation of zidovudine bio-micro dwarfs using a novel bio-muco resident from Artocarpus heterophyllus. 2011;
- Rodklongtan A, La?ongkham O, Nitisinprasert S, Chitprasert P. Enhancement of Lactobacillus reuteri KUB?AC5 survival in broiler gastrointestinal tract by microencapsulation with alginate–chitosan semi?interpenetrating polymer networks. J Appl Microbiol. 2014;117(1):227–38.
- Wu QX, Xu X, Xie Q, Tong WY, Chen Y. Evaluation of chitosan hydrochloride-alginate as enteric micro-probiotic-carrier with dual protective barriers. Int J Biol Macromol. 2016;93:665–71.
- Çakmak H, Özselek Y, Turan OY, F?ratl?gil E, Karbancio?lu-Güler F. Whey protein isolate edible films incorporated with essential oils: Antimicrobial activity and barrier properties. Polym Degrad Stab. 2020;179:109285.
- Zhang B, Liu Y, Wang H, Liu W, Cheong K leong, Teng B. Characterization of seaweed polysaccharide-based bilayer films containing essential oils with antibacterial activity. LWT. 2021;150:111961.
- Alfaro-Galarza O, López-Villegas EO, Rivero-Perez N, Tapia-Maruri D, Jiménez-Aparicio AR, Palma-Rodríguez HM, et al. Protective effects of the use of taro and rice starch as wall material on the viability of encapsulated Lactobacillus paracasei subsp. Paracasei. LWT. 2020;117:108686.
- Ashwar BA, Gani A, Gani A, Shah A, Masoodi FA. Production of RS4 from rice starch and its utilization as an encapsulating agent for targeted delivery of probiotics. Food Chem. 2018;239:287–94.
- Dafe A, Etemadi H, Dilmaghani A, Mahdavinia GR. Investigation of pectin/starch hydrogel as a carrier for oral delivery of probiotic bacteria. Int J Biol Macromol. 2017;97:536–43.
- Benavent-Gil Y, Rodrigo D, Rosell CM. Thermal stabilization of probiotics by adsorption onto porous starches. Carbohydr Polym. 2018;197:558–64.
- Zanjani MAK, Ehsani MR, Ghiassi Tarzi B, Sharifan A. Promoting Lactobacillus casei and Bifidobacterium adolescentis survival by microencapsulation with different starches and chitosan and poly L?lysine coatings in ice cream. J Food Process Preserv. 2018;42(1):e13318.
- Lancuški A, Ammar AA, Avrahami R, Vilensky R, Vasilyev G, Zussman E. Design of starch-formate compound fibers as encapsulation platform for biotherapeutics. Carbohydr Polym. 2017;158:68–76.
- Reyes V, Chotiko A, Chouljenko A, Sathivel S. Viability of Lactobacillus acidophilus NRRL B-4495 encapsulated with high maize starch, maltodextrin, and gum arabic. Lwt. 2018;96:642–7.
- Thangrongthong S, Puttarat N, Ladda B, Itthisoponkul T, Pinket W, Kasemwong K, et al. Microencapsulation of probiotic Lactobacillus brevis ST-69 producing GABA using alginate supplemented with nanocrystalline starch. Food Sci Biotechnol. 2020;29:1475–82.
- Sultana K, Godward G, Reynolds N, Arumugaswamy R, Peiris P, Kailasapathy K. Encapsulation of probiotic bacteria with alginate–starch and evaluation of survival in simulated gastrointestinal conditions and in yoghurt. Int J Food Microbiol. 2000;62(1–2):47–55.
- Haroosh HJ, Dong Y, Jasim S, Ramakrishna S. Morphological structures and drug release effect of multiple electrospun nanofibre membrane systems based on PLA, PCL, and PCL/magnetic nanoparticle composites. J Nanomater. 2022;2022(1):5190163.


 Subhasish Pramanik*
Subhasish Pramanik*










 10.5281/zenodo.13143255
10.5281/zenodo.13143255